Exploring Liraglutide in Lithium–Pilocarpine-Induced Temporal Lobe Epilepsy Model in Rats: Impact on Inflammation, Mitochondrial Function, and Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals and Drugs
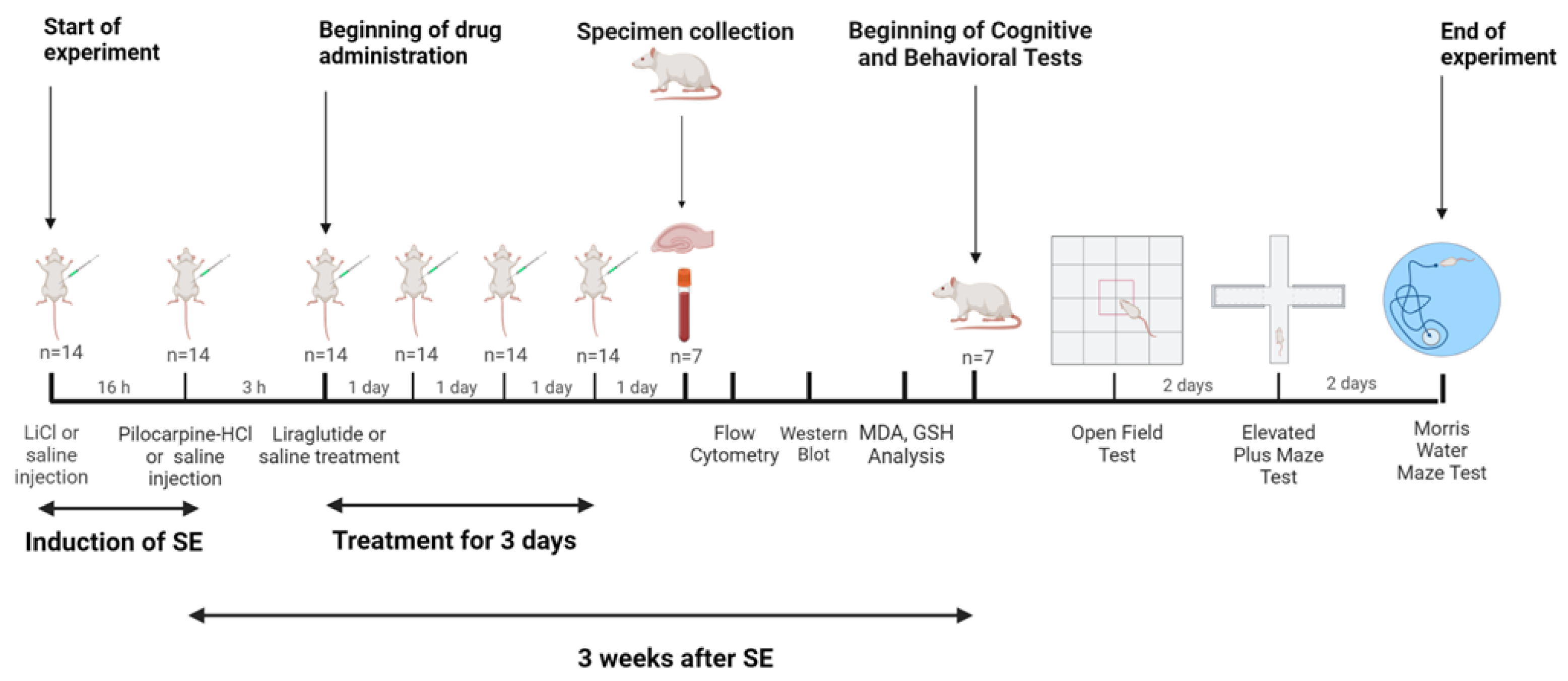
2.3. Study Design
2.4. Lithium–Pilocarpine-Model of TLE
2.5. Flow Cytometry
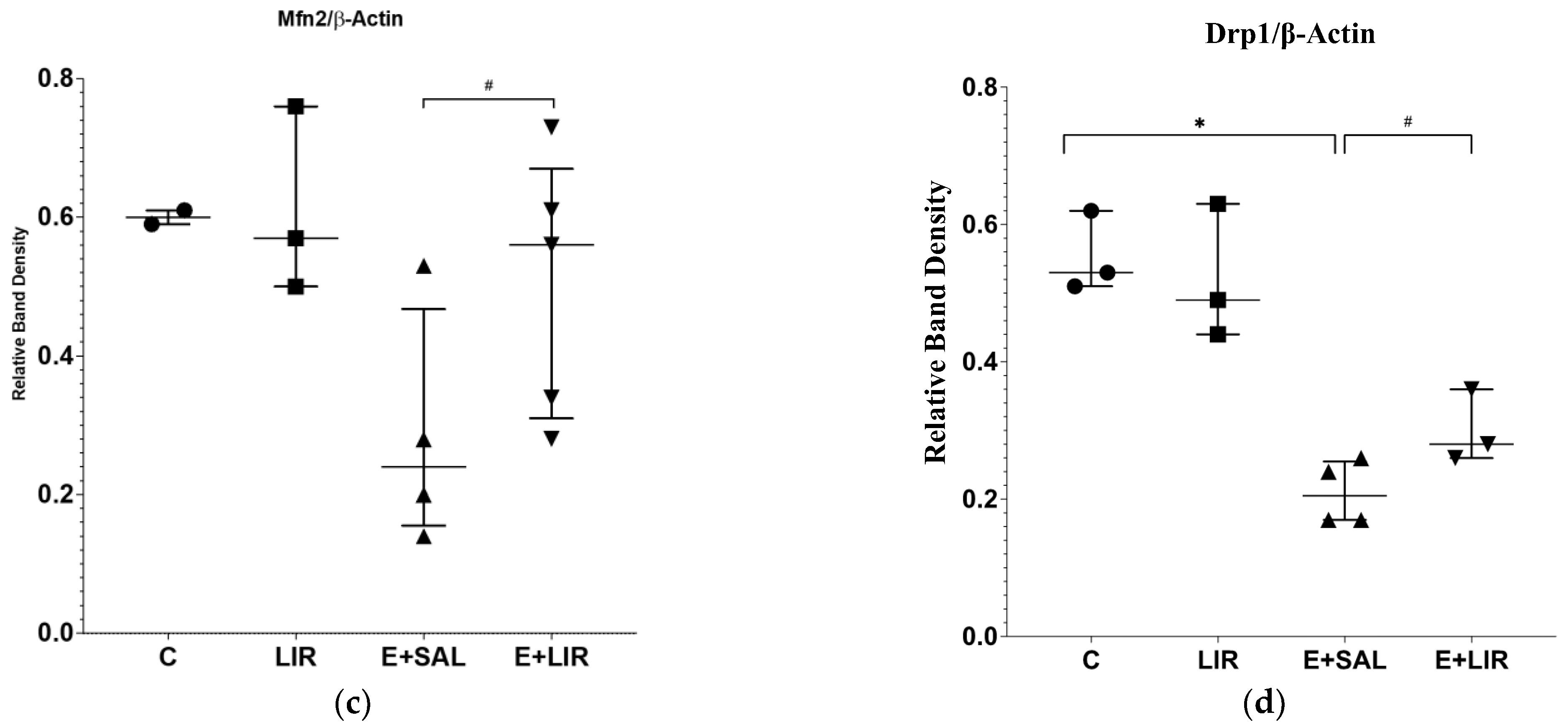
2.6. Western Blotting
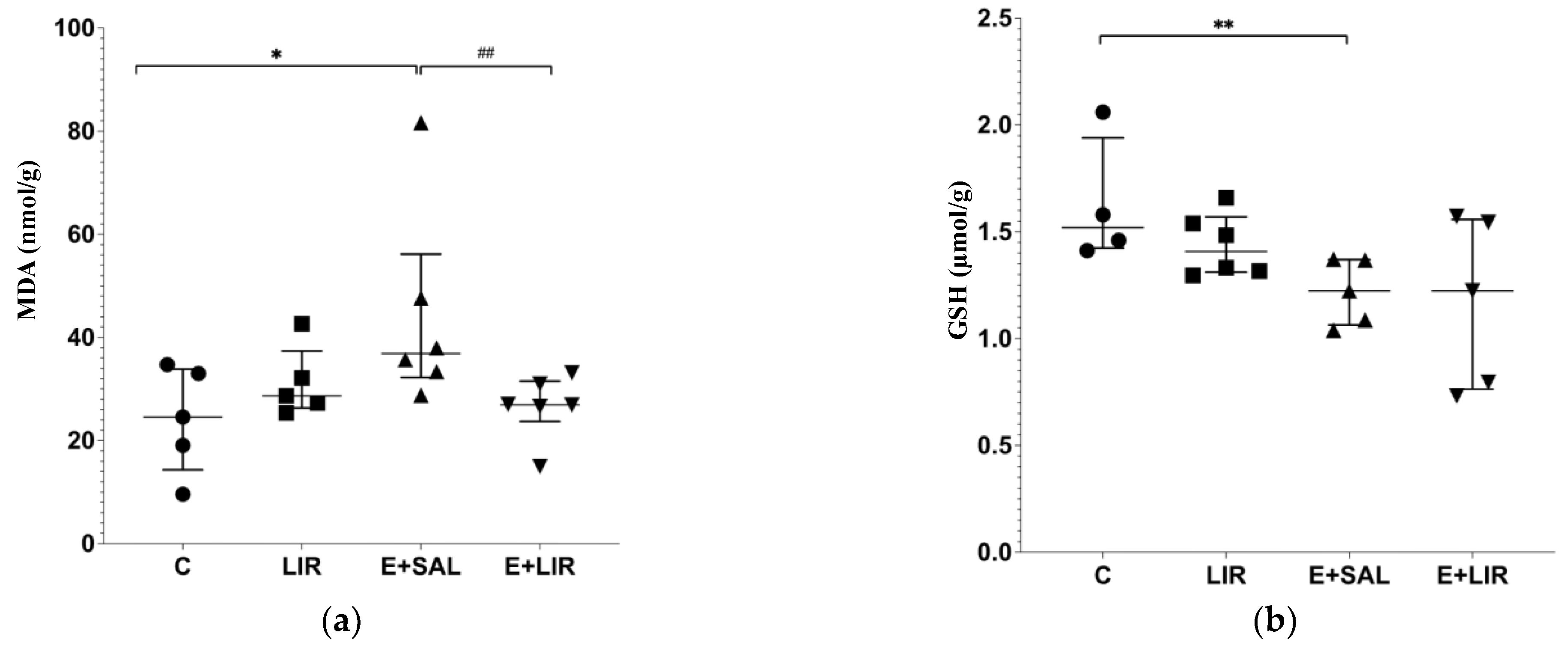
2.7. Determination of Malondialdehyde (MDA) and Glutathione (GSH) Levels in the Hippocampus
2.8. Cognitive and Behavioral Experiments
2.9. Statistical Analysis
3. Results
3.1. Liraglutide Treatment Decreased Inflammation in the Hippocampal Tissues of Rats with Epilepsy
3.2. Liraglutide Treatment Recruited Antioxidant Pathways in the Hippocampal Tissues of Rats with Epilepsy
3.3. Liraglutide Treatment Changed Mitochondrial Dynamics in the Hippocampal Tissues of Rats with Epilepsy
3.4. Liraglutide’s Impact on Mitochondrial Function in PBMCs
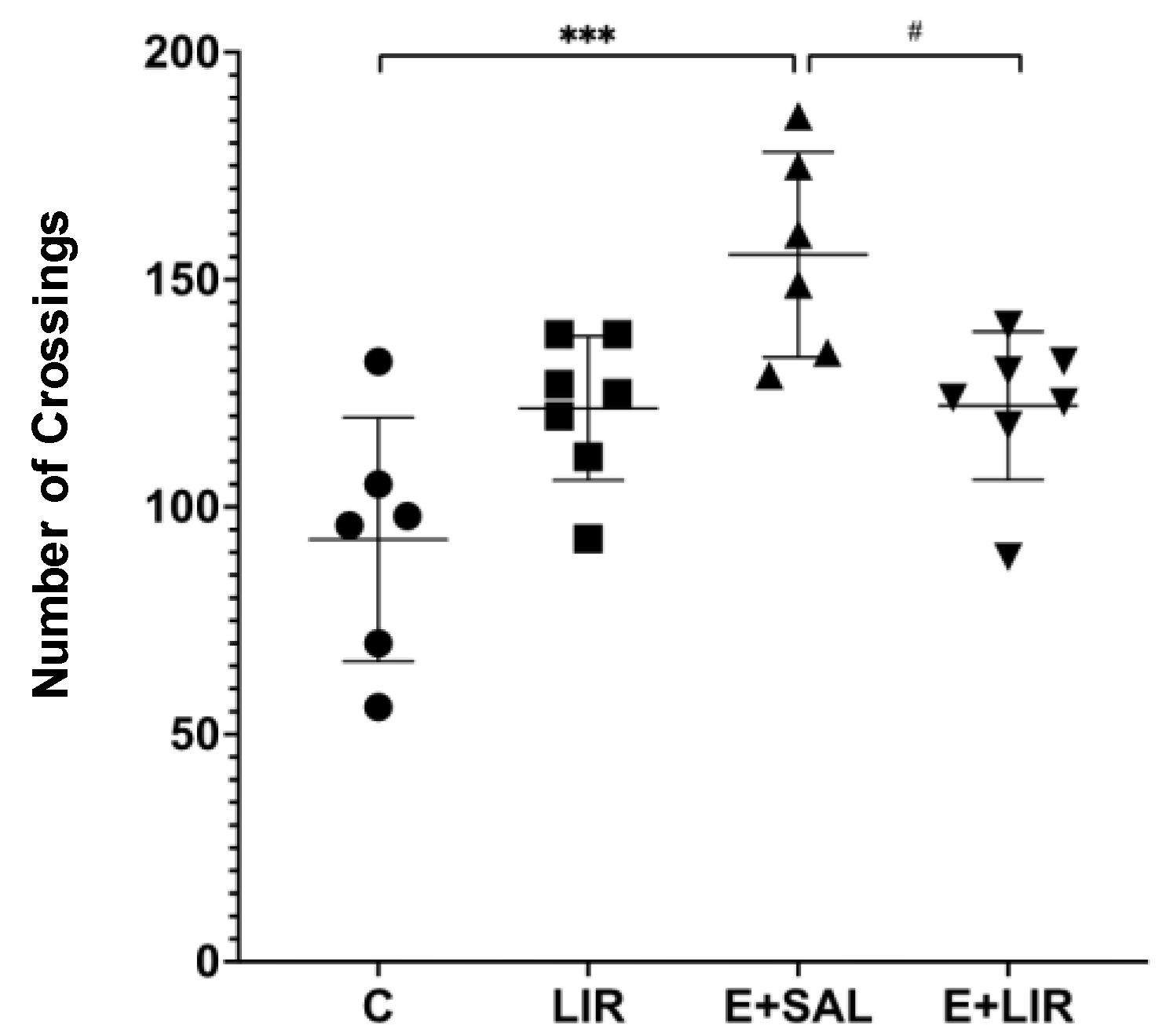
3.5. Liraglutide Treatment Reversed the Increased Locomotor Activity in Rats with Epilepsy
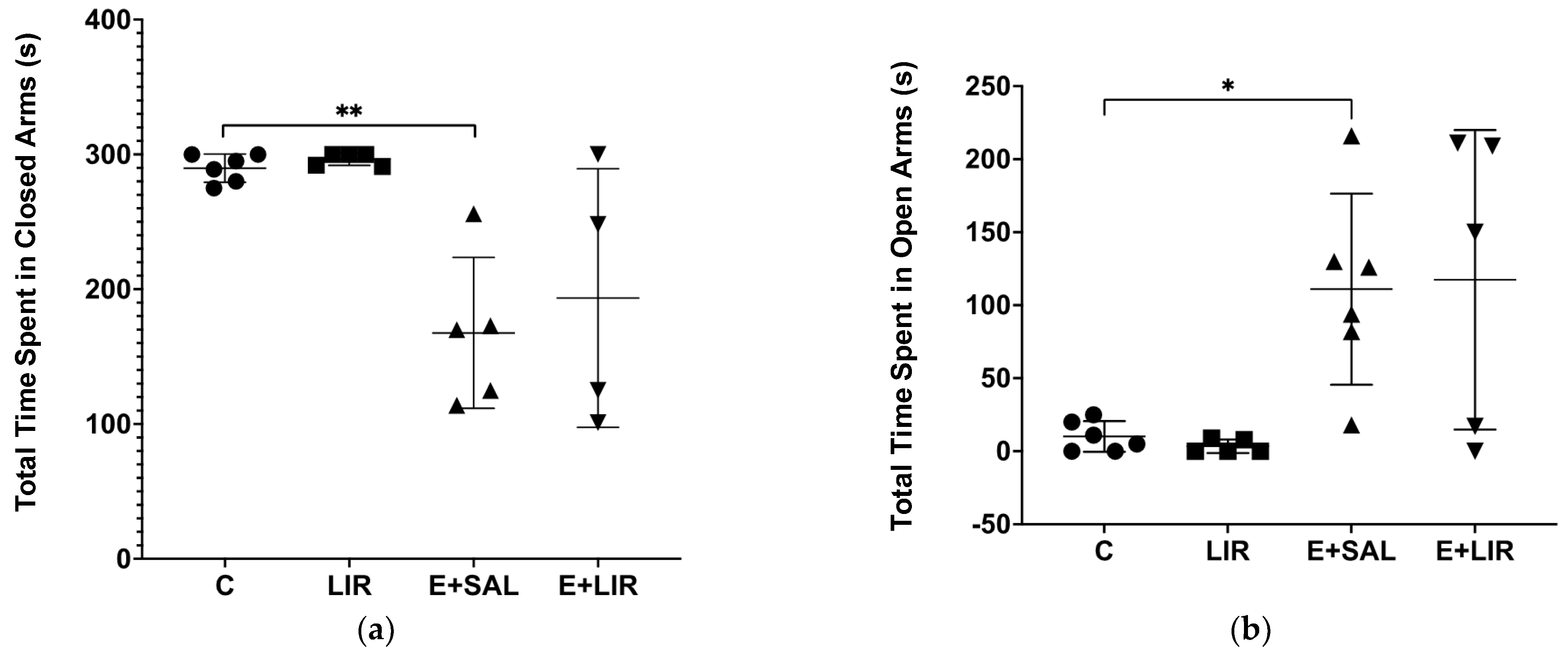
3.6. Liraglutide Treatment Did Not Affect Anxiety-Related Behavior in Rats with Epilepsy
3.7. Effects of Liraglutide Treatment on Learning and Memory in Rats with Epilepsy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Amlerova, J.; Sroubek, J.; Angelucci, F.; Hort, J. Evidences for a Role of Gut Microbiota in Pathogenesis and Management of Epilepsy. Int. J. Mol. Sci. 2021, 22, 5576. [Google Scholar] [CrossRef] [PubMed]
- Keezer, M.R.; Sisodiya, S.M.; Sander, J.W. Comorbidities of epilepsy: Current concepts and future perspectives. Lancet Neurol. 2016, 15, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Giussani, G. Treatment of epilepsy in light of the most recent advances. Lancet Neurol. 2019, 18, 7–8. [Google Scholar] [CrossRef]
- Blair, R.D. Temporal lobe epilepsy semiology. Epilepsy Res. Treat. 2012, 2012, 751510. [Google Scholar] [CrossRef]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F.; et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef]
- Bartolomei, F.; Wendling, F.; Regis, J.; Gavaret, M.; Guye, M.; Chauvel, P. Pre-ictal synchronicity in limbic networks of mesial temporal lobe epilepsy. Epilepsy Res. 2004, 61, 89–104. [Google Scholar] [CrossRef]
- Folbergrova, J.; Kunz, W.S. Mitochondrial dysfunction in epilepsy. Mitochondrion 2012, 12, 35–40. [Google Scholar] [CrossRef]
- Otahal, J.; Folbergrova, J.; Kovacs, R.; Kunz, W.S.; Maggio, N. Epileptic focus and alteration of metabolism. Int. Rev. Neurobiol. 2014, 114, 209–243. [Google Scholar]
- Kang, H.C.; Lee, Y.M.; Kim, H.D. Mitochondrial disease and epilepsy. Brain Dev. 2013, 35, 757–761. [Google Scholar] [CrossRef]
- Jahani-Asl, A.; Cheung, E.C.; Neuspiel, M.; MacLaurin, J.G.; Fortin, A.; Park, D.S.; McBride, H.M.; Slack, R.S. Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. J. Biol. Chem. 2007, 282, 23788–23798. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Rivero, J.M.; Villanueva-Paz, M.; de la Cruz-Ojeda, P.; de la Mata, M.; Cotan, D.; Oropesa-Avila, M.; de Lavera, I.; Alvarez-Cordoba, M.; Luzon-Hidalgo, R.; Sanchez-Alcazar, J.A. Mitochondrial Dynamics in Mitochondrial Diseases. Diseases 2016, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, G.; Chen, L.; Kim, S.; Yu, J.; Hu, G.; Chen, J.; Huang, Y.; Zheng, G.; Huang, S. The Role of NLRP3 and IL-1beta in Refractory Epilepsy Brain Injury. Front. Neurol. 2019, 10, 1418. [Google Scholar]
- Li, H.Y.; Zhong, Y.F.; Wu, S.Y.; Shi, N. NF-E2 related factor 2 activation and heme oxygenase-1 induction by tert-butylhydroquinone protect against deltamethrin-mediated oxidative stress in PC12 cells. Chem. Res. Toxicol. 2007, 20, 1242–1251. [Google Scholar] [CrossRef]
- Jhang, J.J.; Yen, G.C. The role of Nrf2 in NLRP3 inflammasome activation. Cell Mol. Immunol. 2017, 14, 1011–1012. [Google Scholar] [CrossRef]
- Hennig, P.; Garstkiewicz, M.; Grossi, S.; Di Filippo, M.; French, L.E.; Beer, H.D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018, 19, 562. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, D. Autistic spectrum disorders and mitochondrial encephalopathies. Acta Paediatr. 2008, 97, 859–860. [Google Scholar] [CrossRef]
- Leuner, K.; Schulz, K.; Schütt, T.; Pantel, J.; Prvulovic, D.; Rhein, V.; Savaskan, E.; Czech, C.; Eckert, A.; Müller, W.E. Peripheral mitochondrial dysfunction in Alzheimer’s disease: Focus on lymphocytes. Mol. Neurobiol. 2012, 46, 194–204. [Google Scholar] [CrossRef]
- Smith, A.M.; Depp, C.; Ryan, B.J.; Johnston, G.I.; Alegre-Abarrategui, J.; Evetts, S.; Rolinski, M.; Baig, F.; Ruffmann, C.; Simon, A.K.; et al. Mitochondrial dysfunction and increased glycolysis in prodromal and early Parkinson’s blood cells. Mov. Disord. 2018, 33, 1580–1590. [Google Scholar] [CrossRef]
- Badawi, G.A.; Abd El Fattah, M.A.; Zaki, H.F.; El Sayed, M.I. Sitagliptin and liraglutide reversed nigrostriatal degeneration of rodent brain in rotenone-induced Parkinson’s disease. Inflammopharmacology 2017, 25, 369–382. [Google Scholar] [CrossRef]
- Chen, A.; Chen, Z.; Xia, Y.; Lu, D.; Yang, X.; Sun, A.; Zou, Y.; Qian, J.; Ge, J. Liraglutide attenuates NLRP3 inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS pathway in H9c2 cells. Biochem. Biophys. Res. Commun. 2018, 499, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Koshal, P.; Jamwal, S.; Kumar, P. Glucagon-like Peptide-1 (GLP-1) and neurotransmitters signaling in epilepsy: An insight review. Neuropharmacology 2018, 136 Pt B, 271–279. [Google Scholar] [CrossRef]
- McClean, P.L.; Parthsarathy, V.; Faivre, E.; Holscher, C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 6587–6594. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.M.; Eldosoky, M.; El-Shafey, M.; El-Mesery, M.; Abbas, K.M.; Ali, A.N.; Helal, G.M.; Abulseoud, O.A. Effects of GLP-1 Receptor Activation on a Pentylenetetrazole-Kindling Rat Model. Brain Sci. 2019, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Citraro, R.; Iannone, M.; Leo, A.; De Caro, C.; Nesci, V.; Tallarico, M.; Abdalla, K.; Palma, E.; Arturi, F.; De Sarro, G.; et al. Evaluation of the effects of liraglutide on the development of epilepsy and behavioural alterations in two animal models of epileptogenesis. Brain Res. Bull. 2019, 153, 133–142. [Google Scholar] [CrossRef]
- Wu, P.; Dong, Y.; Chen, J.; Guan, T.; Cao, B.; Zhang, Y.; Qi, Y.; Guan, Z.; Wang, Y. Liraglutide Regulates Mitochondrial Quality Control System Through PGC-1α in a Mouse Model of Parkinson’s Disease. Neurotox. Res. 2022, 40, 286–297. [Google Scholar] [CrossRef]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef]
- Glien, M.; Brandt, C.; Potschka, H.; Voigt, H.; Ebert, U.; Loscher, W. Repeated low-dose treatment of rats with pilocarpine: Low mortality but high proportion of rats developing epilepsy. Epilepsy Res. 2001, 46, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.O.; Mortari, M.R.; Carolino, R.O.; Coutinho-Netto, J.; Dos Santos, W.F. Glutamate binding is altered in hippocampus and cortex of Wistar rats after pilocarpine-induced Status Epilepticus. Neurosci. Lett. 2007, 424, 51–54. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Wamil, A.C.G.; Kleinrok, Z.; De Wied, D. Beneficial effects of ACTH4-10 on pilocarpine induced seizures. Neurosci. Res. Commun. 1989, 4, 109–116. [Google Scholar]
- Tan, Y.S.; Lei, Y.L. Isolation of Tumor-Infiltrating Lymphocytes by Ficoll-Paque Density Gradient Centrifugation. Methods Mol. Biol. 2019, 1960, 93–99. [Google Scholar] [PubMed]
- Kolgazi, M.; Cantali-Ozturk, C.; Deniz, R.; Ozdemir-Kumral, Z.N.; Yuksel, M.; Sirvanci, S.; Yegen, B.C. Nesfatin-1 alleviates gastric damage via direct antioxidant mechanisms. J. Surg. Res. 2015, 193, 111–118. [Google Scholar] [CrossRef]
- Hall, C.; Ballachey, E.L. A study of the rat’s behavior in a field. A contribution to method in comparative psychology. Univ. Calif. Publ. Psychol. 1932, 6, 1–12. [Google Scholar]
- Berton, O.; Ramos, A.; Chaouloff, F.; Mormde, P. Behavioral reactivity to social and nonsocial stimulations: A multivariate analysis of six inbred rat strains. Behav. Genet. 1997, 27, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.G. Interactions of three benzodiazepine receptor inverse agonists with ethanol in a plus-maze test of anxiety. Pharmacol. Biochem. Behav. 1988, 30, 701–706. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Yörük, M.A.; Okkay, U.; Savaş, A.B.; Bayram, C.; Sezen, S.; Ertuğrul, M.S.; Hacımüftüoğlu, A. Behavioral Tests Used in Experimental Animal Models. Anatol. J. Biol. 2022, 3, 14–22. [Google Scholar]
- Foreman, N.; Gillett, R. A Handbook of Spatial Research Paradigms and Methodologies; Psychology Press: Hove, UK, 1997; Volume 2. [Google Scholar]
- Chakravarty, S.; Rizvi, S.I. Day and Night GSH and MDA Levels in Healthy Adults and Effects of Different Doses of Melatonin on These Parameters. Int. J. Cell Biol. 2011, 2011, 404591. [Google Scholar] [CrossRef]
- Karamercan, M.A.; Weiss, S.L.; Villarroel, J.P.; Guan, Y.; Werlin, E.; Figueredo, R.; Becker, L.B.; Sims, C. Can peripheral blood mononuclear cells be used as a proxy for mitochondrial dysfunction in vital organs during hemorrhagic shock and resuscitation? Shock 2013, 40, 476–484. [Google Scholar] [CrossRef]
- Friedman, A.; Dingledine, R. Molecular cascades that mediate the influence of inflammation on epilepsy. Epilepsia 2011, 52 (Suppl. S3), 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Chen, S.D.; Lin, K.J.; Chuang, Y.C. Seizure-Induced Oxidative Stress in Status Epilepticus: Is Antioxidant Beneficial? Antioxidants 2020, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Si, Z.; Li, X.; Lu, L.; Pan, Y.; Liu, J. FK506 Attenuated Pilocarpine-Induced Epilepsy by Reducing Inflammation in Rats. Front. Neurol. 2019, 10, 971. [Google Scholar] [CrossRef]
- Cristina de Brito Toscano, E.; Leandro Marciano Vieira, E.; Boni Rocha Dias, B.; Vidigal Caliari, M.; Paula Goncalves, A.; Varela Giannetti, A.; Mauricio Siqueira, J.; Kimie Suemoto, C.; Elaine Paraizo Leite, R.; Nitrini, R.; et al. NLRP3 and NLRP1 inflammasomes are up-regulated in patients with mesial temporal lobe epilepsy and may contribute to overexpression of caspase-1 and IL-beta in sclerotic hippocampi. Brain Res. 2021, 1752, 147230. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.F.; Tan, L.; Tan, M.S.; Jiang, T.; Tan, C.C.; Li, M.M.; Wang, H.F.; Yu, J.T. Inhibition of the NLRP3 inflammasome provides neuroprotection in rats following amygdala kindling-induced status epilepticus. J. Neuroinflamm. 2014, 11, 212. [Google Scholar] [CrossRef]
- Yue, J.; Wei, Y.J.; Yang, X.L.; Liu, S.Y.; Yang, H.; Zhang, C.Q. NLRP3 inflammasome and endoplasmic reticulum stress in the epileptogenic zone in temporal lobe epilepsy: Molecular insights into their interdependence. Neuropathol. Appl. Neurobiol. 2020, 46, 770–785. [Google Scholar] [CrossRef]
- Erdogan, M.A.; Erdogan, A.; Erbas, O. The Anti-Seizure Effect of Liraglutide on Ptz-Induced Convulsions Through its Anti-Oxidant and Anti-Inflammatory Properties. Neurochem. Res. 2023, 48, 188–195. [Google Scholar] [CrossRef]
- Hadjiyanni, I.; Siminovitch, K.A.; Danska, J.S.; Drucker, D.J. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia 2010, 53, 730–740. [Google Scholar] [CrossRef]
- He, L.; Wong, C.K.; Cheung, K.K.; Yau, H.C.; Fu, A.; Zhao, H.-L.; Leung, K.M.; Kong, A.P.; Wong, G.W.; Chan, P.K.; et al. Anti-inflammatory effects of exendin-4, a glucagon-like peptide-1 analog, on human peripheral lymphocytes in patients with type 2 diabetes. J. Diabetes Investig. 2013, 4, 382–392. [Google Scholar] [CrossRef]
- Huang, J.; Yi, H.; Zhao, C.; Zhang, Y.; Zhu, L.; Liu, B.; He, P.; Zhou, M. Glucagon-like peptide-1 receptor (GLP-1R) signaling ameliorates dysfunctional immunity in COPD patients. Int. J. Chron Obstruct. Pulmon. Dis. 2018, 13, 3191–3202. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Irie, J.; Tagawa, H.; Kusumoto, Y.; Kato, M.; Kobayashi, N.; Tanaka, K.; Kikuchi, R.; Fujita, M.; Nakajima, Y.; et al. GLP-1 receptor agonist, liraglutide, ameliorates hepatosteatosis induced by anti-CD3 antibody in female mice. J. Diabetes Complicat. 2017, 31, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Zobel, E.H.; Ripa, R.S.; von Scholten, B.J.; Curavic, V.R.; Kjaer, A.; Hansen, T.W.; Rossing, P.; Størling, J. Effect of liraglutide on expression of inflammatory genes in type 2 diabetes. Sci. Rep. 2021, 11, 18522. [Google Scholar] [CrossRef] [PubMed]
- Almarshad, F.M.; Ram, D. Liraglutide-Induced Depression with Suicidality in an Obese Adult: A Case Report. Galician Med. J. 2024, 31, 1–6. [Google Scholar] [CrossRef]
- Cuomo, A.; Bolognesi, S.; Goracci, A.; Ciuoli, C.; Crescenzi, B.B.; Maina, G.; Rosso, G.; Facchi, E.; Maccora, C.; Giordano, N.; et al. Feasibility, Adherence and Efficacy of Liraglutide Treatment in a Sample of Individuals with Mood Disorders and Obesity. Front. Psychiatry 2018, 9, 784. [Google Scholar] [CrossRef]
- He, Y.; Liang, F.; Wang, Y.; Wei, Y.; Ma, T. Liraglutide-associated depression in a patient with type 2 diabetes: A case report and discussion. Medicine 2024, 103, e37928. [Google Scholar] [CrossRef]
- Jones, B. The therapeutic potential of GLP-1 receptor biased agonism. Br. J. Pharmacol. 2022, 179, 492–510. [Google Scholar] [CrossRef]
- Freitas, R.M.; Vasconcelos, S.M.M.; Souza, F.C.F.; Viana, G.S.B.; Fonteles, M.M.F. Oxidative stress in the hippocampus after pilocarpine-induced status epilepticus in Wistar rats. FEBS J. 2005, 272, 1307–1312. [Google Scholar] [CrossRef]
- Santos, I.M.S.; Tomé, A.R.; Saldanha, G.B.; Ferreira, P.M.P.; Militão, G.C.G.; Freitas, R.M. Oxidative stress in the hippocampus during experimental seizures can be ameliorated with the antioxidant ascorbic acid. Oxid. Med. Cell. Longev. 2009, 2, 214–221. [Google Scholar] [CrossRef]
- Koshal, P.; Kumar, P. Neurochemical modulation involved in the beneficial effect of liraglutide, GLP-1 agonist on PTZ kindling epilepsy-induced comorbidities in mice. Mol. Cell. Biochem. 2016, 415, 77–87. [Google Scholar] [CrossRef]
- Mazhar, F.; Malhi, S.M.; Simjee, S.U. Comparative studies on the effects of clinically used anticonvulsants on the oxidative stress biomarkers in pentylenetetrazole-induced kindling model of epileptogenesis in mice. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Bharal, N.; Khurana, S.; Mediratta, P.K.; Sharma, K.K. Anticonvulsant and antioxidant actions of trimetazidine in pentylenetetrazole-induced kindling model in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Hogan, A.E.; Tobin, A.M.; Ahern, T.; Corrigan, M.A.; Gaoatswe, G.; Jackson, R.; O‘Reilly, V.; Lynch, L.; Doherty, D.G.; Moynagh, P.N.; et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: Lessons from obesity, diabetes and psoriasis. Diabetologia 2011, 54, 2745–2754. [Google Scholar] [CrossRef]
- Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Tuo, J.; Zhang, F.; Tai, Z.; Liu, X.; Qiu, X.; Zhang, H.; Yang, J.; Wang, J.; et al. PGC-1α Affects Epileptic Seizures by Regulating Mitochondrial Fusion in Epileptic Rats. Neurochem. Res. 2023, 48, 1361–1369. [Google Scholar] [CrossRef]
- Córdova-Dávalos, L.; Carrera-Calvo, D.; Solis-Navarrete, J.; Mercado-Gómez, O.F.; Arriaga-Ávila, V.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Guevara-Guzmán, R. Status epilepticus triggers early mitochondrial fusion in the rat hippocampus in a lithium-pilocarpine model. Epilepsy Res. 2016, 123, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Kim, J.E. PDI-mediated S-nitrosylation of DRP1 facilitates DRP1-S616 phosphorylation and mitochondrial fission in CA1 neurons. Cell Death Dis. 2018, 9, 869. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, J.; Tang, S.; Zheng, Y.; Zhou, X.; Tian, F.; Xu, Z. Dynamic-related protein 1 inhibitor eases epileptic seizures and can regulate equilibrative nucleoside transporter 1 expression. BMC Neurol. 2020, 20, 353. [Google Scholar] [CrossRef]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin mitochondrial quality control: A source of regional vulnerability in Parkinson’s disease. Mol. Neurodegener. 2020, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Orosco, L.A.; Panula, P.J. Oxidative stress and regulation of Pink1 in zebrafish (Danio rerio). PLoS ONE 2013, 8, e81851. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Lv, J.; Zheng, Z.; Liu, Y.; Zhang, Y.; Luo, C.; Qi, L.; Qin, B.; Liu, C. Mechanisms of microRNA-142 in mitochondrial autophagy and hippocampal damage in a rat model of epilepsy. Int. J. Mol. Med. 2021, 47, 98. [Google Scholar] [CrossRef]
- Sedky, A.A.; Magdy, Y. Reduction in TNF alpha and oxidative stress by liraglutide: Impact on ketamine-induced cognitive dysfunction and hyperlocomotion in rats. Life Sci. 2021, 278, 119523. [Google Scholar] [CrossRef]
- Szyndler, J.; Wierzba-Bobrowicz, T.; Skórzewska, A.; Maciejak, P.; Walkowiak, J.; Lechowicz, W.; Turzyńska, D.; Bidziński, A.; Płaźnik, A. Behavioral, biochemical and histological studies in a model of pilocarpine-induced spontaneous recurrent seizures. Pharmacol. Biochem. Behav. 2005, 81, 15–23. [Google Scholar] [CrossRef]
- Stewart, L.S.; Leung, L.S. Temporal lobe seizures alter the amplitude and timing of rat behavioral rhythms. Epilepsy Behav. 2003, 4, 153–160. [Google Scholar] [CrossRef]
- Guerrero-Hreins, E.; Goldstone, A.P.; Brown, R.M.; Sumithran, P. The therapeutic potential of GLP-1 analogues for stress-related eating and role of GLP-1 in stress, emotion and mood: A review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110303. [Google Scholar] [CrossRef]
- Weina, H.; Yuhu, N.; Christian, H.; Birong, L.; Feiyu, S.; Le, W. Liraglutide attenuates the depressive- and anxiety-like behaviour in the corticosterone induced depression model via improving hippocampal neural plasticity. Brain Res. 2018, 1694, 55–62. [Google Scholar] [CrossRef]
- Krass, M.; Rünkorg, K.; Vasar, E.; Volke, V. Acute administration of GLP-1 receptor agonists induces hypolocomotion but not anxiety in mice. Acta Neuropsychiatr. 2012, 24, 296–300. [Google Scholar] [CrossRef]
- Strawn, J.R.; D’Alessio, D.A.; Keck Jr, P.E.; Seeley, R.J. Failure of glucagon-like peptide-1 to induce panic attacks or anxiety in patients with panic disorder. J. Psychiatr. Res. 2008, 42, 787–789. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.G.; Filho, A.J.M.C.; Oliveira, J.V.S.; de Souza, D.A.A.; Lopes, I.S.; de Carvalho, M.A.J.; de Lima, K.A.; Sousa, F.C.F.; Vasconcelos, S.M.M.; Macedo, D.; et al. Prevention of pentylenetetrazole-induced kindling and behavioral comorbidities in mice by levetiracetam combined with the GLP-1 agonist liraglutide: Involvement of brain antioxidant and BDNF upregulating properties. Biomed. Pharmacother. 2019, 109, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Beldhuis, H.J.; Everts, H.G.; Van der Zee, E.A.; Luiten, P.G.; Bohus, B. Amygdala kindling-induced seizures selectively impair spatial memory. 2. Effects on hippocampal neuronal and glial muscarinic acetylcholine receptor. Hippocampus 1992, 2, 411–419. [Google Scholar] [CrossRef]
- Erdoğan, F.; Gölgeli, A.; Arman, F.; Ersoy, A.O. The effects of pentylenetetrazole-induced status epilepticus on behavior, emotional memory, and learning in rats. Epilepsy Behav. 2004, 5, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, I.V.; Zubareva, O.E.; Kalemenev, S.V.; Lavrentyeva, V.V.; Dyomina, A.V.; Karepanov, A.A.; Zaitsev, A.V. Impairments in cognitive functions and emotional and social behaviors in a rat lithium-pilocarpine model of temporal lobe epilepsy. Behav. Brain Res. 2019, 372, 112044. [Google Scholar] [CrossRef]
- Seo, M.K.; Jeong, S.; Seog, D.H.; Lee, J.A.; Lee, J.H.; Lee, Y.; McIntyre, R.S.; Park, S.W.; Lee, J.G. Effects of liraglutide on depressive behavior in a mouse depression model and cognition in the probe trial of Morris water maze test. J. Affect. Disord. 2023, 324, 8–15. [Google Scholar] [CrossRef]
- Chen, S.; Sun, J.; Zhao, G.; Guo, A.; Chen, Y.; Fu, R.; Deng, Y. Liraglutide Improves Water Maze Learning and Memory Performance While Reduces Hyperphosphorylation of Tau and Neurofilaments in APP/PS1/Tau Triple Transgenic Mice. Neurochem. Res. 2017, 42, 2326–2335. [Google Scholar] [CrossRef]
- Babic, I.; Gorak, A.; Engel, M.; Sellers, D.; Else, P.; Osborne, A.L.; Pai, N.; Huang, X.F.; Nealon, J.; Weston-Green, K. Liraglutide prevents metabolic side-effects and improves recognition and working memory during antipsychotic treatment in rats. J. Psychopharmacol. 2018, 32, 578–590. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antmen, F.M.; Fedaioglu, Z.; Acar, D.; Sayar, A.K.; Yavuz, I.E.; Ada, E.; Karakose, B.; Rzayeva, L.; Demircan, S.; Kardouh, F.; et al. Exploring Liraglutide in Lithium–Pilocarpine-Induced Temporal Lobe Epilepsy Model in Rats: Impact on Inflammation, Mitochondrial Function, and Behavior. Biomedicines 2024, 12, 2205. https://doi.org/10.3390/biomedicines12102205
Antmen FM, Fedaioglu Z, Acar D, Sayar AK, Yavuz IE, Ada E, Karakose B, Rzayeva L, Demircan S, Kardouh F, et al. Exploring Liraglutide in Lithium–Pilocarpine-Induced Temporal Lobe Epilepsy Model in Rats: Impact on Inflammation, Mitochondrial Function, and Behavior. Biomedicines. 2024; 12(10):2205. https://doi.org/10.3390/biomedicines12102205
Chicago/Turabian StyleAntmen, Fatma Merve, Zeynep Fedaioglu, Dilan Acar, Ahmed Kerem Sayar, Ilayda Esma Yavuz, Ece Ada, Bengisu Karakose, Lale Rzayeva, Sevcan Demircan, Farah Kardouh, and et al. 2024. "Exploring Liraglutide in Lithium–Pilocarpine-Induced Temporal Lobe Epilepsy Model in Rats: Impact on Inflammation, Mitochondrial Function, and Behavior" Biomedicines 12, no. 10: 2205. https://doi.org/10.3390/biomedicines12102205
APA StyleAntmen, F. M., Fedaioglu, Z., Acar, D., Sayar, A. K., Yavuz, I. E., Ada, E., Karakose, B., Rzayeva, L., Demircan, S., Kardouh, F., Senay, S., Kolgazi, M., Suyen, G., & Oz-Arslan, D. (2024). Exploring Liraglutide in Lithium–Pilocarpine-Induced Temporal Lobe Epilepsy Model in Rats: Impact on Inflammation, Mitochondrial Function, and Behavior. Biomedicines, 12(10), 2205. https://doi.org/10.3390/biomedicines12102205






