Exploring Intra-Articular Administration of Monoclonal Antibodies as a Novel Approach to Osteoarthritis Treatment: A Systematic Review
Abstract
:1. Introduction
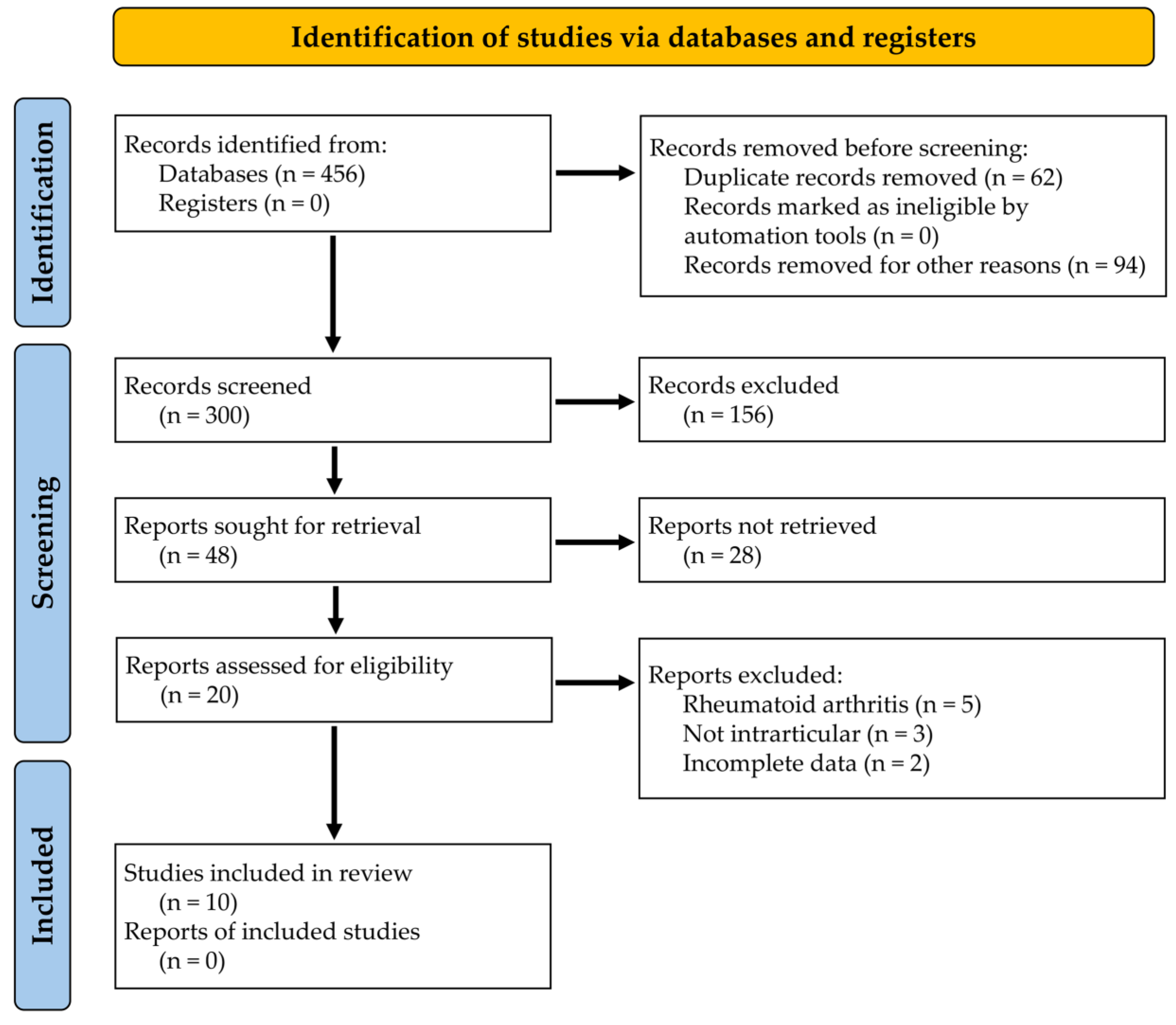
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Solmi, M.; Smith, T.O.; Noale, M.; Cooper, C.; Maggi, S. Association between lower limb osteoarthritis and incidence of depressive symptoms: Data from the osteoarthritis initiative. Age Ageing 2017, 46, 470–476. [Google Scholar] [CrossRef]
- Shichman, I.; Askew, N.; Habibi, A.; Nherera, L.; Macaulay, W.; Seyler, T.; Schwarzkopf, R. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2040–2060. Arthroplast. Today 2023, 21, 101152. [Google Scholar] [CrossRef] [PubMed]
- Scaturro, D.; Vitagliani, F.; Caracappa, D.; Tomasello, S.; Chiaramonte, R.; Vecchio, M.; Camarda, L.; Mauro, G.L. Rehabilitation approach in robot assisted total knee arthroplasty: An observational study. BMC Musculoskelet. Disord. 2023, 24, 140. [Google Scholar] [CrossRef] [PubMed]
- Sahin, N.; Yesil, H. Regenerative methods in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101824. [Google Scholar] [CrossRef]
- Scaturro, D.; Vitagliani, F.; Terrana, P.; Tomasello, S.; Falco, V.; Cuntrera, D.; Spoto, I.; Midiri, M.; Letizia Mauro, G. Hybrid Hyaluronic Acid versus High Molecular Weight Hyaluronic Acid for the Treatment of Hip Osteoarthritis in Overweight/Obese Patients. J. Funct. Morphol. Kinesiol. 2022, 7, 20. [Google Scholar] [CrossRef]
- Scaturro, D.; Vitagliani, F.; Terrana, P.; Cuntrera, D.; Falco, V.; Tomasello, S.; Letizia Mauro, G. Intra-Articular Hybrid Hyaluronic Acid Injection Treatment in Overweight Patients with Knee Osteoarthritis: A Single-Center, Open-Label, Prospective Study. Appl. Sci. 2021, 11, 8711. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, M.; Meng, H.Y.; Wang, A.Y.; Guo, Q.Y.; Wang, Y.; Peng, J. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthr. Cartil. 2013, 21, 1627–1637. [Google Scholar] [CrossRef]
- Andia, I.; Martin, J.I.; Maffulli, N. Platelet-rich Plasma and Mesenchymal Stem Cells: Exciting, But … are we there Yet? Sports Med. Arthrosc. 2018, 26, 59–63. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C. The Current Role of Disease-modifying Osteoarthritis Drugs. Arch. Bone Jt. Surg. 2023, 11, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E.; Schnitzer, T.J.; Birbara, C.A.; Mokhtarani, M.; Shelton, D.L.; Smith, M.D.; Brown, M.T. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 2010, 363, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Sabha, M.; Siaton, B.C.; Hochberg, M.C. Lorecivivint, an intra-articular potential disease-modifying osteoarthritis drug. Expert Opin. Investig. Drugs 2020, 29, 1339–1346. [Google Scholar] [CrossRef]
- Tamilarasan, A.G.; Cunningham, G.; Irving, P.M.; Samaan, M.A. Recent advances in monoclonal antibody therapy in IBD: Practical issues. Frontline Gastroenterol. 2019, 10, 409–416. [Google Scholar] [CrossRef]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- Bobrowicz, M.; Zagozdzon, R.; Domagala, J.; Vasconcelos-Berg, R.; Guenova, E.; Winiarska, M. Monoclonal Antibodies in Dermatooncology-State of the Art and Future Perspectives. Cancers 2019, 11, 1420. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Bedingfield, S.K.; Colazo, J.M.; Yu, F.; Liu, D.D.; Jackson, M.A.; Himmel, L.E.; Cho, H.; Crofford, L.J.; Hasty, K.A.; Duvall, C.L. Amelioration of post-traumatic osteoarthritis via nanoparticle depots delivering small interfering RNA to damaged cartilage. Nat. Biomed. Eng. 2021, 5, 1069–1083. [Google Scholar] [CrossRef]
- Vadalà, G.; Ambrosio, L.; Cattani, C.; Bernardini, R.; Giacalone, A.; Papalia, R.; Denaro, V. Bevacizumab Arrests Osteoarthritis Progression in a Rabbit Model: A Dose-Escalation Study. J. Clin. Med. 2021, 10, 2825. [Google Scholar] [CrossRef]
- Li, W.; Lin, J.; Wang, Z.; Ren, S.; Wu, X.; Yu, F.; Weng, J.; Zeng, H. Bevacizumab tested for treatment of knee osteoarthritis via inhibition of synovial vascular hyperplasia in rabbits. J. Orthop. Transl. 2019, 19, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Sato, M.; Kobayashi, M.; Yokoyama, M.; Tani, Y.; Mochida, J. Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Arthritis Res. Ther. 2014, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Onodera, T.; Terkawi, M.A.; Iwasaki, K.; Hishimura, R.; Liang, D.; Miyazaki, T.; Iwasaki, N. Local Administration of Low-Dose Nerve Growth Factor Antibody Reduced Pain in a Rat Osteoarthritis Model. Int. J. Mol. Sci. 2021, 22, 2552. [Google Scholar] [CrossRef] [PubMed]
- van Helvoort, E.M.; de Visser, H.M.; Lafeber, F.P.J.G.; Coeleveld, K.; Versteeg, S.; Weinans, H.H.; Popov-Celeketic, J.; Eijkelkamp, N.; Mastbergen, S.C. IL4-10 Fusion Protein Shows DMOAD Activity in a Rat Osteoarthritis Model. Cartilage 2021, 13, 1155S–1164S. [Google Scholar] [CrossRef]
- van Helvoort, E.M.; Popov-Celeketic, J.; Eijkelkamp, N.; Coeleveld, K.; Tryfonidou, M.A.; Wijne, C.D.; Hack, C.E.; Lafeber, F.P.J.G.; Mastbergen, S.C. Canine IL4-10 fusion protein provides disease modifying activity in a canine model of OA; an exploratory study. PLoS ONE 2019, 14, e0219587. [Google Scholar] [CrossRef]
- Zhang, Q.; Lv, H.H.; Chen, A.; Liu, F.; Wu, X. Efficacy of infliximab in a rabbit model of osteoarthritis. Connect. Tissue Res. 2012, 53, 355–358. [Google Scholar] [CrossRef]
- Urech, D.M.; Feige, U.; Ewert, S.; Schlosser, V.; Ottiger, M.; Polzer, K.; Schett, G.; Lichtlen, P. Anti-inflammatory and cartilage-protecting effects of an intra-articularly injected anti-TNF{alpha} single-chain Fv antibody (ESBA105) designed for local therapeutic use. Ann. Rheum. Dis. 2010, 69, 443–449. [Google Scholar] [CrossRef]
- Chiusaroli, R.; Visentini, M.; Galimberti, C.; Casseler, C.; Mennuni, L.; Covaceuszach, S.; Lanza, M.; Ugolini, G.; Caselli, G.; Rovati, L.C.; et al. Targeting of ADAMTS5’s ancillary domain with the recombinant mAb CRB0017 ameliorates disease progression in a spontaneous murine model of osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1807–1810. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef]
- Wang, Y.; Wagner, E.S.; Yu, D.; Chen, K.J.; Keel, T.J.; Pownder, S.L.; Koff, M.F.; Cheetham, J.; Samaroo, K.J.; Reesink, H.L. Assessment of osteoarthritis functional outcomes and intra-articular injection volume in the rat anterior cruciate ligament transection model. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2022, 40, 2004–2014. [Google Scholar] [CrossRef]
- Wyatt, L.A.; Nwosu, L.N.; Wilson, D.; Hill, R.; Spendlove, I.; Bennett, A.J.; Scammell, B.E.; Walsh, D.A. Molecular expression patterns in the synovium and their association with advanced symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2019, 27, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Hamilton, J.L.; Kc, R.; Berendsen, A.D.; Duan, X.; Cheong, C.W.; Li, X.; Im, H.-J.; Olsen, B.R. Vascular Endothelial Growth Factor in Cartilage Development and Osteoarthritis. Sci. Rep. 2017, 7, 13027. [Google Scholar] [CrossRef] [PubMed]
- Estee, M.M.; Cicuttini, F.M.; Page, M.J.; Wluka, A.E.; Wang, Y. Efficacy of tumor necrosis factor inhibitors in hand osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Osteoarthr. Cartil. Open 2023, 5, 100404. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Stoppiello, L.A.; Mapp, P.I.; Wilson, D.; Hill, R.; Scammell, B.E.; Walsh, D.A. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol. 2014, 66, 3018–3027. [Google Scholar] [CrossRef]
- Vincent, T.L. Peripheral pain mechanisms in osteoarthritis. Pain 2020, 161 (Suppl. S1), S138–S146. [Google Scholar] [CrossRef]
- Sanga, P.; Katz, N.; Polverejan, E.; Wang, S.; Kelly, K.M.; Haeussler, J.; Thipphawong, J. Long-Term Safety and Efficacy of Fulranumab in Patients With Moderate-to-Severe Osteoarthritis Pain: A Phase II Randomized, Double-Blind, Placebo-Controlled Extension Study. Arthritis Rheumatol. 2017, 69, 763–773. [Google Scholar] [CrossRef]
- Fan, Z.-R.; Ma, J.-X.; Wang, Y.; Chen, H.-T.; Lang, S.; Ma, X.-L. Efficacy and safety of tanezumab administered as a fixed dosing regimen in patients with knee or hip osteoarthritis: A meta-analysis of randomized controlled phase III trials. Clin. Rheumatol. 2021, 40, 2155–2165. [Google Scholar] [CrossRef]
- Sotozawa, M.; Kumagai, K.; Ishikawa, K.; Yamada, S.; Inoue, Y.; Inaba, Y. Bevacizumab suppressed degenerative changes in articular cartilage explants from patients with osteoarthritis of the knee. J. Orthop. Surg. Res. 2023, 18, 25. [Google Scholar] [CrossRef]
- Vincent, T.L. IL-1 in osteoarthritis: Time for a critical review of the literature. F1000Research 2019, 8, F1000. [Google Scholar] [CrossRef]
- Magdelaine-Beuzelin, C.; Pinault, C.; Paintaud, G.; Watier, H. Therapeutic antibodies in ophthalmology: Old is new again. MAbs 2010, 2, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, E.; Maehara, M.; Watanabe, M.; Sato, M. Candidates for Intra-Articular Administration Therapeutics and Therapies of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 3594. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Blanco, F.J.; Guermazi, A.; Miki, K.; Yamabe, T.; Viktrup, L.; Junor, R.; Carey, W.; Brown, M.T.; West, C.R.; et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: Efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period. Ann. Rheum. Dis. 2020, 79, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.L.; Li, Y.; Ning, G.Z.; Yuan, Z.F.; Chen, L.X.; Bi, M.C.; Sun, J.C.; Feng, S.Q. Tanezumab for Patients with Osteoarthritis of the Knee: A Meta-Analysis. PLoS ONE 2016, 11, e0157105. [Google Scholar] [CrossRef] [PubMed]
- Al Khayyat, S.G.; Conticini, E.; Falsetti, P.; Fogliame, G.; Gentileschi, S.; Baldi, C.; Bardelli, M.; Migliore, A.; Cantarini, L.; Frediani, B. Intra-articular injections of biological disease-modifying anti-rheumatic drugs in inflammatory arthropathies: An up-to-date narrative review. Joint Bone Spine 2024, 90, 105598, Erratum in: Joint Bone Spine 2024, 91, 105745. [Google Scholar] [CrossRef]
- Salem, R.M.; El-deeb, A.E.; Elsergany, M.; Elsaadany, H.; El-Khouly, R. Intra-articular injection of etanercept versus glucocorticoids in rheumatoid arthritis patients. Clin. Rheumatol. 2021, 40, 557–564. [Google Scholar] [CrossRef]
| First Author | Year | Animal Model | Number of Animals | Control Group | OA Model | Type of Antibody | Concentration | Volume | Frequency | Number of Injections | Follow-Up | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bedingfield et al. [19] | 2021 | mice | 9 | Y | CML * | Anti-MMP-13 | 0.5 mg/mL | - | weekly | 6 | 6 w | MMP13 ↓ |
| Vadalà et al. [20] | 2021 | rabbit | 18 | Y | ACL § | anti-VEGF | 6.25 to 25 mg/mL | 800 μL | weekly | 4 | 12 w | MMP13 ↓, coll II ↑, aggrecan ↑, OARSI ↑ |
| Li et al. [21] | 2019 | rabbit | 8 | Y | 5 w plaster | anti-VEGF | 10 mg/mL | 400 μL | 3 weeks | 2 | 6 w | MMP1 ↓, VEGF ↓ |
| Nagai et al. [22] | 2014 | rabbit | 6 | Y | ACLT § | anti-VEGF | 25 mg/mL | 1000 μL | weekly | 4 | 12 w | Pain ↓ |
| Tian et al. [23] | 2021 | rat | 18 | Y | MIA † injection for 2 weeks | anti-NGF | - | - | weekly | 4 | - | Pain ↓ |
| van Helvoort et al. [24] | 2021 | rat | 10 | Y | Groove model | anti-IL4-10 | 0.02 mg/mL | 25 µL | weekly | 10 | 10 w | Pain ↓ |
| van Helvoort et al. [25] | 2019 | canine | 4 | Y | Groove model | anti-IL4-10 | 0.02 mg/mL | 500 μL | weekly | 10 | 10 w | Pain ↓ |
| Zhang et al. [26] | 2012 | rabbit | 20 | Y | Hulth technique (medial meniscus resections) ACLT | anti-TNF-α | 10 to 20 mg/mL | 500 μL | weekly | 3 | 12 w | Mankin ↑ |
| Urech et al. [27] | 2010 | rat | 6 | Y | TNF-α injection | anti-TNF-α | - | 40 μL | - | 1 | 48 h | - |
| Chiusaroli et al. [28] | 2013 | mice | 41 | Y | Old mouse | anti-ADAMTS5 | 0.3 to 3 mg/mL | 4 μL | 6 weeks | 2 | Mankin ↑, OARSI ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smakaj, A.; Gasbarra, E.; Cardelli, T.; Salvati, C.; Bonanni, R.; Cariati, I.; Iundusi, R.; Tarantino, U. Exploring Intra-Articular Administration of Monoclonal Antibodies as a Novel Approach to Osteoarthritis Treatment: A Systematic Review. Biomedicines 2024, 12, 2217. https://doi.org/10.3390/biomedicines12102217
Smakaj A, Gasbarra E, Cardelli T, Salvati C, Bonanni R, Cariati I, Iundusi R, Tarantino U. Exploring Intra-Articular Administration of Monoclonal Antibodies as a Novel Approach to Osteoarthritis Treatment: A Systematic Review. Biomedicines. 2024; 12(10):2217. https://doi.org/10.3390/biomedicines12102217
Chicago/Turabian StyleSmakaj, Amarildo, Elena Gasbarra, Tommaso Cardelli, Chiara Salvati, Roberto Bonanni, Ida Cariati, Riccardo Iundusi, and Umberto Tarantino. 2024. "Exploring Intra-Articular Administration of Monoclonal Antibodies as a Novel Approach to Osteoarthritis Treatment: A Systematic Review" Biomedicines 12, no. 10: 2217. https://doi.org/10.3390/biomedicines12102217
APA StyleSmakaj, A., Gasbarra, E., Cardelli, T., Salvati, C., Bonanni, R., Cariati, I., Iundusi, R., & Tarantino, U. (2024). Exploring Intra-Articular Administration of Monoclonal Antibodies as a Novel Approach to Osteoarthritis Treatment: A Systematic Review. Biomedicines, 12(10), 2217. https://doi.org/10.3390/biomedicines12102217







