MPO-DNA Complexes and cf-DNA in Patients with Sepsis and Their Clinical Value
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Preparation
2.3. Serum MPO-DNA, Cell-Free DNA (cf-DNA) Assay
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
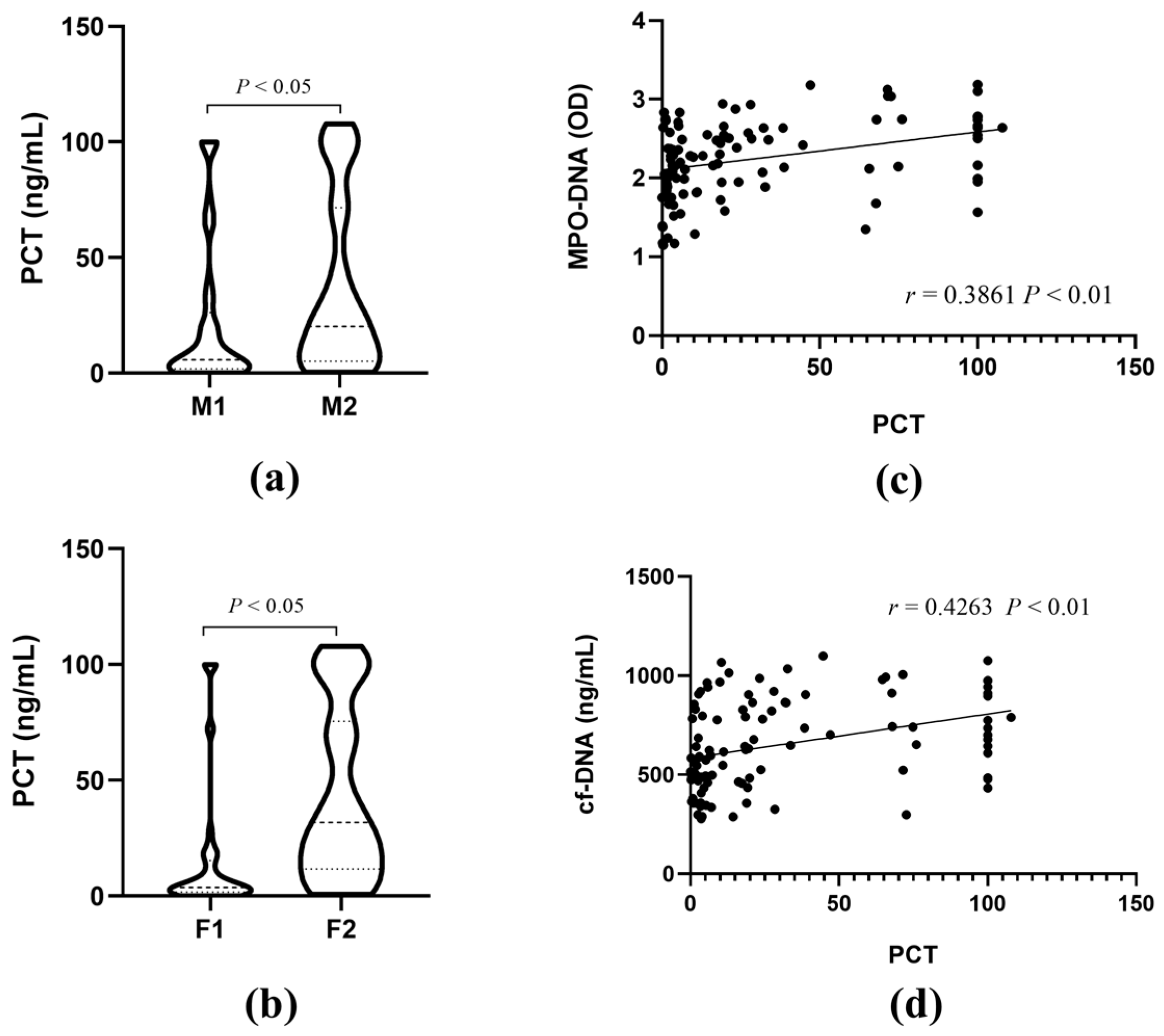
3.2. The Correlations of MPO-DNA and cf-DNA with PCT
3.3. The Correlations of MPO-DNA and cf-DNA with Sepsis Severity
3.4. Correlation Analysis between MPO-DNA and cf-DNA Levels and Clinical Indicators
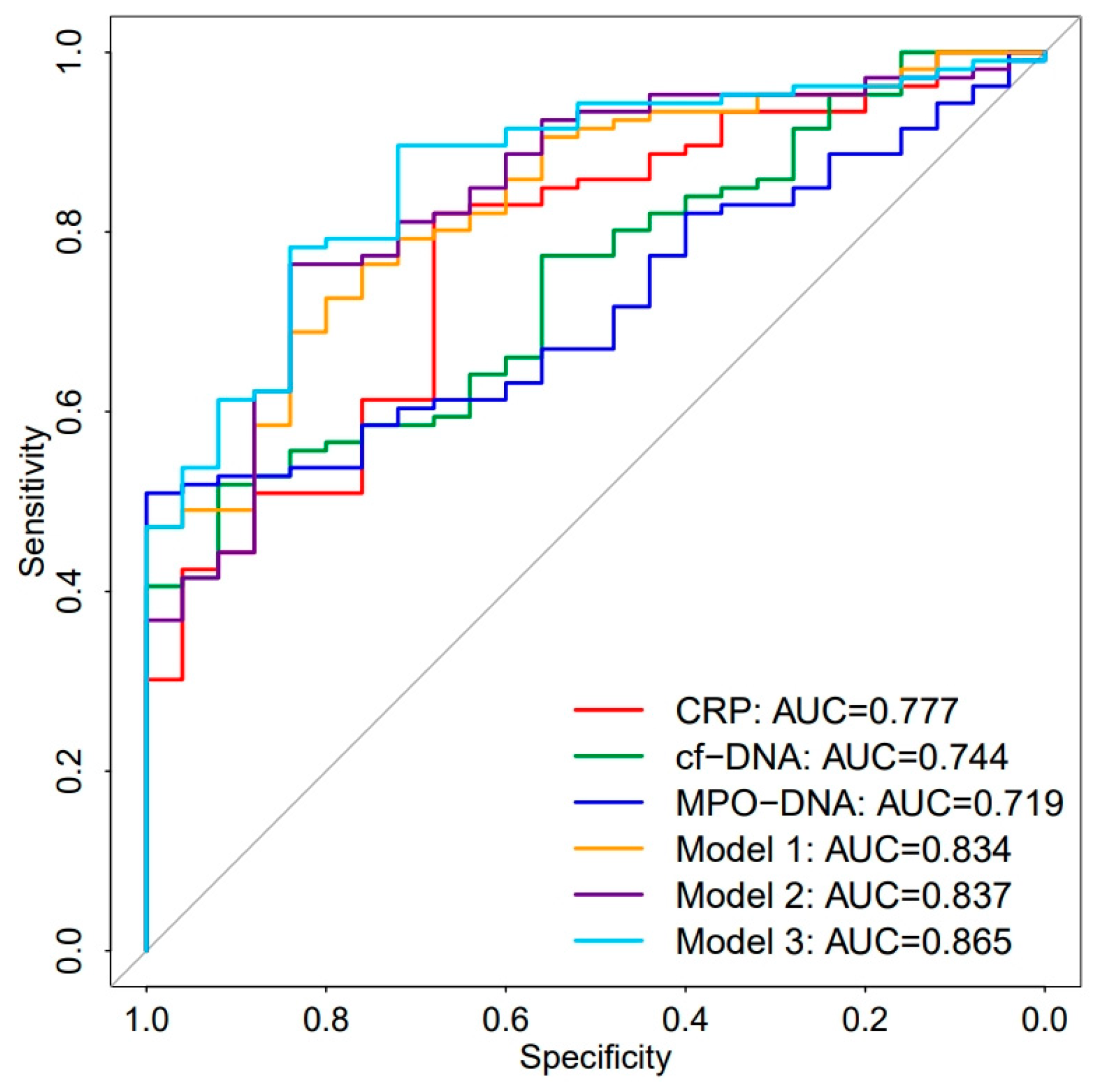
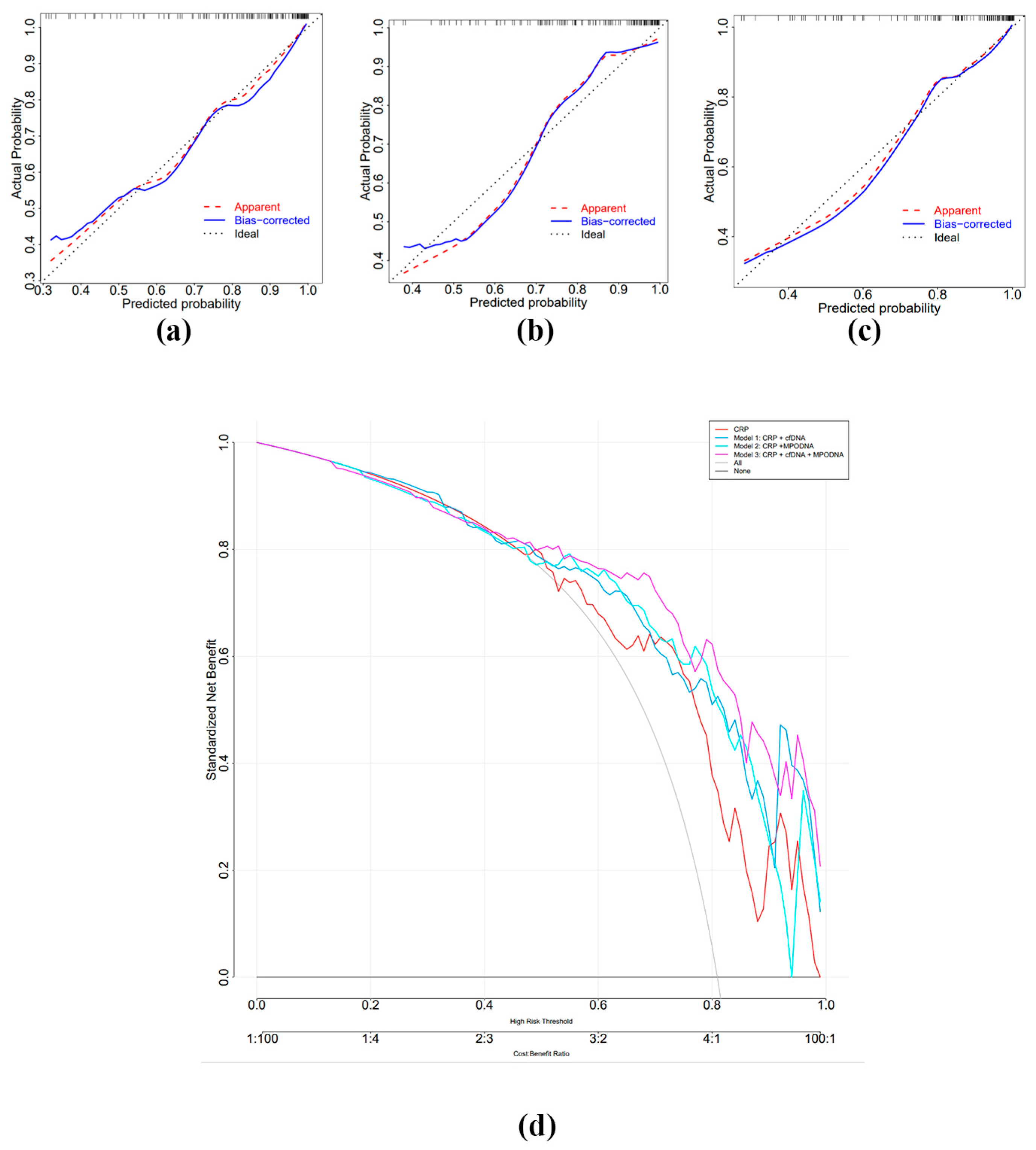
3.5. Diagnostic Value of MPO-DNA and cf-DNA in Sepsis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, S.-Y.; Sun, J.-H.; Zhang, H.-C.; Cai, Q.-L.; Gao, C.; Li, L.; Cao, J.; Xu, F.; Zhou, Y.; et al. Sepsis-induced immunosuppression: Mechanisms, diagnosis and current treatment options. Mil. Med. Res. 2022, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Cajander, S.; Kox, M.; Scicluna, B.P.; Weigand, M.A.; Mora, R.A.; Flohé, S.B.; Martin-Loeches, I.; Lachmann, G.; Girardis, M.; Garcia-Salido, A.; et al. Profiling the dysregulated immune response in sepsis: Overcoming challenges to achieve the goal of precision medicine. Lancet Respir. Med. 2024, 12, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Aschenbrenner, A.C.; Bauer, M.; Bock, C.; Calandra, T.; Gat-Viks, I.; Kyriazopoulou, E.; Lupse, M.; Monneret, G.; Pickkers, P.; et al. The pathophysiology of sepsis and precision-medicine-based immunotherapy. Nat. Immunol. 2024, 25, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Leligdowicz, A.; Dodek, P.M.; Norena, M.; Wong, H.; Kumar, A.; Kumar, A. Association between source of infection and hospital mortality in patients who have septic shock. Am. J. Respir. Crit. Care Med. 2014, 189, 1204–1213. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, Y.; Tang, J.; Lunsford, L.D.; Li, T.; Tang, R.; He, J.; Xu, P.; Faramand, A.; Xu, J.; et al. Association of Corticosteroid Treatment with Outcomes in Adult Patients With Sepsis: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 213–223. [Google Scholar] [CrossRef]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H. Sepsis-induced Coagulopathy and Disseminated Intravascular Coagulation. Anesthesiology 2020, 132, 1238–1245. [Google Scholar] [CrossRef]
- Nourshargh, S.; Renshaw, S.A.; Imhof, B.A. Reverse Migration of Neutrophils: Where, When, How, and Why? Trends Immunol. 2016, 37, 273–286. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Tabrizi, Z.A.; Khosrojerdi, A.; Aslani, S.; Hemmatzadeh, M.; Babaie, F.; Bairami, A.; Shomali, N.; Hosseinzadeh, R.; Safari, R.; Mohammadi, H. Multi-facets of neutrophil extracellular trap in infectious diseases: Moving beyond immunity. Microb. Pathog. 2021, 158, 105066. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Duan, Z.; Wang, X.; Chu, C.; Yang, C.; Chen, F.; Wang, D.; Wang, C.; Li, Q.; Ding, W. Neutrophil extracellular traps impair intestinal barrier functions in sepsis by regulating TLR9-mediated endoplasmic reticulum stress pathway. Cell Death Dis. 2021, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yu, Y.; Qu, M.; Qiu, Z.; Zhang, H.; Miao, C.; Guo, K. Neutrophil extracellular traps contribute to immunothrombosis formation via the STING pathway in sepsis-associated lung injury. Cell Death Discov. 2023, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Lefrançais, E.; Mallavia, B.; Zhuo, H.; Calfee, C.S.; Looney, M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. J. Clin. Investig. 2018, 3, 98178. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Nakazawa, D.; Shida, H.; Miyoshi, A.; Kusunoki, Y.; Tomaru, U.; Ishizu, A. NETosis markers: Quest for specific, objective, and quantitative markers. Clin. Chim. Acta 2016, 459, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Saukkonen, K.; Lakkisto, P.; Pettila, V.; Varpula, M.; Karlsson, S.; Ruokonen, E.; Pulkki, K.; for the Finnsepsis Study Group. Cell-free plasma DNA as a predictor of outcome in severe sepsis and septic shock. Clin. Chem. 2008, 54, 1000–1007. [Google Scholar] [CrossRef]
- Maruchi, Y.; Tsuda, M.; Mori, H.; Takenaka, N.; Gocho, T.; Huq, M.A.; Takeyama, N. Plasma myeloperoxidase-conjugated DNA level predicts outcomes and organ dysfunction in patients with septic shock. Crit. Care 2018, 22, 176. [Google Scholar] [CrossRef]
- Jiménez-Alcázar, M.; Kim, N.; Fuchs, T.A. Circulating Extracellular DNA: Cause or Consequence of Thrombosis? Semin. Thromb. Hemost. 2017, 43, 553–561. [Google Scholar] [CrossRef]
- Stoimenou, M.; Tzoros, G.; Skendros, P.; Chrysanthopoulou, A. Methods for the Assessment of NET Formation: From Neutrophil Biology to Translational Research. Int. J. Mol. Sci. 2022, 23, 15823. [Google Scholar] [CrossRef]
- Karakike, E.; Kyriazopoulou, E.; Tsangaris, I.; Routsi, C.; Vincent, J.-L.; Giamarellos-Bourboulis, E.J. The early change of SOFA score as a prognostic marker of 28-day sepsis mortality: Analysis through a derivation and a validation cohort. Crit. Care 2019, 23, 387. [Google Scholar] [CrossRef]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Jones, T.M.; Hamad, Y.; Pande, A.; Varon, J.; O’bRien, C.; Anderson, D.J.; Warren, D.K.; Dantes, R.B.; Epstein, L.; et al. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Netw. Open 2019, 2, e187571. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Denning, N.-L.; Aziz, M.; Gurien, S.D.; Wang, P. DAMPs and NETs in Sepsis. Front. Immunol. 2019, 10, 2536. [Google Scholar] [CrossRef]
- Lerman, Y.V.; Kim, M. Neutrophil migration under normal and sepsis conditions. Cardiovasc. Hematol. Disord. Targets 2015, 15, 19–28. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ueki, S.; Kamide, Y.; Miyabe, Y.; Fukuchi, M.; Yokoyama, Y.; Furukawa, T.; Azuma, N.; Oka, N.; Takeuchi, H.; et al. Increased Circulating Cell-Free DNA in Eosinophilic Granulomatosis With Polyangiitis: Implications for Eosinophil Extracellular Traps and Immunothrombosis. Front. Immunol. 2021, 12, 801897. [Google Scholar] [CrossRef]
- Hayden, H.; Ibrahim, N.; Klopf, J.; Zagrapan, B.; Mauracher, L.-M.; Hell, L.; Hofbauer, T.M.; Ondracek, A.S.; Schoergenhofer, C.; Jilma, B.; et al. ELISA detection of MPO-DNA complexes in human plasma is error-prone and yields limited information on neutrophil extracellular traps formed in vivo. PLoS ONE 2021, 16, e0250265. [Google Scholar] [CrossRef]
- Reshetnyak, T.; Nurbaeva, K.; Ptashnik, I.; Kudriaeva, A.; Belogurov, A.; Lila, A.; Nasonov, E. Markers of NETosis in Patients with Systemic Lupus Erythematosus and Antiphospholipid Syndrome. Int. J. Mol. Sci. 2023, 24, 9210. [Google Scholar] [CrossRef]
- Bressan, A.; Faggin, E.; Donato, M.; Tonon, L.; Buso, R.; Nardin, C.; Tiepolo, M.; Cinetto, F.; Scarpa, R.; Agostini, C.; et al. NETosis in Acute Thrombotic Disorders. Semin. Thromb. Hemost. 2023, 49, 709–715. [Google Scholar] [CrossRef]
- Shi, Y.; Mao, H.; Miao, W.; Deng, J.; Gao, Q.; Zeng, S.; Ma, L.; Han, Y.; Ji, W.; Li, Y.; et al. Potential Association of Neutrophil Extracellular Traps With Cognitive Impairment in Cerebral Small Vessel Disease. J. Gerontol. Ser. A 2023, 78, 1999–2006. [Google Scholar] [CrossRef]
- Nakazawa, D.; Shida, H.; Tomaru, U.; Yoshida, M.; Nishio, S.; Atsumi, T.; Ishizu, A. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA–associated microscopic polyangiitis. J. Am. Soc. Nephrol. 2014, 25, 990–997. [Google Scholar] [CrossRef] [PubMed]
- von Meijenfeldt, F.A.; Stravitz, R.T.; Zhang, J.; Adelmeijer, J.; Zen, Y.; Durkalski, V.; Lee, W.M.; Lisman, T. Generation of neutrophil extracellular traps in patients with acute liver failure is associated with poor outcome. Hepatology 2022, 75, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Borissoff, J.I.; Joosen, I.A.; Versteylen, M.O.; Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Gallant, M.; Martinod, K.; ten Cate, H.; Hofstra, L.; et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arter. Thromb. Vasc. Biol. 2013, 33, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Yamashita, K.; Mizugishi, K.; Watanabe, T.; Sakamoto, S.; Kitano, T.; Kondo, T.; Kawabata, H.; Kadowaki, N.; Takaori-Kondo, A. Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2013, 19, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Margraf, S.; Lögters, T.; Reipen, J.; Altrichter, J.; Scholz, M.; Windolf, J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): A potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock 2008, 30, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, X.; Guo, P.; Chen, Y.; Li, J.; Tao, T. The accuracy assessment of presepsin (sCD14-ST) for mortality prediction in adult patients with sepsis and a head-to-head comparison to PCT: A meta-analysis. Ther. Clin. Risk Manag. 2019, 15, 741–753. [Google Scholar] [CrossRef]
- Gai, L.; Tong, Y.; Yan, B.Q. Research on the diagnostic effect of PCT level in serum on patients with sepsis due to different pathogenic causes. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4238–4242. [Google Scholar]
- Zhang, H.; Liu, J.; Zhou, Y.; Qu, M.; Wang, Y.; Guo, K.; Shen, R.; Sun, Z.; Cata, J.P.; Yang, S.; et al. Neutrophil extracellular traps mediate m6A modification and regulates sepsis-associated acute lung injury by activating ferroptosis in alveolar epithelial cells. Int. J. Biol. Sci. 2022, 18, 3337–3357. [Google Scholar] [CrossRef]
- Mai, S.H.C.; Khan, M.; Dwivedi, D.J.; Ross, C.A.; Zhou, J.; Gould, T.J.; Gross, P.L.; Weitz, J.I.; Fox-Robichaud, A.E.; Liaw, P.C. Delayed but not Early Treatment with DNase Reduces Organ Damage and Improves Outcome in a Murine Model of Sepsis. Shock 2015, 44, 166–172. [Google Scholar] [CrossRef]
- Boone, B.A.; Murthy, P.; Miller-Ocuin, J.; Doerfler, W.R.; Ellis, J.T.; Liang, X.; Ross, M.A.; Wallace, C.T.; Sperry, J.L.; Lotze, M.T.; et al. Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps. BMC Cancer 2018, 18, 678. [Google Scholar] [CrossRef]
- Qiao, X.; Kashiouris, M.G.; L’hEureux, M.; Fisher, B.J.; Leichtle, S.W.; Truwit, J.D.; Nanchal, R.; Hite, R.D.; Morris, P.E.; Martin, G.S.; et al. Biological Effects of Intravenous Vitamin C on Neutrophil Extracellular Traps and the Endothelial Glycocalyx in Patients with Sepsis-Induced ARDS. Nutrients 2022, 14, 4415. [Google Scholar] [CrossRef] [PubMed]


| Variable | Sepsis Group (n = 106) | Non-Sepsis Group (n = 25) | Healthy Controls (n = 51) |
|---|---|---|---|
| Gender (male/female) | 49/57 | 12/13 | 24/27 |
| Age | 64.45 ± 12.30 | 58.28 ± 15.76 | 64.63 ± 15.26 |
| WBC (109/L) | 13.800 (8.8,18.8) * | 9.490 (6.0,14.8) * | 6.000 (4.8,7.2) |
| Neu (%) | 89.800 (83.7,93.2) *,# | 82.800 (70.4,90.0) * | 53.900 (46.9,60.2) |
| RBC (1012/L) | 3.495 (3.0,4.0) * | 3.560 (3.3,4.2) * | 4.640 (4.3,4.9) |
| Hb (g/L) | 106.500 (84.8,119.0) * | 105.000 (96.0,120.0) * | 143.000 (132.0,152.0) |
| PLT (109/L) | 99.500 (57.3,164.0) *,# | 247.000 (195.5,272.0) | 225.000 (177.0,252.0) |
| PCT (ng/mL) | 13.657 (3.0,45.2) *,# | 0.970 (0.3,1.9) * | 0.035 (0.0,0.1) |
| CRP (mg/L) | 126.030 (75.0,166.1) *,# | 44.780 (15.7,108.0) * | 2.870 (1.8,4.8) |
| SAA (mg/L) | 300.000 (162.0,300.0) * | 175.970 (81.9,294.2) * | 4.440 (2.0,7.8) |
| ALT (U/L) | 25.500 (12.8,68.3) | 24.000 (9.0,29.0) | 19.000 (14.0,29.0) |
| AST (U/L) | 41.000 (18.0,88.0) *,# | 24.000 (13.0,33.0) | 21.000 (19.0,27.0) |
| ALB (g/L) | 30.235 (27.8,34.4) *,# | 33.700 (31.2,37.5) * | 45.900 (44.5,47.0) |
| TBiL (μmol/L) | 16.500 (10.3,25.9) | 9.000 (7.0,12.4) * | 13.300 (11.4,16.8) |
| DBiL (μmol/L) | 7.965 (4.6,15.1) *,# | 4.700 (2.9,7.6) | 4.100 (3.3,5.5) |
| Cr (μmol/L) | 141.500 (74.5,266.3) *,# | 74.000 (52.0,90.0) | 69.000 (55.0,80.0) |
| eGFR (mL/min) | 38.970 (17.0,69.4) *,# | 87.490 (62.9,103.1) | 88.880 (78.5,102.0) |
| Ur (mmol/L) | 13.240 (8.0,20.0) *,# | 4.635 (3.7,8.6) | 5.560 (4.7,6.8) |
| UA (μmol/L) | 378.000 (263.3,467.3) # | 246.000 (182.0,356.5) * | 326.000 (292.0,388.0) |
| cf-DNA (ng/mL) | 624.990 (478.7,857.9) *,# | 469.966 (335.0,573.0) * | 292.328 (253.5,376.3) |
| MPO-DNA (OD) | 2.25 ± 0.49 *,# | 1.91 ± 0.34 * | 1.50 ± 0.51 |
| Variables | MPO-DNA | cf-DNA |
|---|---|---|
| WBC (109/L) | 0.006 | 0.025 |
| N (%) | 0.243 * | 0.217 * |
| PLT (109/L) | −0.211 * | −0.314 ** |
| PCT (ng/mL) | 0.386 ** | 0.426 ** |
| CRP (mg/L) | 0.216 * | 0.173 |
| SAA (mg/L) | 0.168 | 0.113 |
| ALT (U/L) | −0.045 | 0.124 |
| AST (U/L) | 0.05 | 0.165 |
| ALB (g/L) | −0.210 * | −0.158 |
| TBiL (μmol/L) | 0.1 | 0.133 |
| DBiL (μmol/L) | 0.200 * | 0.109 |
| Cr (μmol/L) | 0.235 * | 0.299 ** |
| eGFR (mL/min) | −0.258 ** | −0.295 ** |
| Ur (mmol/L) | 0.209 * | 0.375 ** |
| UA (μmol/L) | 0.235 * | 0.222 * |
| PT (s) | 0.245 * | 0.192 * |
| INR | 0.264 ** | 0.195 * |
| PTA (%) | −0.277 ** | −0.192 * |
| NT-proBNP (pg/mL) | 0.284 ** | 0.076 |
| cTnI (ng/L) | 0.211 * | 0.029 |
| CK-MB (ng/mL) | 0.240 * | 0.128 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| WBC | 0.983–1.110 | 0.161 | / | / |
| Neu (%) | 1.010–1.078 | 0.011 | 0.978–1.059 | 0.390 |
| RBC | 0.482–1.348 | 0.411 | / | / |
| PCT | 1.031–1.228 | 0.008 | 0.961–1.145 | 0.288 |
| CRP | 1.010–1.029 | <0.01 | 1.005–1.030 | 0.007 |
| SAA | 1.001–1.009 | 0.029 | 0.991–1.003 | 0.337 |
| MPO-DNA | 1.739–12.880 | 0.002 | 0.912–14.366 | 0.067 |
| cf-DNA | 1.002–1.008 | <0.01 | 1.000–1.007 | 0.046 |
| Variables | AUC | Sensitivity% | Specificity% | 95%CI | Youden Index | Cut-Off |
|---|---|---|---|---|---|---|
| MPO-DNA | 0.719 | 52.8 | 92 | 0.628–0.809 | 0.448 | 2.261 |
| cf-DNA | 0.744 | 55.7 | 84 | 0.650–0.838 | 0.397 | 587.679 |
| CRP | 0.777 | 82.1 | 68 | 0.680–0.873 | 0.501 | 59.515 |
| Model 1 | 0.834 | 76.4 | 76 | 0.755–0.913 | 0.524 | / |
| Model 2 | 0.837 | 76.4 | 84 | 0.753–0.903 | 0.604 | / |
| Model 3 | 0.865 | 78.3 | 84 | 0.794–0.936 | 0.623 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Guo, J.; Shi, C.; Wang, Y.; Zhang, Y.; Zhang, X.; Gong, Z. MPO-DNA Complexes and cf-DNA in Patients with Sepsis and Their Clinical Value. Biomedicines 2024, 12, 2190. https://doi.org/10.3390/biomedicines12102190
Zhang D, Guo J, Shi C, Wang Y, Zhang Y, Zhang X, Gong Z. MPO-DNA Complexes and cf-DNA in Patients with Sepsis and Their Clinical Value. Biomedicines. 2024; 12(10):2190. https://doi.org/10.3390/biomedicines12102190
Chicago/Turabian StyleZhang, Danmei, Jin Guo, Chunxia Shi, Yukun Wang, Yanqiong Zhang, Xiaoya Zhang, and Zuojiong Gong. 2024. "MPO-DNA Complexes and cf-DNA in Patients with Sepsis and Their Clinical Value" Biomedicines 12, no. 10: 2190. https://doi.org/10.3390/biomedicines12102190
APA StyleZhang, D., Guo, J., Shi, C., Wang, Y., Zhang, Y., Zhang, X., & Gong, Z. (2024). MPO-DNA Complexes and cf-DNA in Patients with Sepsis and Their Clinical Value. Biomedicines, 12(10), 2190. https://doi.org/10.3390/biomedicines12102190






