Differential Post-Translational Modifications of Proteins in Bladder Ischemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bladder Ischemia Model
2.2. Sample Preparation for Proteomic Analysis
2.3. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Fractionation and Trypsin Digestion
2.4. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.5. The Clustered Delta Masses
2.6. Statistical Analysis
3. Results
3.1. Validation of Bladder Ischemia in the Rat Model
3.2. Differential Protein Modifications in Bladder Ischemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef]
- Lu, P.; Vogel, C.; Wang, R.; Yao, X.; Marcotte, E.M. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat. Biotechnol. 2007, 25, 117–124. [Google Scholar] [CrossRef]
- Battle, A.; Khan, Z.; Wang, S.H.; Mitrano, A.; Ford, M.J.; Pritchard, J.K.; Gilad, Y. Genomic variation. Impact of regulatory variation from RNA to protein. Science 2015, 347, 664–667. [Google Scholar] [CrossRef]
- Schweppe, R.E.; Haydon, C.E.; Lewis, T.S.; Resing, K.A.; Ahn, N.G. The characterization of protein post-translational modifications by mass spectrometry. Acc. Chem. Res. 2003, 36, 453–461. [Google Scholar] [CrossRef]
- Pinggera, G.M.; Mitterberger, M.; Steiner, E.; Pallwein, L.; Frauscher, F.; Aigner, F.; Bartsch, G.; Strasser, H. Association of lower urinary tract symptoms and chronic ischaemia of the lower urinary tract in elderly women and men: Assessment using colour Doppler ultrasonography. BJU Int. 2008, 102, 470–474. [Google Scholar] [CrossRef]
- De, E.J.; Hou, P.; Estrera, A.L.; Sdringola, S.; Kramer, L.A.; Graves, D.E.; Westney, O.L. Pelvic ischemia is measurable and symptomatic in patients with coronary artery disease: A novel application of dynamic contrast-enhanced magnetic resonance imaging. J. Sex. Med. 2008, 5, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Lopes, T.; Vasconcelos, A.; Costa, D.; Charrua, A.; Neves, J.; Silva, J.; Cruz, F.; Silva, C. The Impact of Chronic Pelvic Ischemia on LUTS and Urinary Levels of Neuroinflammatory, Inflammatory, and Oxidative Stress Markers in Elderly Men: A Case-control Study. Urology 2019, 123, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, N.; Zhu, X.H.; He, Z.S.; Wahafu, W.; Xu, Z.Q.; Yang, Y. Involvement of TL1A and DR3 in induction of ischaemia and inflammation in urinary bladder dysfunction in the elderly. Mol. Med. Rep. 2012, 6, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Mitterberger, M.; Pallwein, L.; Gradl, J.; Frauscher, F.; Neuwirt, H.; Leunhartsberger, N.; Strasser, H.; Bartsch, G.; Pinggera, G.M. Persistent detrusor overactivity after transurethral resection of the prostate is associated with reduced perfusion of the urinary bladder. BJU Int. 2007, 99, 831–835. [Google Scholar] [CrossRef]
- Pinggera, G.M.; Mitterberger, M.; Pallwein, L.; Schuster, A.; Herwig, R.; Frauscher, F.; Bartsch, G.; Strasser, H. Alpha-Blockers improve chronic ischaemia of the lower urinary tract in patients with lower urinary tract symptoms. BJU Int. 2008, 101, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Collado, A.; Batista, J.E.; Garcia-Penit, J.; Gelabert, A.; Arañó, P.; Villavicencio, H. Bladder blood flow and de-obstructive open prostatectomy: Correlation with clinical and urodynamic parameters. Int. Urol. Nephrol. 2005, 37, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Batista, J.E.; Wagner, J.R.; Azadzoi, K.M.; Siroky, M.B. Direct measurement of blood flow in the human bladder. J. Urol. 1996, 155, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Kershen, R.T.; Azadzoi, K.M.; Siroky, M.B. Blood flow, pressure and compliance in the male human bladder. J. Urol. 2002, 168, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Shishido, K.; Sato, Y.; Ogawa, S.; Oguro, T.; Kataoka, M.; Shiomi, H.; Uchida, H.; Haga, N.; Hosoi, T.; et al. The Association between Severity of Atherosclerosis and Lower Urinary Tract Function in Male Patients with Lower Urinary Tract Symptoms. Low. Urin. Tract Symptoms 2012, 4, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Koo, K.C.; Yoo, J.W.; Lee, K.S. Effect of systemic atherosclerosis on overactive bladder symptoms in men with benign prostatic hyperplasia. Low. Urin. Tract Symptoms 2022, 14, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Yabe, M.; Haga, N.; Ogawa, S.; Kataoka, M.; Akaihata, H.; Sato, Y.; Hata, J.; Ishibashi, K.; Kojima, Y. Atherosclerosis as a predictor of delayed recovery from lower urinary tract dysfunction after robot-assisted laparoscopic radical prostatectomy. Neurourol. Urodyn. 2016, 35, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Camões, J.; Coelho, A.; Castro-Diaz, D.; Cruz, F. Lower Urinary Tract Symptoms and Aging: The Impact of Chronic Bladder Ischemia on Overactive Bladder Syndrome. Urol. Int. 2015, 95, 373–379. [Google Scholar] [CrossRef]
- Irwin, P.; Galloway, N.T. Impaired bladder perfusion in interstitial cystitis: A study of blood supply using laser Doppler flowmetry. J. Urol. 1993, 149, 890–892. [Google Scholar] [CrossRef]
- Tsai, E.; Yang, C.; Chen, H.; Wu, C.; Lee, J. Bladder neck circulation by Doppler ultrasonography in postmenopausal women with urinary stress incontinence. Obstet. Gynecol. 2001, 98, 52–56. [Google Scholar] [CrossRef]
- Speich, J.E.; Tarcan, T.; Hashitani, H.; Vahabi, B.; McCloskey, K.D.; Andersson, K.E.; Wein, A.J.; Birder, L.A. Are oxidative stress and ischemia significant causes of bladder damage leading to lower urinary tract dysfunction? Report from the ICI-RS 2019. Neurourol. Urodyn. 2020, 39 (Suppl. S3), S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Hisasue, S.; Ide, H.; Aoki, H.; Muto, S.; Yamaguchi, R.; Tsujimura, A.; Horie, S. The Impact of increased bladder blood flow on storage symptoms after holmium laser enucleation of the prostate. PLoS ONE 2015, 10, e0129111. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E.; Boedtkjer, D.B.; Forman, A. The link between vascular dysfunction, bladder ischemia, and aging bladder dysfunction. Ther. Adv. Urol. 2017, 9, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Andersson, K.E.; Lin, C.L.; Kao, C.H.; Wu, H.C. Association of lower urinary tract syndrome with peripheral arterial occlusive disease. PLoS ONE 2017, 12, e0170288. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Matsumoto, S.; Kita, M.; Hashizume, K.; Kakizaki, H. Improvement of overactive bladder symptoms and bladder ischemia with dutasteride in patients with benign prostatic enlargement. Low. Urin. Tract Symptoms 2015, 7, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Watanabe, M.; Kita, M.; Matsumoto, S.; Kakizaki, H. Analysis of bladder vascular resistance before and after prostatic surgery in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Neurourol. Urodyn. 2012, 31, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Emechebe, U.; Giraud, D.; Ammi, A.Y.; Scott, K.L.; Jacobs, J.M.; McDermott, J.E.; Dykan, I.V.; Alkayed, N.J.; Barnes, A.P.; Kaul, S.; et al. Phosphoproteomic response of cardiac endothelial cells to ischemia and ultrasound. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140683. [Google Scholar] [CrossRef] [PubMed]
- Fiskum, G.; Danilov, C.A.; Mehrabian, Z.; Bambrick, L.L.; Kristian, T.; McKenna, M.C.; Hopkins, I.; Richards, E.M.; Rosenthal, R.E. Postischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes. Ann. N. Y. Acad. Sci. 2008, 1147, 129–138. [Google Scholar] [CrossRef]
- Noll, J.M.; Augello, C.J.; Kürüm, E.; Pan, L.; Pavenko, A.; Nam, A.; Ford, B.D. Spatial Analysis of Neural Cell Proteomic Profiles Following Ischemic Stroke in Mice Using High-Plex Digital Spatial Profiling. Mol. Neurobiol. 2022, 59, 7236–7252. [Google Scholar] [CrossRef]
- Kip, A.M.; Valverde, J.M.; Altelaar, M.; Heeren, R.M.A.; Hundscheid, I.H.R.; Dejong, C.H.C.; Olde Damink, S.W.M.; Balluff, B.; Lenaerts, K. Combined Quantitative (Phospho)proteomics and Mass Spectrometry Imaging Reveal Temporal and Spatial Protein Changes in Human Intestinal Ischemia-Reperfusion. J. Proteome Res. 2022, 21, 49–66. [Google Scholar] [CrossRef]
- Yang, J.H.; Choi, H.P.; Yang, A.; Azad, R.; Chen, F.; Liu, Z.; Azadzoi, K.M. Post-Translational Modification Networks of Contractile and Cellular Stress Response Proteins in Bladder Ischemia. Cells 2021, 10, 1031. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Choi, H.P.; Wang, F.; Su, H.; Fei, Z.; Yang, J.H.; Azadzoi, K.M. Quantitative Proteomic Analysis of Differentially Expressed Proteins and Downstream Signaling Pathways in Chronic Bladder Ischemia. J. Urol. 2016, 195, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Niu, W.; Li, Y.; Azadzoi, K.M. Impairment of AMPK-α2 augments detrusor contractions in bladder ischemia. Investig. Clin. Urol. 2021, 62, 600–609. [Google Scholar] [CrossRef]
- Bern, M.; Kil, Y.J.; Becker, C. Byonic: Advanced peptide and protein identification software. Curr. Protoc. Bioinform. 2012, 40, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Fraley, C.; Raftery, A. mclust Version 4 for R: Normal Mixture Modeling for Model-Based Clustering, Classification, and Density Estimation. Available online: https://mclust-org.github.io/mclust (accessed on 23 February 2021).
- Fraley, C.; Raftery, A.E. How Many Clusters? Which Clustering Method? Answers Via Model-Based Cluster Analysis. Comput. J. 1998, 41, 578–588. [Google Scholar] [CrossRef]
- Yang, J.H.; Zhao, Z.; Niu, W.; Choi, H.P.; Azadzoi, K.M. Formation of Double Stranded RNA Provokes Smooth Muscle Contractions and Structural Modifications in Bladder Ischemia. Res. Rep. Urol. 2022, 14, 399–414. [Google Scholar] [CrossRef]
- Azadzoi, K.M.; Chen, B.G.; Radisavljevic, Z.M.; Siroky, M.B. Molecular reactions and ultrastructural damage in the chronically ischemic bladder. J. Urol. 2011, 186, 2115–2122. [Google Scholar] [CrossRef]
- Azadzoi, K.M.; Tarcan, T.; Kozlowski, R.; Krane, R.J.; Siroky, M.B. Overactivity and structural changes in the chronically ischemic bladder. J. Urol. 1999, 162, 1768–1778. [Google Scholar] [CrossRef]
- Nomiya, M.; Yamaguchi, O.; Andersson, K.E.; Sagawa, K.; Aikawa, K.; Shishido, K.; Yanagida, T.; Kushida, N.; Yazaki, J.; Takahashi, N. The effect of atherosclerosis-induced chronic bladder ischemia on bladder function in the rat. Neurourol. Urodyn. 2012, 31, 195–200. [Google Scholar] [CrossRef]
- Azadzoi, K.M.; Yalla, S.V.; Siroky, M.B. Oxidative stress and neurodegeneration in the ischemic overactive bladder. J. Urol. 2007, 178, 710–715. [Google Scholar] [CrossRef]
- Minhas, G.; Sharma, J.; Khan, N. Cellular Stress Response and Immune Signaling in Retinal Ischemia-Reperfusion Injury. Front. Immunol. 2016, 7, 444. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.J.; Metzger, J.M. Uncoupling of increased cellular oxidative stress and myocardial ischemia reperfusion injury by directed sarcolemma stabilization. J. Mol. Cell Cardiol. 2014, 67, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Swan, C.L.; Sistonen, L. Cellular stress response cross talk maintains protein and energy homeostasis. EMBO J. 2015, 34, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.S.; Meng, Z.; Kim, Y.C.; Park, H.W.; Hansen, C.G.; Kim, S.; Lim, D.S.; Guan, K.L. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat. Cell Biol. 2015, 17, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Leavy, O. Signalling: New roles for cell stress sensor. Nat. Rev. Immunol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Soboloff, J.; Madesh, M.; Gill, D.L. Sensing cellular stress through STIM proteins. Nat. Chem. Biol. 2011, 7, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Yamazaki, T.; Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 731–745. [Google Scholar] [CrossRef]
- Kultz, D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005, 67, 225–257. [Google Scholar] [CrossRef]
- Yang, J.H.; Siroky, M.B.; Yalla, S.V.; Azadzoi, K.M. Mitochondrial stress and activation of PI3K and Akt survival pathway in bladder ischemia. Res. Rep. Urol. 2017, 9, 93–100. [Google Scholar] [CrossRef]
- Lothrop, A.P.; Torres, M.P.; Fuchs, S.M. Deciphering post-translational modification codes. FEBS Lett. 2013, 587, 1247–1257. [Google Scholar] [CrossRef]
- Holmgren, M.; Wagg, J.; Bezanilla, F.; Rakowski, R.F.; De Weer, P.; Gadsby, D.C. Three distinct and sequential steps in the release of sodium ions by the Na+/K+-ATPase. Nature 2000, 403, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Zentner, G.E.; Henikoff, S. Regulation of nucleosome dynamics by histone modifications. Nat. Struct. Mol. Biol. 2013, 20, 259–266. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Korboukh, I.; Jin, J.; Huang, J. Targeting protein lysine methylation and demethylation in cancers. Acta Biochim. Biophys. Sin. 2012, 44, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Popovic, D.; Vucic, D.; Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 2014, 20, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; White, M.Y. The role of post-translational modifications in acute and chronic cardiovascular disease. Proteomics Clin. Appl. 2014, 8, 506–521. [Google Scholar] [CrossRef]
- Kristian, T.; Hu, B. The Protein Modification and Degradation Pathways after Brain Ischemia. Transl. Stroke Res. 2018, 9, 199–200. [Google Scholar] [CrossRef]
- Yan, L.J. Protein redox modification as a cellular defense mechanism against tissue ischemic injury. Oxid. Med. Cell Longev. 2014, 2014, 343154. [Google Scholar] [CrossRef]
- Tadeusiewicz, J.; Nowak, P. The role of post-translational modification of fibrinogen in the pathogenesis of thrombosis. Pol. Merkur. Lekarski 2015, 38, 107–112. [Google Scholar]
- Hwang, N.R.; Yim, S.H.; Kim, Y.M.; Jeong, J.; Song, E.J.; Lee, Y.; Lee, J.H.; Choi, S.; Lee, K.J. Oxidative modifications of glyceraldehyde-3-phosphate dehydrogenase play a key role in its multiple cellular functions. Biochem. J. 2009, 423, 253–264. [Google Scholar] [CrossRef]
- Zhao, X.; Sidoli, S.; Wang, L.; Wang, W.; Guo, L.; Jensen, O.N.; Zheng, L. Comparative proteomic analysis of histone post-translational modifications upon ischemia/reperfusion-induced retinal injury. J. Proteome Res. 2014, 13, 2175–2186. [Google Scholar] [CrossRef]
- Iwabuchi, M.; Sheng, H.; Thompson, J.W.; Wang, L.; Dubois, L.G.; Gooden, D.; Moseley, M.; Paschen, W.; Yang, W. Characterization of the ubiquitin-modified proteome regulated by transient forebrain ischemia. J. Cereb. Blood Flow. Metab. 2014, 34, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, K. Post-translational modification of mitochondrial proteins by caloric restriction: Possible involvement in caloric restriction-induced cardioprotection. Trends Cardiovasc. Med. 2013, 23, 18–25. [Google Scholar] [CrossRef]
- Powell, S.R.; Gurzenda, E.M.; Wahezi, S.E. Actin is oxidized during myocardial ischemia. Free Radic. Biol. Med. 2001, 30, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Eaton, P.; Byers, H.L.; Leeds, N.; Ward, M.A.; Shattock, M.J. Detection, quantitation, purification, and identification of cardiac proteins S-thiolated during ischemia and reperfusion. J. Biol. Chem. 2002, 277, 9806–9811. [Google Scholar] [CrossRef] [PubMed]
- DalleDonne, I.; Milzani, A.; Colombo, R. The tert-butyl hydroperoxide-induced oxidation of actin Cys-374 is coupled with structural changes in distant regions of the protein. Biochemistry 1999, 38, 12471–12480. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Boja, E.S.; Tan, W.; Tekle, E.; Fales, H.M.; English, S.; Mieyal, J.J.; Chock, P.B. Reversible glutathionylation regulates actin polymerization in A431 cells. J. Biol. Chem. 2001, 276, 47763–47766. [Google Scholar] [CrossRef] [PubMed]
- Stournaras, C.; Drewes, G.; Blackholm, H.; Merkler, I.; Faulstich, H. Glutathionyl (cysteine-374) actin forms filaments of low mechanical stability. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. 1990, 1037, 86–91. [Google Scholar] [CrossRef]
- Xu, Q.; Huff, L.P.; Fujii, M.; Griendling, K.K. Redox regulation of the actin cytoskeleton and its role in the vascular system. Free Radic. Biol. Med. 2017, 109, 84–107. [Google Scholar] [CrossRef]
- Figueiredo-Freitas, C.; Dulce, R.A.; Foster, M.W.; Liang, J.; Yamashita, A.M.S.; Lima-Rosa, F.L.; Thompson, J.W.; Moseley, M.A.; Hare, J.M.; Nogueira, L.; et al. S-nitrosylation of sarcomeric proteins depresses myofilament Ca2+ sensitivity in intact cardiomyocytes. Antioxid. Redox Signal 2015, 23, 1017–1034. [Google Scholar] [CrossRef]
- Cardaci, S.; Filomeni, G.; Ciriolo, M.R. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J. Cell Sci. 2012, 125, 2115–2125. [Google Scholar] [CrossRef]
- Athéa, Y.; Viollet, B.; Mateo, P.; Rousseau, D.; Novotova, M.; Garnier, A.; Vaulont, S.; Wilding, J.R.; Grynberg, A.; Veksler, V.; et al. AMP-activated protein kinase alpha2 deficiency affects cardiac cardiolipin homeostasis and mitochondrial function. Diabetes 2007, 56, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, X.; Xu, X.; Fassett, J.; Zhu, G.; Viollet, B.; Xu, W.; Wiczer, B.; Bernlohr, D.A.; Bache, R.J.; et al. AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension 2008, 52, 918–924. [Google Scholar] [CrossRef] [PubMed]


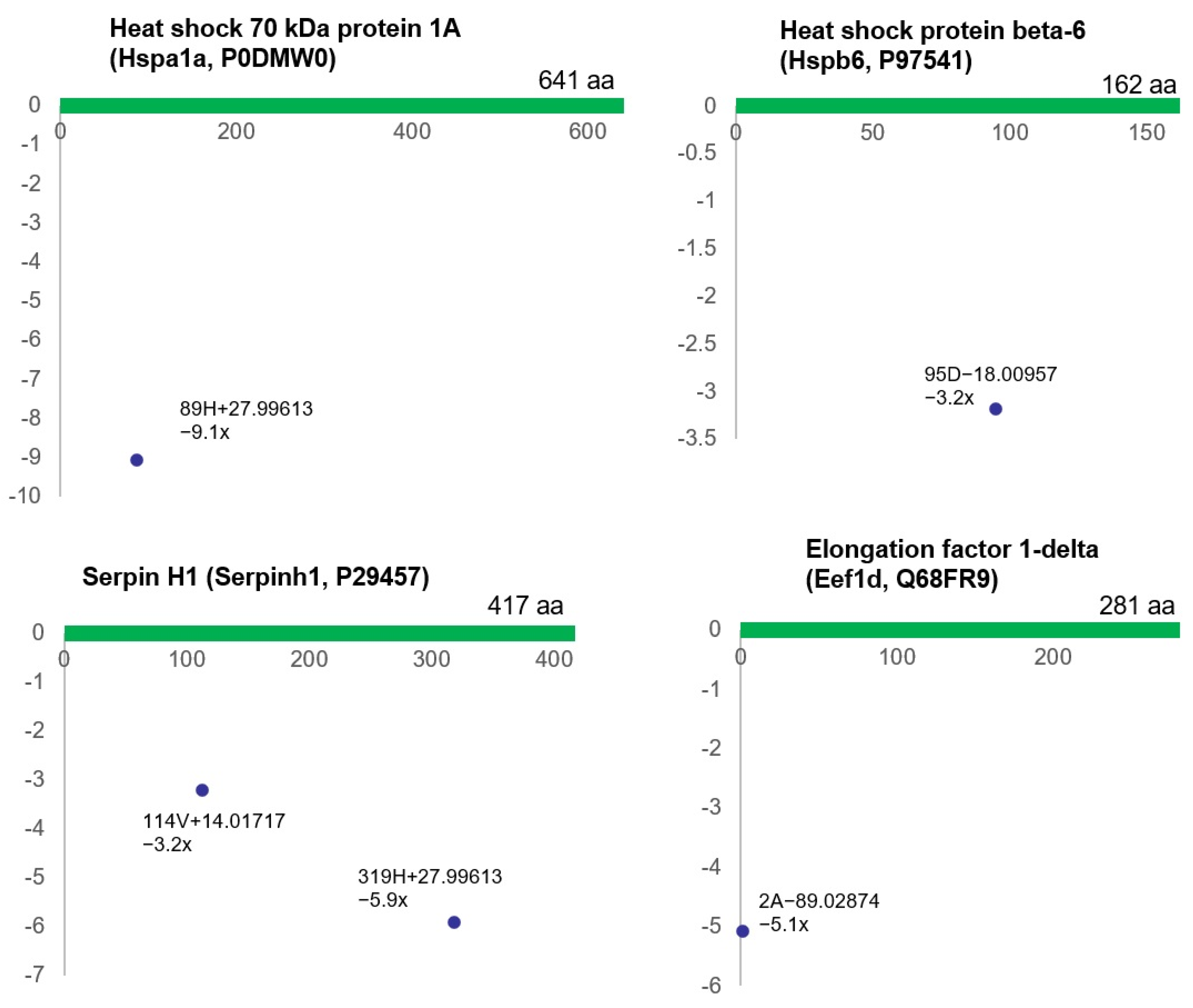
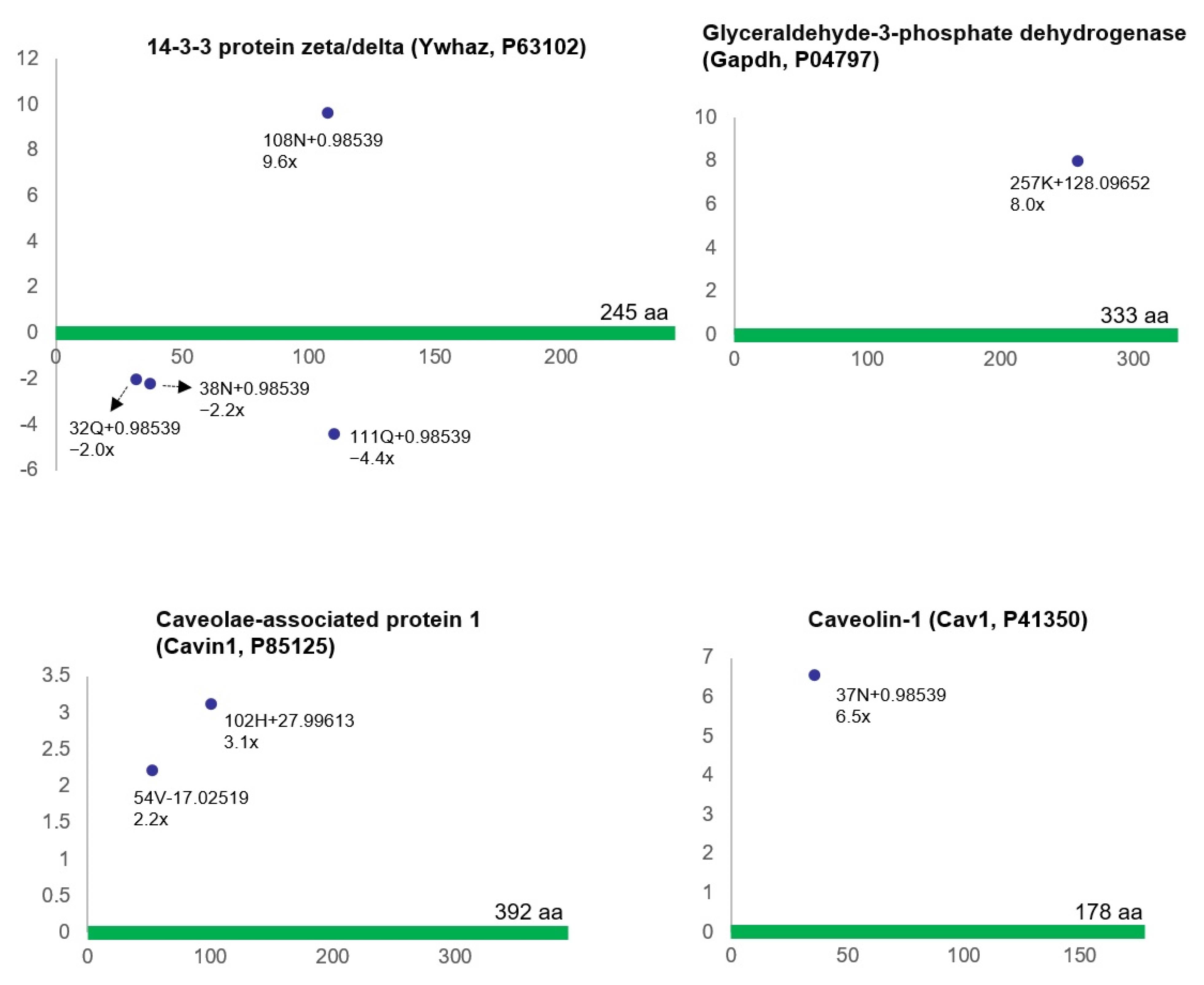
| Proteins Containing Up-Regulated ncAAs | Proteins Containing Down-Regulated ncAAs | ||||||
|---|---|---|---|---|---|---|---|
| Protein Name | Gene Name | Protein ID | a Freq | Protein Name | Gene Name | Protein ID | a Freq |
| Hemoglobin subunit beta-1 | Hbb | P02091 | 5 | ATP synthase subunit beta, mitochondrial | Atp5b | P10719 | 2 |
| 14-3-3 protein zeta/delta | Ywhaz | P63102 | 1 | Albumin | Alb | P02770 | 7 |
| Non-muscle caldesmon | Cald1 | Q62736 | 2 | 40S ribosomal protein S4, X isoform | Rps4x | P62703 | 1 |
| Glyceraldehyde-3-phosphate dehydrogenase | Gapdh | P04797 | 1 | Heat shock 70 kDa protein 1A | Hspa1a | P0DMW0 | 1 |
| Caveolin-1 | Cav1 | P41350 | 1 | Serpin H1 | Serpinh1 | P29457 | 2 |
| Alpha-actinin-1 | Actn1 | Q9Z1P2 | 2 | Alpha-crystallin B chain | Cryab | P23928 | 2 |
| Transgelin | Tagln | P31232 | 6 | L-lactate dehydrogenase A chain | Ldha | P04642 | 2 |
| Albumin | Alb | P02770 | 21 | Creatine kinase B-type | Ckb | P07335 | 1 |
| Polymerase I and transcript release factor | Ptrf | P85125 | 2 | Collagen alpha-2(I) chain | Col1a2 | P02466 | 2 |
| Protein phosphatase 1 regulatory subunit 12A | Ppp1r12a | Q10728 | 3 | Serine protease inhibitor A3K | Serpina3k | P05545 | 3 |
| Major urinary protein | — | P02761 | 1 | Oligoribonuclease, mitochondrial | Rexo2 | Q5U1X1 | 1 |
| Calmodulin-1 | Calm1 | P0DP29 | 2 | ATP synthase subunit alpha, mitochondrial | Atp5a1 | P15999 | 1 |
| Histone H1.0 | H1f0 | P43278 | 1 | Ig gamma-2B chain C region | Igh-1a | P20761 | 1 |
| Glutathione S-transferase P | Gstp1 | P04906 | 1 | Elongation factor 1-delta | Eef1d | Q68FR9 | 1 |
| Cytochrome b5 | Cyb5a | P00173 | 1 | Desmin | Des | P48675 | 4 |
| Histone H1.4 | H1-4 | P15865 | 1 | Sepiapterin reductase | Spr | P18297 | 1 |
| Desmin | Des | P48675 | 5 | Alpha-1-antiproteinase | Serpina1 | P17475 | 4 |
| Hemoglobin subunit alpha-1/2 | Hba1 | P01946 | 4 | Myosin light polypeptide 6 | Myl6 | Q64119 | 1 |
| T-kininogen 2 | — | P08932 | 1 | Lactoylglutathione lyase | Glo1 | Q6P7Q4 | 1 |
| Complement component C9 | C9 | Q62930 | 1 | Selenium-binding protein 1 | Selenbp1 | Q8VIF7 | 1 |
| Actin, cytoplasmic 2 | Actg1 | P63259 | 1 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | Uchl1 | Q00981 | 1 |
| Actin, aortic smooth muscle | Acta2 | P62738 | 7 | Myosin regulatory light polypeptide 9 | Myl9 | Q64122 | 3 |
| Heat shock protein HSP 90-beta | Hsp90ab1 | P34058 | 1 | Gelsolin | Gsn | Q68FP1 | 2 |
| Cysteine and glycine-rich protein 1 | Csrp1 | P47875 | 3 | Phosphoglycerate mutase 1 | Pgam1 | P25113 | 1 |
| Alpha-1-inhibitor 3 | A1i3 | P14046 | 4 | Hemoglobin subunit beta-2 | — | P11517 | 1 |
| Calponin-1 | Cnn1 | Q08290 | 1 | Heat shock protein beta-6 | Hspb6 | P97541 | 1 |
| Tropomyosin beta chain | Tpm2 | P58775 | 3 | Cofilin-1 | Cfl1 | P45592 | 1 |
| Alpha-enolase | Eno1 | P04764 | 1 | 14-3-3 protein zeta/delta | Ywhaz | P63102 | 3 |
| Tubulin alpha-1B chain | Tuba1b | Q6P9V9 | 1 | Four and a half LIM domains protein 1 | Fhl1 | Q9WUH4 | 2 |
| Prothymosin alpha | Ptma | P06302 | 1 | Heat shock cognate 71 kDa protein | Hspa8 | P63018 | 1 |
| Transgelin | Tagln | P31232 | 7 | ||||
| Non-muscle caldesmon | Cald1 | Q62736 | 1 | ||||
| Heat shock protein HSP 90-alpha | Hsp90aa1 | P82995 | 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-P.; Yang, J.-H.; Azadzoi, K.M. Differential Post-Translational Modifications of Proteins in Bladder Ischemia. Biomedicines 2024, 12, 81. https://doi.org/10.3390/biomedicines12010081
Choi H-P, Yang J-H, Azadzoi KM. Differential Post-Translational Modifications of Proteins in Bladder Ischemia. Biomedicines. 2024; 12(1):81. https://doi.org/10.3390/biomedicines12010081
Chicago/Turabian StyleChoi, Han-Pil, Jing-Hua Yang, and Kazem M. Azadzoi. 2024. "Differential Post-Translational Modifications of Proteins in Bladder Ischemia" Biomedicines 12, no. 1: 81. https://doi.org/10.3390/biomedicines12010081





