Abstract
Background: Mucosal leishmaniasis (ML) is a deforming type of American Tegumentary Leishmaniasis caused by Leishmania (Viannia) braziliensis that frequently does not respond to treatment. Despite its relapsing clinical course, few parasites are usually found in mucosal lesions. Host and parasite factors may be responsible for this paradox in the pathogenesis of the disease, allowing for both a low parasite burden and the inability of the host to clear and eliminate the disease. Methods and results: In this work, we present a clinical case of relapsing ML that was treated for 25 years without success with SbV, N-methyl glucamine, sodium stibogluconate, amphotericin B deoxycholate, gabromycin, antimonial plus thalidomide, liposomal amphotericin B, Leishvacin (a vaccine made in Brazil) and miltefosine. In a comparative analysis using nanoscale liquid chromatography coupled with tandem mass spectrometry of protein extracts of L. (V.) braziliensis promastigotes isolated from the patient and from the reference strain (MHOM/BR/94/M15176), we observed increases in ATPase and HSP70 protein levels in the parasite. We also observed an impairment in the production of hydrogen peroxide by peripheral mononuclear blood monocytes (PBMCs), as assessed by the horseradish peroxidase-dependent oxidation of phenol red. Conclusions: We hypothesise that these parasite molecules may be linked to the impairment of host parasiticidal responses, resulting in Leishmania persistence in ML patients.
1. Introduction
Leishmaniasis is a major public health problem worldwide, with an estimated 700,000 to 1,000,000 new cases annually [1]. Mucosal leishmaniasis (ML) is one of the most serious manifestations of American cutaneous leishmaniasis (ACL). It does not seem to heal spontaneously and can cause the destruction of nasal cartilage and soft tissue, with possible facial disfiguration and even mortality [2,3,4].
Treatment failure is a growing problem, and parasitic resistance is the main underlying factor [5]. The proportion of patients with the disease one year after treatment was 30%, according to a retrospective Brazilian hospital study [6,7]. The identification of parasite pathogenic molecules is scientifically valuable since it allows the development of new targeted drugs, but it can also have an immediate clinical impact through its prognostic value in predicting resistance and susceptibility [8,9]. Proteomic and transcriptomic studies of the parasite have suggested possible factors associated with this resistance to treatment, including proteins involved in oxidative stress [9,10] and metabolism [11].
Here, we wanted to explore the possible physiopathological causes of treatment failure in a patient diagnosed with ML caused by Leishmania (V.) braziliensis who had undergone multiple treatments over the course of 25 years. To achieve this goal, we compared the parasites cultured from the patient with those from a control strain subjected to treatment. We measured the expression of proteins to identify molecules potentially involved in drug resistance and assessed hydrogen peroxide production in monocytes from this patient.
2. Materials and Methods
2.1. Culture
The control strain (Leishmania (V.) braziliensis-MHOM/BR/94/M15176) used in this study was provided by the Evandro Chagas Institute, and the experimental strain was collected from a patient in 2009 and cryopreserved in liquid nitrogen in the laboratory until analysis one and a half years later. At the time of sample collection, the patient had active mucosal lesions. Before analysis, both strains were decryopreserved and injected into the feet of C57BL/6 mice, and after the appearance of lesions, the parasites were collected with a 1 mL syringe containing 0.5 mL of 0.9% saline, cultured for five days in McNeal, Novy and Nicolle (NNN) media supplemented with FBS at 22 °C, and then seeded in Schneider medium supplemented with 20% FBS and gentamicin, as described elsewhere [12]. After an initial growth phase of one week, parasite numbers were measured in a Neubauer chamber daily according to the methodology proposed by Brener [13].
2.2. Mass Spectrometry Analysis
2.2.1. Protein Extraction
Protein extraction was performed according to the protocol proposed by Sussulini [14], with modifications. Briefly, a parasite culture suspension in the logarithmic growth phase was centrifuged at 1000× g at 4 °C for 10 min. After three washes with ice-cold PBS, the parasite pellet was solubilized in 1 mL of petroleum ether for 15 min under strong agitation to remove lipids. The proteins were then extracted with 1 mL of 50 mM Tris-HCl (pH 8.8) containing 1.5 mM potassium chloride, 10 mM dithiothreitol-DTT, 1.0 mM phenylmethylsulfonyl fluoride (PMSF), and 0.1% sodium dodecyl sulfate (SDS) (w/v) under shaking for 10 min in an ice bath. After centrifugation, the supernatant (protein extract) was collected, and the proteins were precipitated with acetone overnight. The proteins were quantified via a Qubit™ protein assay kit (Invitrogen, Waltham, MA, USA).
2.2.2. Protein Digestion
The samples were subsequently resuspended in 60 µL of 50 mM sodium bicarbonate. Then, 25 µL of 0.2% RapiGest SF solution (Sigma–Aldrich, St. Louis, MO, USA) was added and vortexed. The mixture was then heated for 15 min at 80 °C and centrifuged. The proteins were reduced by adding 2.5 µL of 100 mM DTT and heating at 60 °C for 30 min. The samples were cooled to room temperature, alkylated with 2.5 µL of 300 mM iodoacetamide, incubated at room temperature in the dark for 30 min, and finally trypsinized with 10 µL of 50 mM trypsin (Madison, WI, USA) for 20 h at 37 °C. The hydrolysis reaction was stopped by the addition of 10 µL of 5% trifluoroacetic acid (TFA), and the samples were incubated at 37 °C for 90 min. The samples were then centrifuged at 14,000 rpm and 6 °C for 30 min at 5425R in a microcentrifuge (Eppendorf, Hamburg, Germany). The supernatant was transferred to a flask, and 5 µL of alcohol dehydrogenase-ADH (1 pmol/µL) and 85 µL of 3% acetonitrile containing 0.1% formic acid were added. The final protein concentration was 250 ng/µL, the ADH concentration was 25 fmol/µL, and the final volume was 200 µL. The full protocol used for protein digestion is presented in the Supplementary Material.
2.2.3. Protein Identification
The resulting peptide mixture was analysed via high-performance liquid chromatography as described by Silva et al [15,16], with modifications as follows. The experiments were performed in reversed-phase mode via a capillary column (NanoAcquity, UPLC-Waters, Milford, MA, USA) with a gradient of 2–97% B (AcN + 0.1% formic acid). The data obtained from the NanoLC-MSE experiments were processed via the ProteinLynx program (version 2.0) from Waters/Micromass (Milford, MA, USA), an application for identifying proteins via a database obtained from the National Center for Biotechnology Information (NCBI). NanoLC-MSE experiments were performed in duplicate, but processing in the ProteinLynx software was performed only once. The analysis of data collected by mass spectrometry of the tryptic peptides and the full protocol for identification are provided in the Supplementary Material (Supplementary Tables S1 and S2).
2.3. Hydrogen Peroxide Production
Hydrogen peroxide (H2O2) production by peripheral mononuclear blood monocytes (PBMCs) before the last treatment was assessed by horseradish peroxidase-dependent oxidation of phenol red, as follows [17]. Triplicate samples, each containing 1.5 × 105 cells, were seeded into 96-well plastic microplates (Corning, NY, USA) containing RPMI 1640. Following a 1 h incubation with a solution composed of 5.5 mM dextrose, 0.5 mM phenol red, and 19 U/mL of horseradish peroxidase type I RZ 1.0 (Sigma–Aldrich, San Luis, MO, USA), with or without the addition of 2 μM phorbol 12-myristate 13-acetate 4-O methyl ether (Sigma–Aldrich, St. Louis, MO, USA), in the presence or absence of 40 μg/L pravastatin sodium, the enzymatic reaction was halted by the addition of 10 μL of 1 N NaOH to each well. The absorbance was then measured at 620 nm via a Multiskan Titertek microplate reader. The average value of the triplicate samples was calculated for each sample and expressed as the optical density (OD) for 1.5 × 105 PBMCs per hour. Consistent results were obtained across the triplicate observations.
3. Results and Discussion
Clinical Case
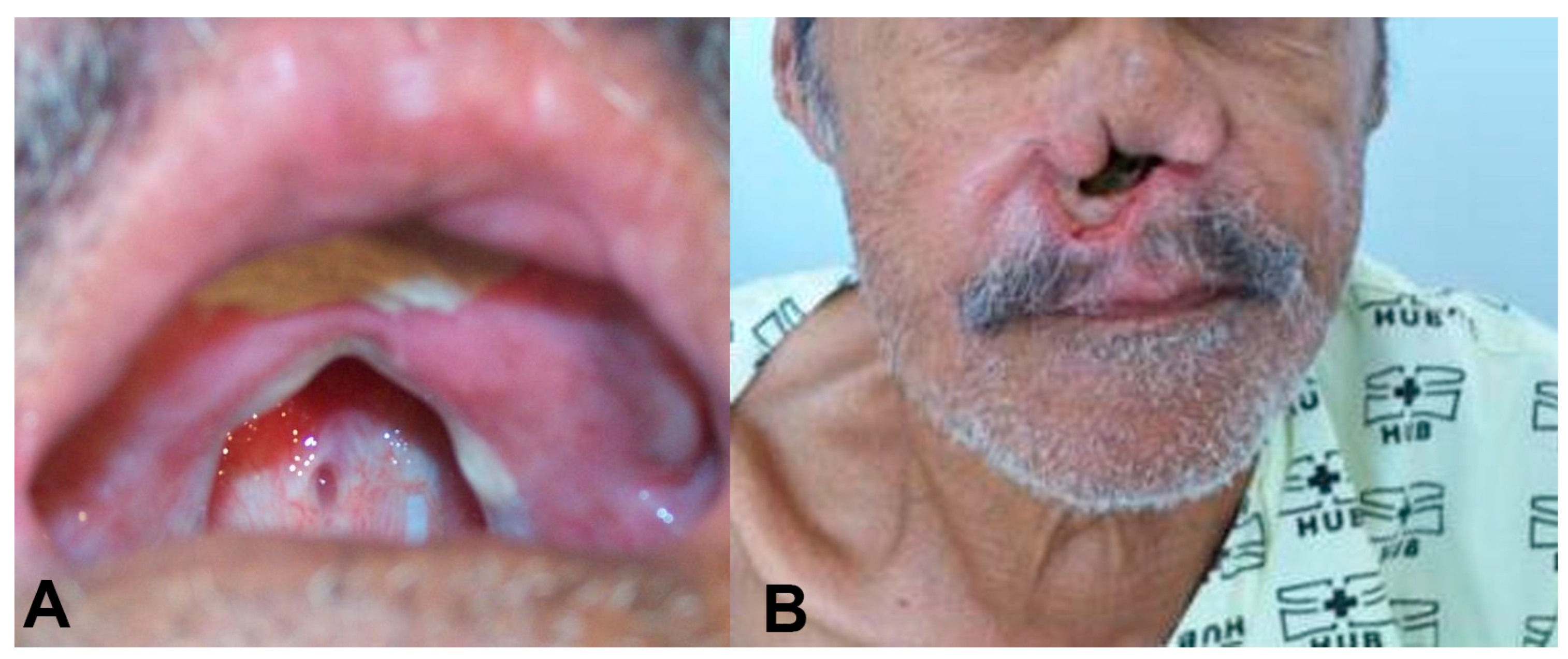
Case: In 1987, a 69-year-old male presented with nasal obstruction, rhinorrhea, dysphagia, and hoarseness. In infancy, he had developed an ulcer in his left leg, with spontaneous healing occurring after approximately four months. Physical examination revealed nasal septum ulcers and infiltration on his lips (Figure 1). Complementary exams, as described by Gomes [18], revealed that the intradermal reaction of the Montenegro (IDRM) area was 6 × 7 mm. Histopathological examination of the skin lesions revealed the presence of lymph-histioplasmacytic dermal infiltrate, and the indirect immunofluorescence ratio for Leishmania was between 1:40 and 1:320 during follow-up. The parasite was identified as Leishmania (V.) braziliensis by isoenzymes and PCR according to the routine care of patients with ATL in the service [19,20,21]. Identification by sequencing of the ITS1 region was also performed in this case (Supplementary Material). Direct examination and culture for fungal infections were negative. The presence of clinical lesions or amastigote forms on direct examination of skin lesions or after inoculation in hamsters was used to define recurrence during the follow-up period.
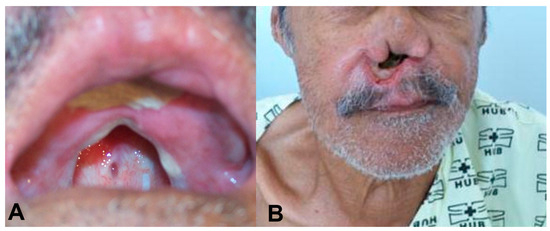
Figure 1.
(A) An oral lesion with destruction of the soft and hard palate. The patient used a palatal prosthesis to allow oral feeding. (B) Complete destruction of the nasal septum.
The patient was initially treated with 20 mg SbV/day for 30 days. After an initial improvement, worsening of the disease was observed. We then treated this patient with a variety of drugs, isolated and in combination, including N-methyl glucamine, sodium stibogluconate, amphotericin B deoxycholate, gabromycin, antimonial plus thalidomide, liposomal amphotericin B, Leishvacin [22] (a vaccine made in Brazil), and miltefosine (Table 1). According to the last systematic review on ATL treatment, there is a dearth of evidence on the treatment of ML precluding any conclusion on the comparative results of different treatment modalities [23]. The treatment strategy adopted had to be based in scarce data available on the treatment of ML. Most of the drugs used had already been tested on humans, with variable success, including itraconazole [24,25], mitefosine [26], allopurinol [24,27], sodium stibogluconate [27], amphotericin B [28], pentamidine [29], pentoxifylline [30], and thalidomide [31]. We also tried immunotherapy, which has previously been shown to reduce treatment duration and side effects when combined with antimonials [32]. On multiple occasions, we used combination therapy, which is believed to reduce the risk of drug resistance [33]. Despite that, the lesions gradually worsened, resulting in septal and soft palate perforation and pharynx infiltration with partial obstruction and shortness of breath. The patient gradually developed drilling of the soft palate, resulting in multiple secondary bacterial and fungal infections with feeding problems, leading to the need for gastrostomy and a palatal prosthesis (Figure 1) to improve nutrition and prevent further complications. Miltefosine treatment [34] at 2 mg/kg/day for 42 days resulted in a clinical cure, weight gain (10% of body weight), negativity of indirect immunofluorescence (IIF), and smear and culture results associated with a regression of histopathological infiltrates [35].

Table 1.
Treatments for American cutaneous leishmaniasis given to the patient for 25 years at the University Hospital of Brasilia, Brazil.
However, disease relapse was observed after 2½ years, as demonstrated by intense histopathological staining of the infiltrate and detection of the parasite in culture and after hamster inoculation. After more than 25 years of follow-up and multiple therapeutic regimens, none of the main drugs used for leishmaniasis [36] or various experimental combinations of drugs were successful in curing this patient. The patient died in 2012 from pancreatic cancer while he still had active mucosal lesions.
To better understand the possible mechanisms behind the treatment failure observed in this patient, we studied patient and parasite factors possibly related to treatment outcome. Samples were collected during active disease and after the last treatment had been performed.
The patient showed a basal production of 18.9 µM H2O2/1.5 × 105 monocytes/h, which decreased to 8.4 µM H2O2/1.5 × 105 monocytes/h after stimulation with PMA. These values were significantly lower than the production observed in individuals with New World human cutaneous leishmaniasis (146 µM H2O2/1.5 × 105 monocytes/h) and similar to those reported in noninfected healthy individuals (37 µM H2O2/1.5 × 105 monocytes/h) [37].
The increase in possible pathogenic molecules in the studied Leishmania strain was coupled with a much lower in vitro production of H2O2 by the patient’s monocytes. H2O2 is a strong microbicidal molecule produced by phagocytes and is involved in immune defence against Leishmania. After phagocytosis of Leishmania, macrophages trigger microbicidal mechanisms such as nitric oxide and oxygen radical production. The oxygen radical might have direct effects on parasites, causing protein denaturation and damage to the cell membrane and DNA. An important mechanism by which H2O2 eliminates microorganisms is the irreversible oxidation of essential proteins such as F0F1 mitochondrial ATPase [38]. The production of oxygen radicals with the disruption of the parasite’s mitochondrial membrane potential is one of the cornerstones of the host’s immune response against Leishmania and is also important during the treatment response [39,40]. Thus, it is not surprising that this patient’s lack of response to treatment was associated with a diminished production of hydrogen peroxide.
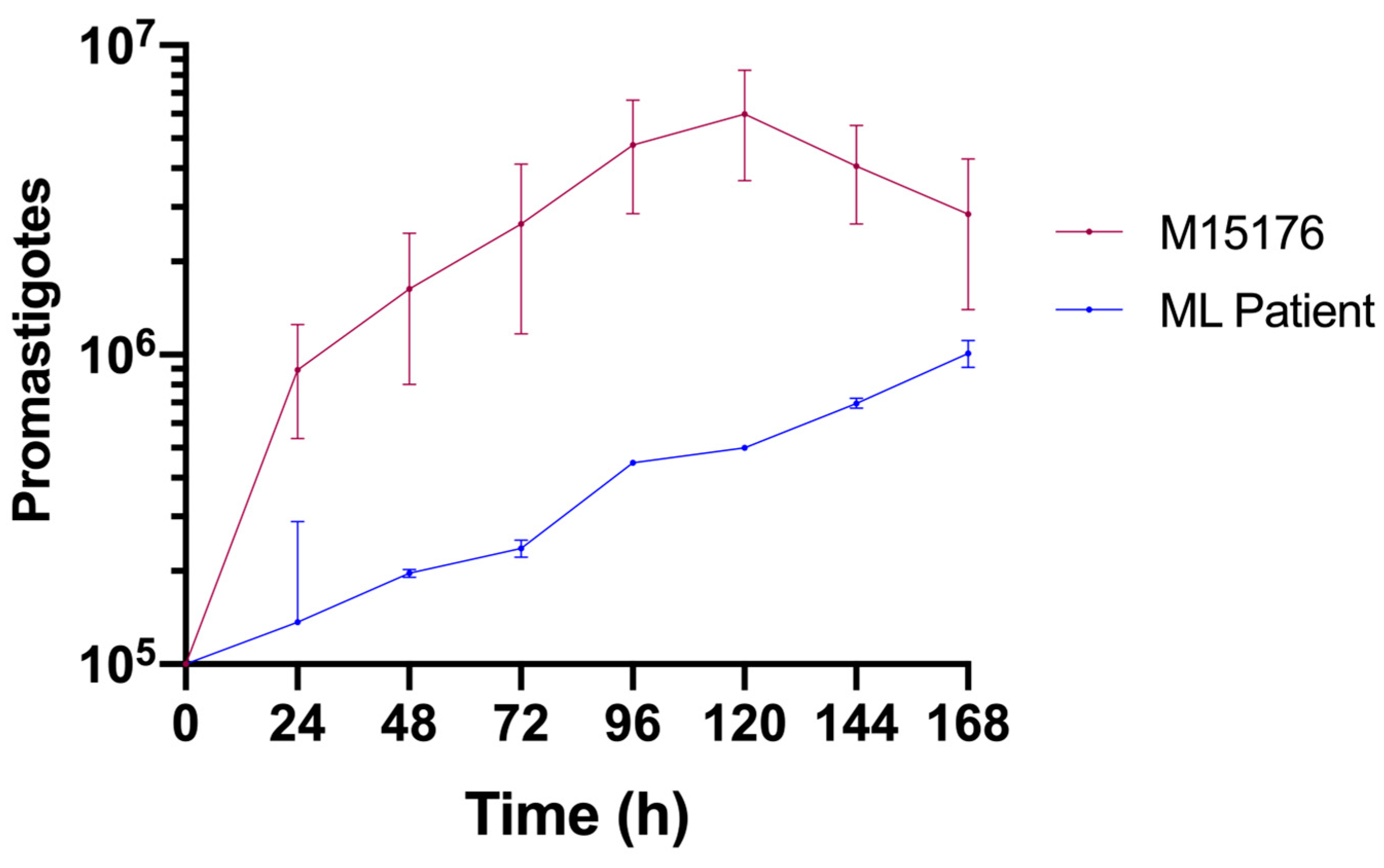
Compared with the control strain, the strain isolated from the ML patient had a lower growth rate (Figure 2). The control strain achieved the metacyclic phase on the fifth day, whereas the ML strain did not achieve this phase during the same period.
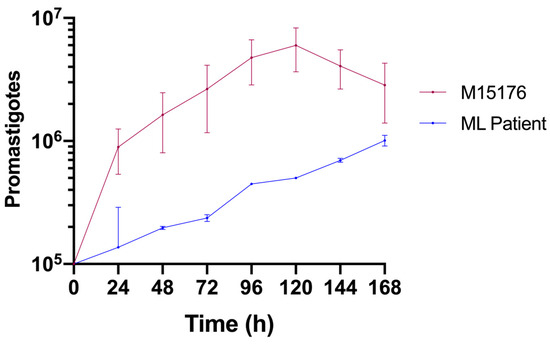
Figure 2.
Comparative growth curves of the L. (V.) braziliensis control (red) and ML (blue) strains in Schneider medium supplemented with 20% FBS.
Compared with the control strain, the Leishmania (V.) braziliensis strain isolated from this patient presented a low proliferative capacity (Figure 2). As has already been shown in other eukaryotes [41], a subpopulation of quiescent Leishmania parasites can be found in persistent infections [42]. This population has been linked to drug resistance and treatment failure in murine and in vitro studies [41]. It is possible that the altered proliferative state of the parasite we studied is one of the adaptative changes that allowed it to persist in this host despite multiple treatment courses.
Results from quantitative mass spectrometry analysis showed an increased abundancy of mtHSP70 and the ATPase alpha subunit in the patient isolate compared with those in the control (Table 2).

Table 2.
Quantitative analysis of selected proteins’ femtomole (fmol).
This high level is consistent with the possibility that these proteins are associated with therapeutic failure. ATPases constitute a highly conserved family of enzymes that are responsible for ATP metabolism in prokaryotic and eukaryotic cells [43]. F ATPases present in the mitochondrial membrane of trypanosomatids catalyse both the synthesis and hydrolysis of ATP and are also called ATP synthases [43]. F ATPases play important roles in Leishmania metabolism and are responsible for both ATP synthesis and the maintenance of the mitochondrial membrane potential; thus, F ATPases are a theoretical target for drug development against leishmaniasis [40]. Experimental studies have not yet established the practical application of this physio-pathological knowledge to diseases caused by protozoans. On the one hand, a low expression of F ATPases could favour parasite survival in some situations. For example, one study has shown that the expression of the F1 ATPase alpha subunit is decreased in benznidazole-resistant Trypanosoma cruzi [44]. Similarly, in Leishmania (L.) donovani, an ATPase inhibitor has been shown to diminish the uptake of pentamidine by the parasite [45]. Similarly, the loss of Leishmania (L.) amazonensis virulence has been associated with increased expression of F ATPase [46]. On the other hand, some studies have shown that drugs that impair F ATPase function have leishmanicidal proprieties. Roy et al. reported that 3,3′-diindolylmethane (DIM), a DNA topoisomerase I inhibitor, disrupts F ATPase function in Leishmania spp. parasites, leading to depolarization of the mitochondrial membrane followed by programmed cell death [47]. This also seems to be the sequence of events responsible for the leishmanicidal proprieties of camptothecin [48]. Similar to what we have shown in this study, a clonal population of antimonony-resistant Leishmania (L.) panamensis also expresses relatively high levels of F ATPases [49]. Studies on the pathogenic potential of ATPase in Leishmania (V.) braziliensis are lacking, but the finding of greater production of this protein in this patient sample seems to favour the possible pathogenic role of the molecule. This result, however, may not be applicable to a wider population, and a larger study including samples from patients responsive and not responsive to treatment is warranted to reach the most definitive conclusions.
The pathogenic role of parasitic HSP70 has already been studied because this protein is highly expressed in Leishmania species, which are responsible for human disease [50]. It is known to promote resistance to oxidative stress but direct immunological effects through extracellular transport [51], leading to macrophage activation, and TNF-alpha production may also play a role [51]. Treatment resistance has also been associated with a specific mutation in the HSP70 gene (T579A), linking 75% of failures to antimony therapy, leading to a sevenfold increase in the risk of failure in the presence of this polymorphism (OR = 7.29; 95% CI = [1.17–45.25]; p = 0.0331) [52]. In a murine model, an HSP70 inhibitor used to treat leishmaniasis has shown promising results [53]. An inhibitor of the protozoan HSP70 Polymyxin B [54] has been shown to have leishmanicidal activity [55], and the possible confirmation of the role of this chaperone in parasite resistance opens the possibility of repurposing this drug, which is already commercially available, for the treatment of refractory leishmaniasis. Our study thus reinforces the potential pathogenic role of this protein and suggests its potential as a therapeutic target and as a marker for poor clinical outcomes.
Regarding our limitations, it could be argued that the multiple clinical and parasitological relapses of ML described were in fact reinfections. Importantly, however, a sterile cure of leishmaniasis is the exception rather than the rule [56], which by itself does not eliminate the possibility that the lesions are the result of a new infecting strain. However, we must also consider that after the first cutaneous ulcer, no more cutaneous lesions occurred in this case. Many authors consider that the main mechanism for parasite infection in the mucosa is through dissemination from a cutaneous lesion [57,58], and a kDNA signature study revealed that parasites from mucosal lesions are more similar to those from cutaneous lesions of the same patient than those from unrelated patients [59]; we believe that the hypothesis of reinfection in this patient is unlikely.
There is genetic variability between parasites isolated from the same patient and those from clones of the same Leishmania reference strain, representing a naturally mixed infection [60]. Changes in the genetic structure of one strain maintained in culture change over time in different DNA batches [61] and can be accompanied by changes in phenotype, such as loss of infectivity and the development of biochemical and antigenic changes [62]. Thus, we question whether our findings are not a result of a selection process rather than a true phenotypic difference between the strains analysed. Importantly, despite the existence of heterogeneity, genetic variability at the interpatient level is less pronounced than that between different strains [59], and different genetic profiles have been associated with phenotypic characteristics of the infecting parasite [63]. Changes in parasites over time are a limitation of in vitro studies such as ours. However, we have taken precautions to limit the modification of parasite biological and biochemical characteristics by avoiding culturing for long periods and by culturing the tested strains in mice before experiments [12,64].
4. Conclusions
Monitoring of this patient revealed that ML caused by Leishmania (V.) braziliensis might be a therapeutic challenge. In this case, both host and parasite factors seemed to be important in determining the lack of response to treatment over 25 years. The dramatic outcome observed illustrates the necessity of new drugs for this neglected disease, indicating possible new therapeutic targets. The targeting of molecules involved in the parasitic oxidative machinery or its molecular integrity is a promising strategy for future drug development. Our study reinforces the possible utility of some compounds already studied in experimental settings, and we hope that our findings lead to new studies of the possible mechanisms behind Leishmania drug resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12102227/s1, Figure S1: Discriminatory sequences in the ITS1 region from the Leishmania gender; Table S1: Proteins identified in L.(V.) braziliensis control strain (MHOM/BR/94/M15176) by analysis carried out with the “Protein Lynx” software using the L.(V.) braziliensis database, from data collected by mass spectrometry of the tryptic peptides; Table S2: Proteins identified in L.(V.) braziliensis isolated from the patient sample by analysis carried out with the “Protein Lynx” software using the L.(V.) braziliensis database, from data collected by mass spectrometry of the tryptic peptides
Author Contributions
A.A.A.U. and R.N.R.S. were responsible for the design and execution of the study and writing of the original draft. A.C.A., J.A.T. and F.V. were responsible for the design and analysis of the proteins and related considerations. A.d.O.S.A. and I.M.D.B. work with host cells, and related considerations should be considered. D.H.B. and S.d.G.A.V.B. worked on the definitive version of the manuscript. R.N.R.S. supervised all the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the CONSELHO NACIONAL DE DESENVOLVIMENTO CIENTÍFICO E TECNOLÓGICO, grant numbers 404594/2021-2, 308618/2021-1, and 09117/2023-4.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Use Ethics Committee (CEUA) of the University of Brasília (number UnBDoC n° 152483/2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author due to privacy concerns.
Conflicts of Interest
The authors declare no conflicts of interest. Felipe Vinecky and Jorge Alex Taquita were employed by the company Embrapa Cenargen. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- World Health Organization. Leishmaniasis World Health Organization Site: World Health Organization. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 13 June 2023).
- Hepburn, N. Cutaneous leishmaniasis. Clin. Exp. Dermatol. 2000, 25, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Aronson, N.E.; Joya, C.A. Cutaneous leishmaniasis: Updates in diagnosis and management. Infect. Dis. Clin. 2019, 33, 101–117. [Google Scholar]
- Marsden, P.D. Mucosal leishmaniasis (“espundia” Escomel, 1911). Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 859–876. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Neglected Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Koff, A.B.; Rosen, T. Treatment of cutaneous leishmaniasis. J. Am. Acad. Dermatol. 1994, 31, 693–708. [Google Scholar] [CrossRef]
- Borges, K.T.; Nogueira, L.S.C.; Sampaio, J.H.D.; Tauil, P.L.; Sampaio, R.N.R. Clinical, epidemiological and therapeuthic study of 402 patients with american cutaneous leishmaniasis attended at University Hospital of Brasilia, DF, Brazil. Bras. Dermatol. 2005, 80, 249–254. [Google Scholar]
- Biyani, N.; Singh, A.K.; Mandal, S.; Chawla, B.; Madhubala, R. Differential expression of proteins in antimony-susceptible and-resistant isolates of Leishmania donovani. Mol. Biochem. Parasitol. 2011, 179, 91–99. [Google Scholar] [CrossRef]
- Moreira, D.d.S.; Xavier, M.V.; Murta, S.M.F. Ascorbate peroxidase overexpression protects Leishmania braziliensis against trivalent antimony effects. Memórias Do Inst. Oswaldo Cruz 2018, 113, e180377. [Google Scholar] [CrossRef]
- Codonho, B.S.; Costa, S.d.S.; Peloso, E.d.F.; Joazeiro, P.P.; Gadelha, F.R.; Giorgio, S. HSP70 of Leishmania amazonensis alters resistance to different stresses and mitochondrial bioenergetics. Memórias Do Inst. Oswaldo Cruz 2016, 111, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Rastrojo, A.; García-Hernández, R.; Vargas, P.; Camacho, E.; Corvo, L.; Imamura, H.; Dujardin, J.C.; Castanys, S.; Aguado, B.; Gamarro, F.; et al. Genomic and transcriptomic alterations in Leishmania donovani lines experimentally resistant to antileishmanial drugs. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 246–264. [Google Scholar] [CrossRef]
- Grimaldi, G.J.; Jaffe, C.L.; Mcmahon-Pratt, D.; Falqueto, A. A simple procedure for the isolation of leishmanial parasites and for the recovery of parasite virulence in avirulent stocks. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 560. [Google Scholar] [CrossRef] [PubMed]
- Brener, Z. Contribuição ao Estudo da Terapêutica Experimental da Doença de Chagas; Biblioteca Virtual em Saúde: Belo Horizonte, Brazil, 1961.
- Sussulini, A.; Garcia, J.S.; Mesko, M.F.; Moraes, D.P.; Flores, É.M.; Pérez, C.A.; Arruda, M.A. Evaluation of soybean seed protein extraction focusing on metalloprotein analysis. Microchim. Acta 2007, 158, 173–180. [Google Scholar] [CrossRef]
- Silva, J.C.; Denny, R.; Dorschel, C.A.; Gorenstein, M.; Kass, I.J.; Li, G.Z.; McKenna, T.; Nold, M.J.; Richardson, K.; Young, P.; et al. Quantitative proteomic analysis by accurate mass retention time pairs. Anal Chem. 2005, 77, 2187–2200. [Google Scholar] [CrossRef]
- Silva, J.C.; Gorenstein, M.V.; Li, G.-Z.; Vissers, J.P.; Geromanos, S.J. Absolute quantification of proteins by LCMSE: A virtue of parallel MS acquisition* S. Mol. Cell. Proteom. 2006, 5, 144–156. [Google Scholar] [CrossRef]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.M.; Paula, N.A.; Morais, O.O.; Soares, K.A.; Roselino, A.M.; Sampaio, R.N. Complementary exams in the diagnosis of American tegumentary leishmaniasis. Bras. Dermatol. 2014, 89, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Cuba, C.C.; Llanos-Cuentas, E.A.; Barreto, A.C.; Magalhães, A.V.; Lago, E.L.; Reed, S.G.; Marsden, P.D. Human mucocutaneous leishmaniasis in Três Braços, Bahia-Brazil: An area of Leishmania braziliensis braziliensis transmission. I. Lab. Diagn. Rev. da Soc. Bras. de Med. Trop. 1984, 17, 161–167. [Google Scholar] [CrossRef]
- FIOCRUZ. Manual of Molecular Procedures: Instituto Oswaldo Cruz 2009; FIOCRUZ: Rio de Janeiro, Brazil, 2009. [Google Scholar]
- Gomes, C.M.; Cesetti, M.V.; de Paula, N.A.; Vernal, S.; Gupta, G.; Sampaio, R.N.; Roselino, A.M. Field Validation of SYBR Green- and TaqMan-Based Real-Time PCR Using Biopsy and Swab Samples to Diagnose American Tegumentary Leishmaniasis in an Area Where Leishmania (Viannia) braziliensis Is Endemic. J. Clin. Microbiol. 2017, 55, 526–534. [Google Scholar] [CrossRef]
- El-On, J. Current status and perspectives of the immunotherapy of leishmaniasis. Isr. Med. Assoc. J. IMAJ 2009, 11, 623–628. [Google Scholar]
- Pinart, M.; Rueda, J.-R.; Romero, G.A.; Pinzón-Flórez, C.E.; Osorio-Arango, K.; Maia-Elkhoury, A.N.S.; Reveiz, L.; Elias, V.M.; Tweed, J.A. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst. Rev. 2020, 8, 1–329. [Google Scholar] [CrossRef]
- Mashayekhi Goyonlo, V.; Derakhshan, Z.; Darchini-Maragheh, E. Treatment of Cutaneous Leishmaniasis with Allopurinol Plus Itraconazole in Iran. Am. J. Trop. Med. Hyg. 2023, 108, 1164–1166. [Google Scholar] [CrossRef] [PubMed]
- Amato, V.S.; Padilha, A.R.; Nicodemo, A.C.; Duarte, M.I.; Valentini, M.; Uip, D.E.; Boulos, M.; Neto, V.A. Use of itraconazole in the treatment of mucocutaneous leishmaniasis: A pilot study. Int. J. Infect. Dis. 2000, 4, 153–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sampaio, R.N.R.; Paula CDRd Porto, C.; Motta JdOCd Pereira, L.I.d.A.; Martins, S.S.; Barroso, D.H.; Freire, G.S.M.; Gomes, C.M. A randomized, open-label clinical trial comparing the long-term effects of miltefosine and meglumine antimoniate for mucosal leishmaniasis. Rev. da Soc. Bras. de Med. Tropical. 2019, 52, e20180292. [Google Scholar] [CrossRef] [PubMed]
- Llanos-Cuentas, A.; Echevarria, J.; Cruz, M.; La Rosa, A.; Campos, P.; Campos, M.; Franke, E.; Berman, J.; Modabber, F.; Marr, J. Efficacy of sodium stibogluconate alone and in combination with allopurinol for treatment of mucocutaneous leishmaniasis. Clin. Infect. Dis. 1997, 25, 677–684. [Google Scholar] [CrossRef]
- Cunha, M.A.; Leão, A.C.; de Cassia Soler, R.; Lindoso, J.A. Efficacy and Safety of Liposomal Amphotericin B for the Treatment of Mucosal Leishmaniasis from the New World: A Retrospective Study. Am. J. Trop. Med. Hyg. 2015, 93, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Tuon, F.F.; Cieslinski, J.; de Souza, R.M.; Imamura, R.; Amato, V.S. Comparative study on liposomal amphotericin B and other therapies in the treatment of mucosal leishmaniasis: A 15-year retrospective cohort study. PLoS ONE 2019, 14, e0218786. [Google Scholar] [CrossRef] [PubMed]
- Lessa, H.A.; Machado, P.; Lima, F.; Cruz, A.A.; Bacellar, O.; Guerreiro, J.; Carvalho, E.M. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am. J. Trop. Med. Hyg. 2001, 65, 87–89. [Google Scholar] [CrossRef]
- Goyonlo, V.M.; Vahabi-Amlashi, S.; Taghavi, F. Successful treatment by adding thalidomide to meglumine antimoniate in a case of refractory anthroponotic mucocutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug Resistance. 2019, 11, 177–179. [Google Scholar] [CrossRef]
- Mayrink, W.; Botelho, A.C.d.C.; Magalhães, P.A.; Batista, S.M.; Lima, A.d.O.; Genaro, O.; Costa, C.A.D.; Melo, M.N.D.; Michalick, M.S.M.; Williams, P.; et al. Immunotherapy, immunochemotherapy and chemotherapy for American cutaneous leishmaniasis treatment. Rev. da Soc. Bras. de Med. Trop. 2006, 39, 14–21. [Google Scholar] [CrossRef]
- Sampaio, R.N.R. Pharmacotherapy in leishmaniasis: Old, new treatments, their impacts and expert opinion. Taylor Fr. 2023, 24, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Toledo, J.; Valda, L.; Balderrama, M.; Rea, I.; Parra, R.; Ardiles, J.; Soto, P.; Gomez, A.; Molleda, F.; et al. Treatment of Bolivian mucosal leishmaniasis with miltefosine. Clin. Infect. Dis. 2007, 44, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Arana, B.A.; Toledo, J.; Rizzo, N.; Vega, J.C.; Diaz, A.; Luz, M.; Gutierrez, P.; Arboleda, M.; Berman, J.D.; et al. Miltefosine for new world cutaneous leishmaniasis. Clin. Infect. Dis. 2004, 38, 1266–1272. [Google Scholar] [CrossRef]
- Sampaio, R.; Marsden, P. Treatment of the mucosal form of leishmaniasis without response to glucantime, with liposomal amphotericin B. Rev. da Soc. Bras. de Med. Trop. 1997, 30, 125–128. [Google Scholar] [CrossRef] [PubMed]
- de Saldanha, R.R.; Martins-Papa, M.C.; Sampaio, R.N.R.; Muniz-Junqueira, M.I. Meglumine antimonate treatment enhances phagocytosis and TNF-α production by monocytes in human cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 596–603. [Google Scholar] [CrossRef]
- Tamarit, J.; Cabiscol, E.; Ros, J. Identification of the major oxidatively damaged proteins inEscherichia coli cells exposed to oxidative stress. J. Biol. Chem. 1998, 273, 3027–3032. [Google Scholar] [CrossRef]
- Muniz-Junqueira, M.I.; de Paula-Coelho, V.N. Meglumine antimonate directly increases phagocytosis, superoxide anion and TNF-α production, but only via TNF-α it indirectly increases nitric oxide production by phagocytes of healthy individuals, in vitro. Int. Immunopharmacol. 2008, 8, 1633–1638. [Google Scholar] [CrossRef]
- De Sarkar, S.; Chatterjee, M. Exploring Endoperoxides as Leishmanicidal Compounds. In Oxidative Stress in Microbial Diseases; Springer: Berlin/Heidelberg, Germany, 2019; p. 453. [Google Scholar]
- Barrett, M.P.; Kyle, D.E.; Sibley, L.D.; Radke, J.B.; Tarleton, R.L. Protozoan persister-like cells and drug treatment failure. Nat. Rev. Microbiol. 2019, 17, 607–620. [Google Scholar] [CrossRef]
- Mandell, M.A.; Beverley, S.M. Continual renewal and replication of persistent Leishmania major parasites in concomitantly immune hosts. Proc. Natl. Acad. Sci. USA 2017, 114, E801–E810. [Google Scholar] [CrossRef]
- Nirody, J.A.; Budin, I.; Rangamani, P. ATP synthase: Evolution, energetics, and membrane interactions. J. Gen. Physiol. 2020, 152, e201912475. [Google Scholar] [CrossRef]
- Lima, D.A.; Gonçalves, L.O.; Reis-Cunha, J.L.; Guimarães, P.A.S.; Ruiz, J.C.; Liarte, D.B.; Murta, S.M.F. Transcriptomic analysis of benznidazole-resistant and susceptible Trypanosoma cruzi populations. Parasit. Vectors 2023, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Padmanabhan, P.K.; Sahani, M.H.; Barrett, M.P.; Madhubala, R. Roles for mitochondria in pentamidine susceptibility and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 2006, 145, 1–10. [Google Scholar] [CrossRef]
- Magalhães, R.D.M.; Duarte, M.C.; Mattos, E.C.; Martins, V.T.; Lage, P.S.; Chávez-Fumagalli, M.A.; Lage, D.P.; Menezes-Souza, D.; Regis, W.C.; Manso Alves, M.J.; et al. Identification of Differentially Expressed Proteins from Leishmania amazonensis Associated with the Loss of Virulence of the Parasites. PLoS Neglected Trop. Dis. 2014, 8, e2764. [Google Scholar] [CrossRef]
- Roy, A.; Ganguly, A.; BoseDasgupta, S.; Das, B.B.; Pal, C.; Jaisankar, P.; Majumder, H.K. Mitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3, 3′-diindolylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol. Pharmacol. 2008, 74, 1292–1307. [Google Scholar] [CrossRef] [PubMed]
- Bodley, A.L.; McGarry, M.W.; Shapiro, T.A. Drug cytotoxicity assay for African trypanosomes and Leishmania species. J. Infect. Dis. 1995, 172, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Gongora, R.; Vasquez, J.-J.; Drummelsmith, J.; Burchmore, R.; Roy, G.; Ouellette, M.; Gomez, M.A.; Saravia, N.G. Discovery of factors linked to antimony resistance in Leishmania panamensis through differential proteome analysis. Mol. Biochem. Parasitol. 2012, 183, 166–176. [Google Scholar] [CrossRef]
- Brandau, S.; Dresel, A.; Clos, J. High constitutive levels of heat-shock proteins in human-pathogenic parasites of the genus Leishmania. Biochem. J. 1995, 310, 225–232. [Google Scholar] [CrossRef]
- Cuervo, P.; De Jesus, J.B.; Saboia-Vahia, L.; Mendonça-Lima, L.; Domont, G.B.; Cupolillo, E. Proteomic characterization of the released/secreted proteins of Leishmania (Viannia) braziliensis promastigotes. J. Proteom. 2009, 73, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.C.; Ribeiro-Alves, M.; Romero, G.A.; Dávila, A.M.; Cupolillo, E. Assessment of drug resistance related genes as candidate markers for treatment outcome prediction of cutaneous leishmaniasis in Brazil. Acta Trop. 2013, 126, 132–141. [Google Scholar] [CrossRef]
- Inacio, J.D.; Gervazoni, L.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. The effect of (-)-epigallocatechin 3-O-gallate in vitro and in vivo in Leishmania braziliensis: Involvement of reactive oxygen species as a mechanism of action. PLoS Neglected Trop. Dis. 2014, 8, e3093. [Google Scholar] [CrossRef]
- Zininga, T.; Pooe, O.J.; Makhado, P.B.; Ramatsui, L.; Prinsloo, E.; Achilonu, I.; Dirr, H.; Shonhai, A. Polymyxin B inhibits the chaperone activity of Plasmodium falciparum Hsp70. Cell Stress Chaperones 2017, 22, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Souza Ribeiro Costa, J.; Medeiros, M.; Yamashiro-Kanashiro, E.H.; Rocha, M.C.; Cotrim, P.C.; Stephano, M.A.; Lancellotti, M.; Tavares, G.D.; Oliveira-Nascimento, L. Biodegradable nanocarriers coated with polymyxin B: Evaluation of leishmanicidal and antibacterial potential. PLoS Neglected Trop. Dis. 2019, 13, e0007388. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.G.; de Brito, M.E.; Rodrigues, E.H.; Bandeira, V.; Jardim, M.L.; Abath, F.G. Persistence of Leishmania parasites in scars after clinical cure of american cutaneous leishmaniasis: Is there a sterile cure? J. Infect. Dis. 2004, 189, 1018–1023. [Google Scholar] [CrossRef]
- Marsden, P.D.; Llanos-Cuentas, E.A.; Lago, E.L.; Cuba, C.C.; Barreto, A.C.; Costa, J.M.; Jones, T.C. Human mucocutaneous leishmaniasis in Três Braços, Bahia-Brazil. An area of Leishmania braziliensis braziliensis transmission. III-Mucosal disease presentation and initial evolution. Rev. Soc. Bras. Med. Trop. 1984, 17, 179–186. [Google Scholar] [CrossRef]
- Amato, V.; Tuon, F.; Imamura, R.; Abegao de Camargo, R.; Duarte, M.; Neto, V. Mucosal leishmaniasis: Description of case management approaches and analysis of risk factors for treatment failure in a cohort of 140 patients in Brazil. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.S.; Valete-Rosalino, C.M.; Pacheco, S.J.; Costa, F.A.C.; Schubach, A.O.; Pacheco, R.S. American tegumentary leishmaniasis caused by Leishmania (Viannia) braziliensis: Assessment of parasite genetic variability at intra-and inter-patient levels. Parasites Vectors 2013, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.S.; Martinez, J.E.; Valderrama, L.; Momen, H.; Saravia, N.G. Genotypic polymorphisms in experimental metastatic dermal leishmaniasis. Mol. Biochem. Parasitol. 1995, 69, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Boité, M.C.; de Oliveira, T.S.; Ferreira, G.E.M.; Trannin, M.; dos Santos, B.N.; Porrozzi, R.; Cupolillo, E. Polymorphisms and ambiguous sites present in DNA sequences of Leishmania clones: Looking closer. Infect. Genet. Evol. 2014, 25, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Tesh, R. Leishmaniases of the New World: Current concepts and implications for future research. Clin. Microbiol. Rev. 1993, 6, 230–250. [Google Scholar] [CrossRef]
- Quaresma, P.F.; de Brito, C.F.A.; Rugani, J.M.N.; de Moura Freire, J.; de Paula Baptista, R.; Moreno, E.C.; Gontijo, R.C.; Rego, F.D.; Diniz, J.E.; Melo, M.N.; et al. Distinct genetic profiles of Leishmania (Viannia) braziliensis associate with clinical variations in cutaneous-leishmaniasis patients from an endemic area in Brazil. Parasitology 2018, 145, 1161–1169. [Google Scholar] [CrossRef]
- Sacks, D.L.; Melby, P.C. Animal models for the analysis of immune responses to leishmaniasis. Curr. Protoc. Immunol. 1998, 28, 19.2.1–19.2.20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).