Hyperglycaemia and Its Prognostic Value in Patients with COVID-19 Admitted to the Hospital in Lithuania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection and Variables
2.3. Main Outcome
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, S.; Demmer, R.T. Impaired glucose regulation, SARS-CoV-2 infections and adverse COVID-19 outcomes. Transl. Res. 2022, 241, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Del Prato, S. COVID-19 in people with diabetes:Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020, 8, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Behl, T.; Sharma, N.; Singh, S.; Grewal, A.S.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Bungau, S. COVID-19 and diabetes: Association intensify risk factors fo rmorbidity and mortality. Biomed. Pharmacother. 2022, 151, 113089. [Google Scholar] [CrossRef] [PubMed]
- Vedantam, D.; Poman, D.S.; Motwani, L.; Asif, N.; Patel, A.; Anne, K.K. Stress-InducedHyperglycemia: Consequences and Management. Cureus 2022, 14, e26714. [Google Scholar] [PubMed]
- Plummer, M.P.; Bellomo, R.; Cousins, C.E.; Annink, C.E.; Sundararajan, K.; Reddi, B.A.; Raj, J.P.; Chapman, M.J.; Horowitz, M.; Deane, A.M. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med. 2014, 40, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Khalfallah, M.; Abdelmageed, R.; Elgendy, E.; Hafez, Y.M. Incidence, predictors and outcomes of stress hyperglycemia in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Diabetes Vasc. Dis. Res. 2020, 17, 1479164119883983. [Google Scholar] [CrossRef]
- Li, J.; Quan, K.; Wang, Y.; Zhao, X.; Li, Z.; Pan, Y.; Li, H.; Liu, L.; Wang, Y. Effect of Stress Hyperglycemiaon Neurological Deficit and Mortality in the Acute Ischemic Stroke People with and Without Diabetes. Front. Neurol. 2020, 11, 576895. [Google Scholar] [CrossRef]
- Mamtani, M.; Kulkarni, H.; Bihari, S.; Prakash, S.; Chavan, S.; Huckson, S.; Pilcher, D. Degree of hyperglycemia independently associates with hospital mortality and length of stay in critically ill, nondiabetic patients: Results from the ANZICSCORE binational registry. J. Crit. Care 2020, 55, 149–156. [Google Scholar] [CrossRef]
- Müller, J.A.; Groß, R.; Conzelmann, C.; Krüger, J.; Merle, U.; Steinhart, J.; Weil, T.; Koepke, L.; Bozzo, C.P.; Read, C.; et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 2021, 3, 149–165. [Google Scholar] [CrossRef]
- Matias, A.A.; Manique, I.; Sabino, T.; Rego, T.; Mihon, C.; Panarra, A.; Rizzo, M.; Silva-Nunes, J. Absolute Hyperglycemia versus Stress Hyperglycemia Ratio for the Prognosis of Hospitalized Patients with COVID-19 in the First Months of the Pandemic: A Retrospective Study. Diabetes Ther. 2022, 14, 335–346. [Google Scholar] [CrossRef]
- Manique, I.; Abegão Matias, A.; Bouça, B.; Rego, T.; Cortez, L.; Sabino, T.; Panarra, A.; Rizzo, M.; Silva-Nunes, J. Does the Hyperglycemia Impact on COVID-19 Outcomes Depend upon the Presence of Diabetes? An Observational Study. Metabolites 2022, 12, 1116. [Google Scholar] [CrossRef]
- Liu, S.P.; Zhang, Q.; Wang, W.; Zhang, M.; Liu, C.; Xiao, X.; Liu, Z.; Hu, W.-M.; Jin, P. Hyperglycemia is astrong predictor of poor prognosis in COVID-19. Diabetes Res. Clin. Pract. 2020, 167, 108338. [Google Scholar] [CrossRef]
- Wang, S.; Ma, P.; Zhang, S.; Song, S.; Wang, Z.; Ma, Y.; Xu, J.; Wu, F.; Duan, L.; Yin, Z.; et al. Fasting blood glucose on admission is an independent predictor for 28-daymortality in patients with COVID-19 without previous diagnosis of diabetes: A multi-centre retrospective study. Diabetologia 2020, 63, 2102–2111. [Google Scholar] [CrossRef]
- Mamtani, M.; Athavale, A.M.; Abraham, M.; Vernik, J.; Amarah, A.R.; Ruiz, J.P.; Joshi, A.J.; Itteera, M.; Zhukovski, S.D.; Madaiah, R.P.; et al. Association of hyperglycaemia with hospital mortality in nondiabetic COVID-19 patients: A cohort study. Diabetes Metab. 2021, 47, 101254. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, W.; Xia, P.; Xu, Y.; Li, L.; Li, Q.; Yang, L.; Wei, Q.; Wang, H.; Li, H.; et al. Impaired Fasting Glucose and Diabetes Are Related to Higher Risks of Complications and MortalityAmong Patients with Coronavirus Disease 2019. Front. Endocrinol. 2020, 11, 525. [Google Scholar] [CrossRef]
- Kubiliute, I.; Vitkauskaite, M.; Urboniene, J.; Svetikas, L.; Zablockiene, B.; Jancoriene, L. Clinical characteristics and predictors for in-hospital mortality in adult COVID-19 patients: A retrospective single center cohort study in Vilnius, Lithuania. PLoS ONE 2023, 18, e0290656. [Google Scholar] [CrossRef]
- McGuinness, O.P. Defective glucose homeostasis during infection. Annu. Rev. Nutr. 2005, 25, 9–35. [Google Scholar] [CrossRef]
- Boonen, E.; Vanden Berghe, G. Endocrine responses to critical illness:Novel insights and therapeutic implications. J. Clin. Endocrinol. Metab. 2014, 99, 1569–1582. [Google Scholar] [CrossRef]
- Gunst, J.; De Bruyn, A.; Vanden Berghe, G. Glucose control in the ICU. Curr. Opin. Anaesthesiol. 2019, 32, 156–162. [Google Scholar] [CrossRef]
- Sasidharakurup, H.; Kumar, G.; Nair, B.; Diwakar, S. Mathematical Modeling of Severe Acute Respiratory Syndrome Coronavirus 2 Infection Network with Cytokine Storm, Oxidative Stress, Thrombosis, Insulin Resistance, and Nitric Oxide Pathways. OMICS 2021, 25, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Rajsfus, B.F.; Mohana-Borges, R.; Allonso, D. Diabetogenic viruses: Linking viruses to diabetes mellitus. Heliyon 2023, 9, e15021. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Dalamaga, M.; Liu, J. SARS-CoV-2 adipose tissue infection and hyperglycemia: A further step towards the understanding of severe COVID-19. Metab. Open 2022, 13, 100163. [Google Scholar] [CrossRef]
- He, X.; Liu, C.; Peng, J.; Li, Z.; Li, F.; Wang, J.; Hu, A.; Peng, M.; Huang, K.; Fan, D.; et al. COVID-19 induces new-onset insulin resistance and lipid metabolic dysregulation via regulation of secreted metabolic factors. Signal Transduct. Target. Ther. 2021, 6, 427. [Google Scholar] [CrossRef]
- Ilias, I.; Jahaj, E.; Kokkoris, S.; Zervakis, D.; Temperikidis, P.; Magira, E.; Pratikaki, M.; Vassiliou, A.G.; Routsi, C.; Kotanidou, A.; et al. Clinical Study of Hyperglycemia and SARS-CoV-2 Infection in Intensive Care Unit Patients. In Vivo 2020, 34, 3029–3032. [Google Scholar] [CrossRef]
- Ilias, I.; Diamantopoulos, A.; Pratikaki, M.; Botoula, E.; Jahaj, E.; Athanasiou, N.; Pratikaki, M.; Vassiliou, A.G.; Routsi, C.; Kotanidou, A.; et al. Glycemia, Beta-Cell Function and Sensitivity to Insulin in Mildly to Critically Ill COVID-19 Patients. Medicina 2021, 57, 68. [Google Scholar] [CrossRef]
- Montefusco, L.; Ben Nasr, M.; D’Addio, F.; Loretelli, C.; Rossi, A.; Pastore, I.; Daniele, G.; Abdelsalam, A.; Maestroni, A.; Dell’Acqua, M.; et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021, 3, 774–785. [Google Scholar] [CrossRef]
- Iacobellis, G.; Penaherrera, C.A.; Bermudez, L.E.; Bernal Mizrachi, E. Admission hyperglycemia and radiological findings of SARS-CoV2 in patients with and without diabetes. Diabetes Res. Clin. Pract. 2020, 164, 108185. [Google Scholar] [CrossRef]
- Fadini, G.P.; Morieri, M.L.; Boscari, F.; Fioretto, P.; Maran, A.; Busetto, L.; Bonora, B.M.; Selmin, E.; Arcidiacono, G.; Pinelli, S.; et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res. Clin. Pract. 2020, 168, 108374. [Google Scholar] [CrossRef] [PubMed]
- Coppelli, A.; Giannarelli, R.; Aragona, M.; Penno, G.; Falcone, M.; Tiseo, G.; Ghiadoni, L.; Barbieri, G.; Monzani, F.; Virdis, A.; et al. Hyperglycemia at Hospital Admission Is Associated with Severity of the Prognosis in Patients Hospitalized for COVID-19: The Pisa COVID-19 Study. Diabetes Care 2020, 43, 2345–2348. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Z.; Zhang, J. Hyperglycemia at admission is astrong predictor of mortality and severe/critical complications in COVID-19 patients: Ameta-analysis. Biosci. Rep. 2021, 41, BSR20203584. [Google Scholar] [CrossRef] [PubMed]
- Nateghi, S.; Gomari, M.M.; Roudsari, Y.J.; Foroughi, A.; Mansouri, F.; Shiva, A.; Nasrollahizadeh, A.; Nasiri, Z.; Faraji, N. Moderately hyperglycemia as an independent prognostic factor for the worse outcome of COVID-19. Prim. Care Diabetes 2022, 16, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, S.; Chen, T.; Cui, Z.; Shi, N.; Zhong, X.; Qiu, K.; Zhang, J.; Zeng, T.; Chen, L.; et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes. Metab. 2020, 22, 1897–1906. [Google Scholar] [CrossRef]
- Morse, J.; Gay, W.; Korwek, K.M.; McLean, L.E.; Poland, R.E.; Guy, J.; Sands, K.; Perlin, J.B. Hyperglycaemia increases mortality risk in non-diabetic patients with COVID-19 even more than in diabeticpatients. Endocrinol. Diabetes Metab. 2021, 4, e00291. [Google Scholar] [CrossRef]
- Demeulemeester, F.; de Punder, K.; van Heijningen, M.; van Doesburg, F. Obesity as a Risk Factor for Severe COVID-19 and Complications: A Review. Cells 2021, 10, 933. [Google Scholar] [CrossRef]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Akácsos-Szász, O.Z.; Pál, S.; Nyulas, K.I.; Nemes-Nagy, E.; Fárr, A.M.; Dénes, L.; Szilveszter, M.; Bán, E.G.; Tilinca, M.C.; Simon-Szabó, Z. Pathways of Coagulopathy and Inflammatory Response in SARS-CoV-2 Infection among Type2 Diabetic Patients. Int. J. Mol. Sci. 2023, 24, 4319. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Featuresof 20 133 UK patients in hospital with COVID-19 using the ISARICWHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Müller-Wieland, D.; Marx, N.; Dreher, M.; Fritzen, K.; Schnell, O. COVID-19 and Cardiovascular Comorbidities. Exp. Clin. Endocrinol. Diabetes 2022, 130, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Bader, F.; Manla, Y.; Atallah, B.; Starling, R.C. Heart failure and COVID-19. Heart Fail. Rev. 2021, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, A.; Tascini, C.; Colussi, G.; Peghin, M.; Graziano, E.; De Carlo, C.; Bulfone, L.; Antonello, M.; Sozio, E.; Fabris, M.; et al. Relationship between cytokine release and stress hyperglycemia in patients hospitalized with COVID-19infection. Front. Med. 2022, 9, 988686. [Google Scholar] [CrossRef]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020, 146, 518–534.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Wang, Z.; Liu, N.; He, L.; Zhang, H. Increased risk of new-onset diabetes in patients with COVID-19: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1170156. [Google Scholar] [CrossRef] [PubMed]
- Gerganova, A.; Assyov, Y.; Kamenov, Z. Stress Hyperglycemia, Diabetes Mellitus and COVID-19 Infection: RiskFactors, Clinical Outcomes and Post-Discharge Implications. Front. Clin. Diabetes Healthc. 2022, 3, 826006. [Google Scholar] [CrossRef]
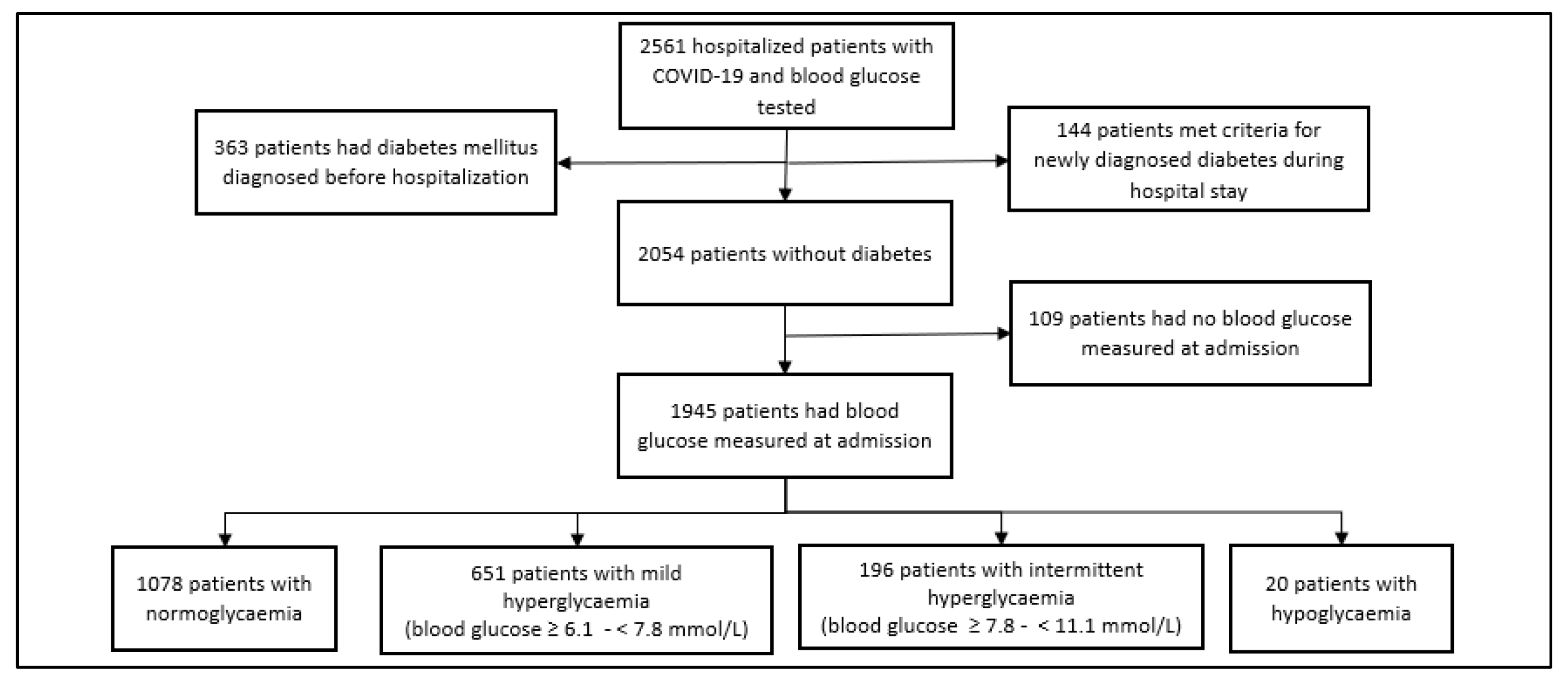
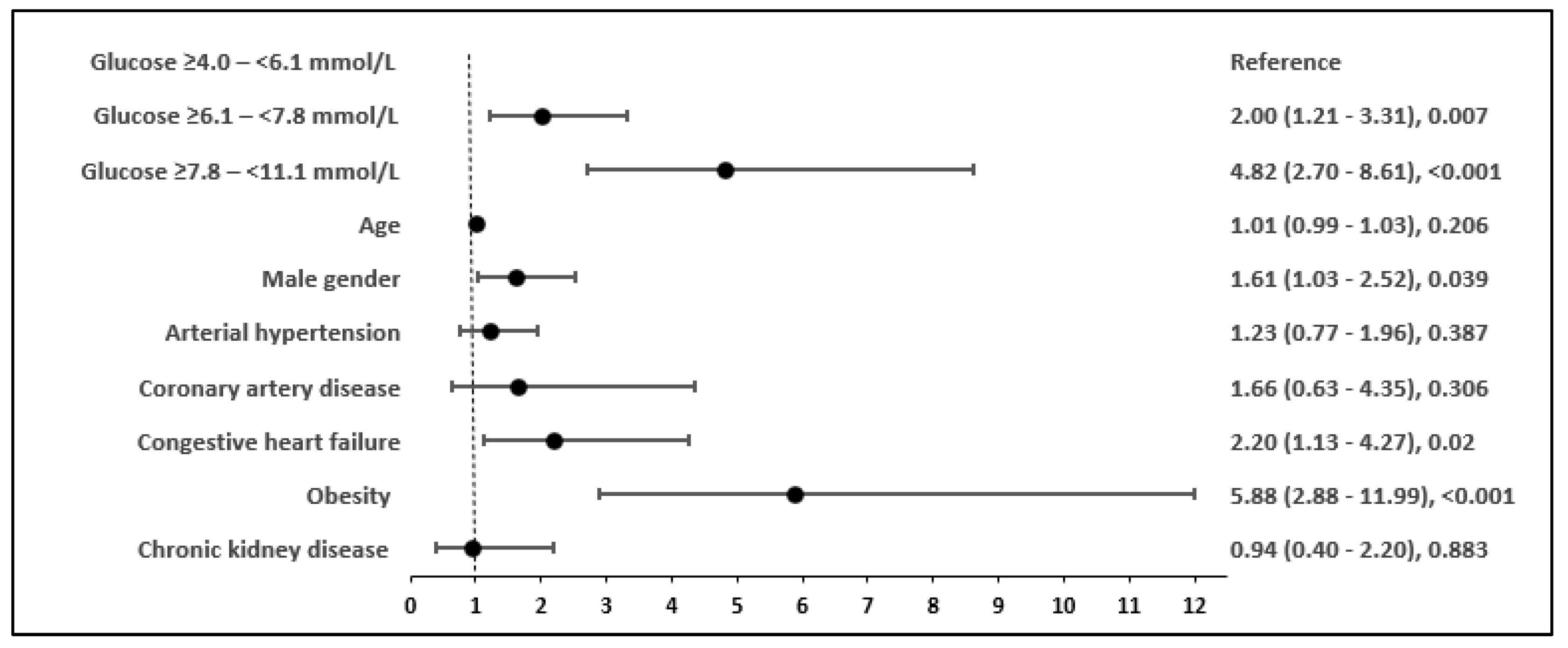
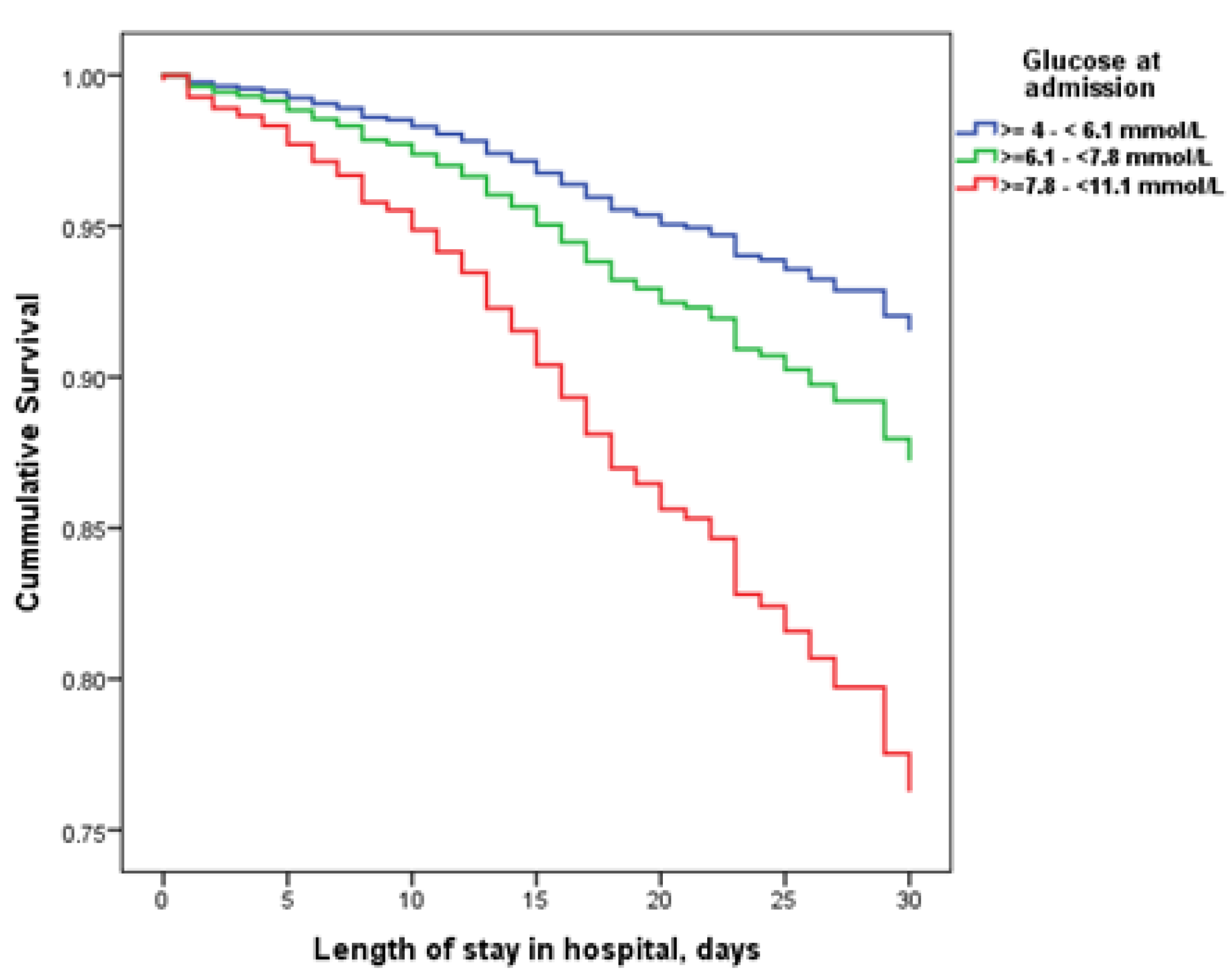
| Demographic and Clinical Characteristic (2561 Patients) | N (%) | Laboratory Characteristics | N | Median (IQR) |
|---|---|---|---|---|
| Age, years, median (IQR) | 60 (49–70) | Haemoglobin, g/L | 2561 | 138 (124–149) |
| Male | 1412 (55.1) | WBC, ×109/L | 2561 | 6.48 (4.84–9.03) |
| Female | 1149 (44.9) | Neutrophils, ×109/L | 2561 | 4.76 (3.30–7.13) |
| Any concommitant condition | 1252 (48.9) | Lymphocytes, ×109/L | 2561 | 1 (0.70–1.40) |
| Arterial hypertension | 983 (38.4) | NLR | 2558 | 4.70 (2.86–8.05) |
| Coronary artery disease | 90 (3.5) | Platelets, ×109/L | 2561 | 198 (153–258) |
| Congestive heart failure | 198 (7.7) | Glucose, mmol/L | 2561 | 6.12 (5.43–7.28) |
| Diabetes mellitus | 363 (14.2) | Creatinine, µmol/L | 2557 | 82 (67–105) |
| Obesity | 123 (4.8) | Urea, mmol/L | 2371 | 5.73 (4.12–8.74) |
| COPD | 42 (1.6) | eGFR, mL/min/1.73 m2 | 2513 | 83 (58.95–96) |
| Chronic kidney disease | 205 (8.0) | Sodium, mmol/L | 2551 | 140 (137–143) |
| Previous stroke | 32 (1.2) | Potassium, mmol/L | 2551 | 4.20 (3.90–4.60) |
| Invasive mechanical ventilation | 192 (7.5) | ALT, U/L | 2500 | 31.42 (19.65–52) |
| Antibiotics use | 1863 (72.7) | AST, U/L | 2485 | 36 (26–57) |
| Antivirals (remdesivir) | 808 (31.6) | AST to ALT ratio | 2483 | 1.17 (0.87–1.67) |
| Systemic steroids | 1630 (63.6) | LDH, U/L | 2302 | 303 (236–408.04) |
| In-hospital mortality | 313 (12.2) | CRP, mg/L | 2558 | 62.15 (22.78–124.88) |
| Length of hospital stay, days, median (IQR) | 11 (7–16) | Ferritin, µg/L | 2367 | 479.85 (236–1009.44) |
| IL-6, ng/L | 2254 | 29.60 (14.40–57.30) | ||
| D-dimer, µg/L | 2344 | 510 (305–985) | ||
| Troponin I, ng/L | 2122 | 10 (5–26) |
| Demographic and Clinical Characteristic | Normoglycaemia N = 1078 | Mild Hyperglycaemia, N = 651 | Intermittent Hyperglycaemia, N = 196 | p-Value 1 | p-Value 2 | p-Value 3 |
|---|---|---|---|---|---|---|
| Age in years, median (IQR) | 55 (43–66) | 60 (50–68) | 67 (59–76) | <0.001 | <0.001 | <0.001 |
| Male | 604 (56.0%) | 379 (58.2%) | 108 (55.1%) | 0.373 | 0.810 | 0.439 |
| Female | 474 (44.0%) | 272 (41.8%) | 88 (44.9%) | 0.373 | 0.810 | 0.439 |
| Any underlying condition | 373 (34.6%) | 307 (47.2%) | 119 (60.7%) | <0.001 | <0.001 | 0.001 |
| Arterial hypertension | 299 (27.7%) | 246 (37.8%) | 96 (49.0%) | <0.001 | <0.001 | 0.005 |
| Coronary artery disease | 23 (2.1%) | 19 (2.9%) | 10 (5.1%) | 0.304 | 0.016 | 0.141 |
| Congestive heart failure | 48 (4.5%) | 38 (5.8%) | 21 (10.7%) | 0.200 | <0.001 | 0.019 |
| Obesity | 24 (2.2%) | 22 (3.4%) | 12 (6.1%) | 0.149 | 0.002 | 0.086 |
| COPD | 12 (1.1%) | 10 (1.5%) | 2 (1.0%) | 0.447 | 1.000 | 0.743 |
| Chronic kidney disease | 58 (5.4%) | 30 (4.6%) | 18 (9.2%) | 0.479 | 0.039 | 0.015 |
| Previous stroke | 8 (0.7%) | 5 (0.8%) | 5 (2.6%) | 1.000 | 0.037 | 0.057 |
| Invasive mechanical ventilation | 29 (2.7%) | 38 (5.8%) | 29 (14.8%) | 0.001 | <0.001 | <0.001 |
| Antibiotics | 763 (70.8%) | 486 (74.7%) | 141 (71.9%) | 0.081 | 0.742 | 0.447 |
| Antivirals (remdesivir) | 326 (30.2%) | 242 (37.2%) | 64 (32.7%) | 0.003 | 0.500 | 0.248 |
| Systemic steroids | 634 (58.8%) | 465 (71.4%) | 134 (68.4%) | <0.001 | 0.012 | 0.409 |
| In-hospital mortality | 57 (5.3%) | 61 (9.4%) | 44 (22.4%) | 0.001 | <0.001 | <0.001 |
| Length of hospital stay, days, median (IQR) | 10 (7–14) | 11 (8–16) | 11.50 (7–16) | 0.001 | 0.059 | 0.996 |
| Laboratory Characteristics | Normoglycaemia | Mild Hyperglycaemia | Intermittent Hyperglycaemia | p-Value 1 | p-Value 2 | p-Value 3 | |||
|---|---|---|---|---|---|---|---|---|---|
| n | Value, Median (IQR) | n | Value, Median (IQR) | n | Value, Median (IQR) | ||||
| Haemoglobin, g/L | 1078 | 139 (125–150) | 651 | 140 (127–151) | 196 | 141 (124–151) | 0.120 | 0.693 | 0.621 |
| WBC, ×109/L | 1078 | 5.87 (4.43–7.62) | 651 | 6.54 (5.05–8.91) | 196 | 8.22 (6.07–11.68) | <0.001 | <0.001 | <0.001 |
| Neutrophils, ×109/L | 1078 | 4.10 (2.90–5.80) | 651 | 5 (3.60–7.16) | 196 | 6.60 (4.50–9.90) | <0.001 | <0.001 | <0.001 |
| Lymphocytes, ×109/L | 1078 | 1.07 (0.80–1.46) | 651 | 0.93 (0.66–1.26) | 196 | 0.90 (0.60–1.33) | <0.001 | <0.001 | 0.732 |
| NLR | 1076 | 3.73 (2.45–5.91) | 650 | 5.20 (3.30–8.46) | 196 | 7.47 (4.50–12.54) | <0.001 | <0.001 | <0.001 |
| Platelets, ×109/L | 1078 | 194 (151–254) | 651 | 195 (153–253) | 196 | 213.50 (168.25–277.75) | 0.832 | 0.001 | 0.003 |
| Glucose at admission, mmol/L | 1078 | 5.46 (5.09–5.77) | 651 | 6.60 (6.33–7.06) | 196 | 8.70 (8.16–9.57) | <0.001 | <0.001 | <0.001 |
| Creatinine, µmol/L | 1075 | 78.2 (65–96) | 651 | 81.01 (68–98.93) | 196 | 87 (70.25–130.93) | 0.015 | <0.001 | 0.001 |
| Urea, mmol/L | 970 | 4.98 (3.71–6.68) | 608 | 5.60 (4.10–7.82) | 182 | 7.13 (4.88–14.15) | <0.001 | <0.001 | <0.001 |
| eGFR | 1052 | 88.95 (70–99.50) | 638 | 83.60 (63.28–95.45) | 194 | 68.05 (41–88.15) | <0.001 | <0.001 | <0.001 |
| Sodium, mmol/L | 1073 | 141 (138–143) | 650 | 140 (137–143) | 196 | 139 (136–142) | 0.007 | <0.001 | 0.012 |
| Potassium, mmol/L | 1073 | 4.20 (3.90–4.53) | 650 | 4.10 (3.80–4.44) | 196 | 4.10 (3.77–4.50) | <0.001 | 0.013 | 0.882 |
| ALT, U/L | 1059 | 30.11 (19–49) | 633 | 35 (22–55.66) | 190 | 38.50 (21–57) | <0.001 | 0.002 | 0.780 |
| AST, U/L | 1050 | 33.12 (24–50) | 629 | 39 (29–60.15) | 188 | 41 (28–76.75) | <0.001 | <0.001 | 0.531 |
| AST to ALT ratio | 1050 | 1.13 (0.86–1.58) | 628 | 1.13 (0.88–1.58) | 188 | 1.25 (0.85–1.73) | 0.806 | 0.123 | 0.178 |
| LDH, U/L | 993 | 282 (220–359) | 592 | 318 (263–420.81) | 165 | 362 (253.50–534) | <0.001 | <0.001 | 0.024 |
| CRP, mg/L | 1077 | 48.65 (17.1–94.9) | 650 | 68.21 (31.34–129.73) | 196 | 100.05 (42.53–181.85) | <0.001 | <0.001 | 0.001 |
| Ferritin, µg/L | 1007 | 409.33 (204.63–802.70) | 612 | 568.50 (296.25–1223.25) | 170 | 659.98 (294.24–1402.31) | <0.001 | <0.001 | 0.288 |
| IL-6, ng/L | 958 | 26.15 (13.57–48.5) | 581 | 32.40 (15.80–61.24) | 161 | 36.90 (14.5–77.1) | <0.001 | 0.003 | 0.454 |
| D-dimer, µg/L | 990 | 420 (260– 726.25) | 614 | 522.50 (320–926.25) | 179 | 780 (365–1745) | <0.001 | <0.001 | <0.001 |
| Troponin I, ng/L | 899 | 7.97 (4–15) | 561 | 10 (6–22) | 161 | 20.20 (8.25–116) | <0.001 | <0.001 | <0.001 |
| Characteristic | In-Hospital Mortality | |
|---|---|---|
| HR (95% CI) | p | |
| Normoglycaemia | Reference | |
| Mild hyperglycaemia | 1.62 (1.10–2.39) | 0.015 |
| Intermittent hyperglycaemia | 3.04 (2.01–4.60) | <0.001 |
| Age in years | 1.06 (1.05–1.08) | <0.001 |
| Male gender | 1.07 (0.77–1.49) | 0.689 |
| Hypertension | 0.94 (0.67–1.33) | 0.729 |
| Coronary artery disease | 1.23 (0.69–2.18) | 0.487 |
| Congestive heart failure | 1.97 (1.33–2.93) | 0.001 |
| Obesity | 2.94 (1.56–5.57) | 0.001 |
| Previous stroke | 3.86 (1.95–7.65) | <0.001 |
| Chronic kidney disease | 0.61 (0.36–1.04) | 0.069 |
| Antivirals (Remdesivir) | 0.56 (0.37–0.85) | 0.006 |
| Systemic steroids | 1.36 (0.94–1.99) | 0.108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabuliene, L.; Kubiliute, I.; Urbonas, M.; Jancoriene, L.; Urboniene, J.; Ilias, I. Hyperglycaemia and Its Prognostic Value in Patients with COVID-19 Admitted to the Hospital in Lithuania. Biomedicines 2024, 12, 55. https://doi.org/10.3390/biomedicines12010055
Zabuliene L, Kubiliute I, Urbonas M, Jancoriene L, Urboniene J, Ilias I. Hyperglycaemia and Its Prognostic Value in Patients with COVID-19 Admitted to the Hospital in Lithuania. Biomedicines. 2024; 12(1):55. https://doi.org/10.3390/biomedicines12010055
Chicago/Turabian StyleZabuliene, Lina, Ieva Kubiliute, Mykolas Urbonas, Ligita Jancoriene, Jurgita Urboniene, and Ioannis Ilias. 2024. "Hyperglycaemia and Its Prognostic Value in Patients with COVID-19 Admitted to the Hospital in Lithuania" Biomedicines 12, no. 1: 55. https://doi.org/10.3390/biomedicines12010055
APA StyleZabuliene, L., Kubiliute, I., Urbonas, M., Jancoriene, L., Urboniene, J., & Ilias, I. (2024). Hyperglycaemia and Its Prognostic Value in Patients with COVID-19 Admitted to the Hospital in Lithuania. Biomedicines, 12(1), 55. https://doi.org/10.3390/biomedicines12010055







