TGM2-Mediated Autophagy Contributes to the Radio-Resistance of Non-Small Cell Lung Cancer Stem-like Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Cell Sorting
2.2. Ionizing Radiation (IR)
2.3. Cell Counting Kit-8 (CCK-8) Assay
2.4. EdU Assay
2.5. Migration and Invasion
2.6. Measurement of Autophagic Activity and Drugs of Autophagy
2.7. Western Blotting
2.8. Nontargeted LC–MS-Based Metabolomics
2.9. RNA Interference
2.10. RT-qPCR and PCR Array
2.11. Immunofluorescence (IF)
2.12. Bioinformatics Analysis
2.13. Co-Immunoprecipitation (Co-IP)
2.14. Animal Experiments
2.15. Statistical Analysis
3. Results
3.1. Identification of “Stemness” of CD44+A549 Cells
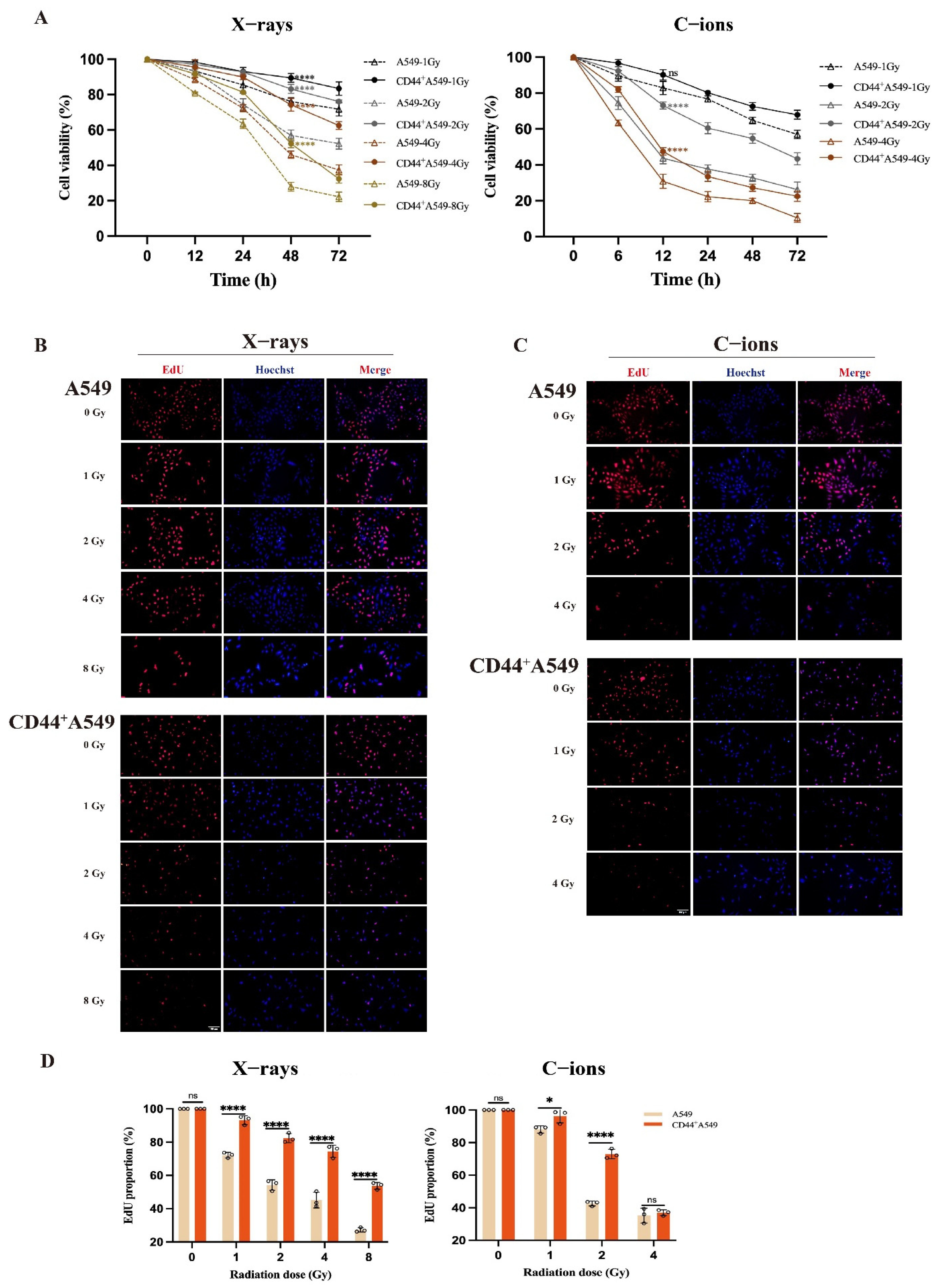
3.2. CD44+A549 Cells Exhibit Greater Radio-Resistance than A549 Cells, and C-Ions Could Overcome This Radio-Resistance
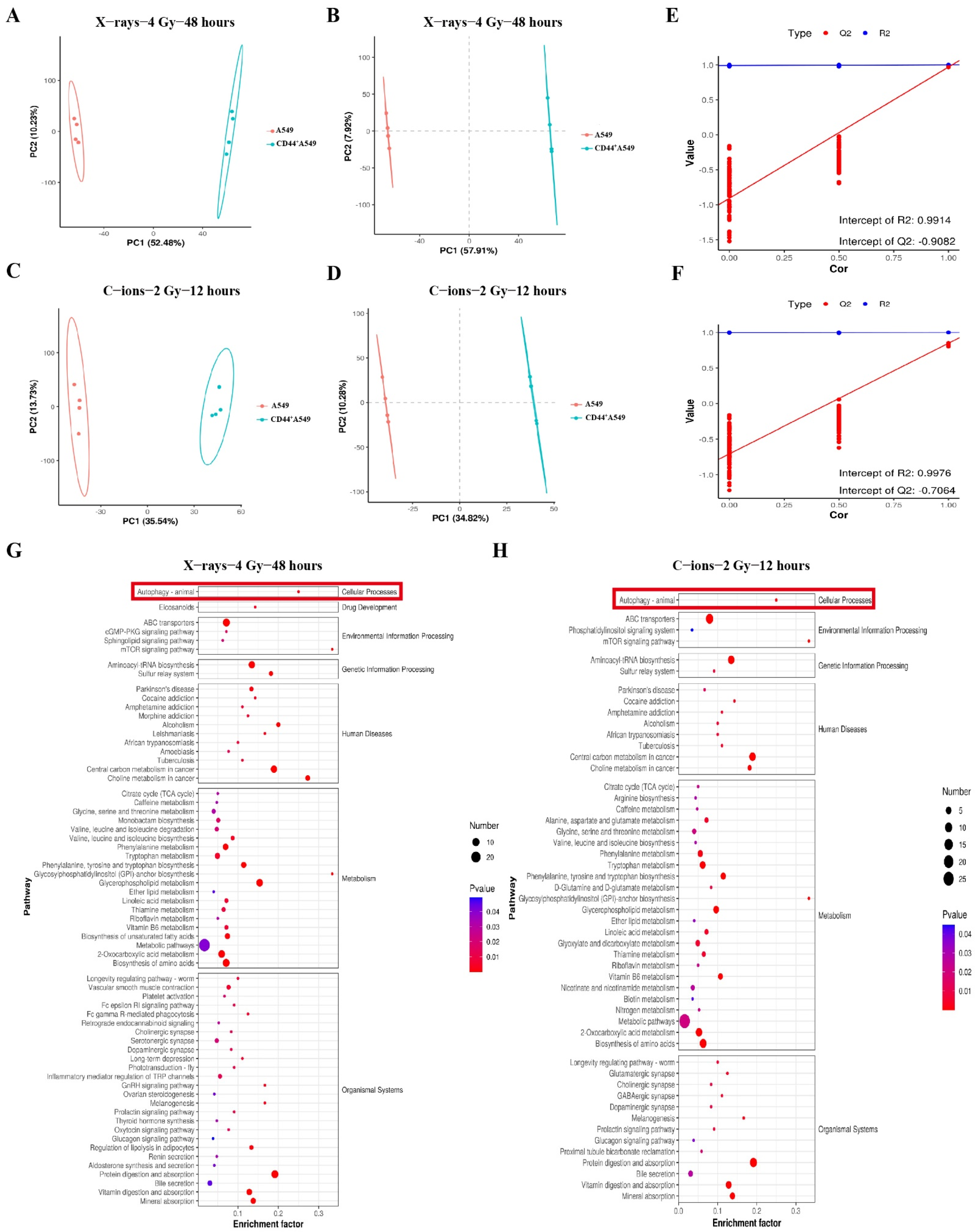
3.3. CD44+A549 Cells Possess High Levels of Autophagy, CD44+A549 Cells Regulates Its Radiation Sensitivity through Autophagy
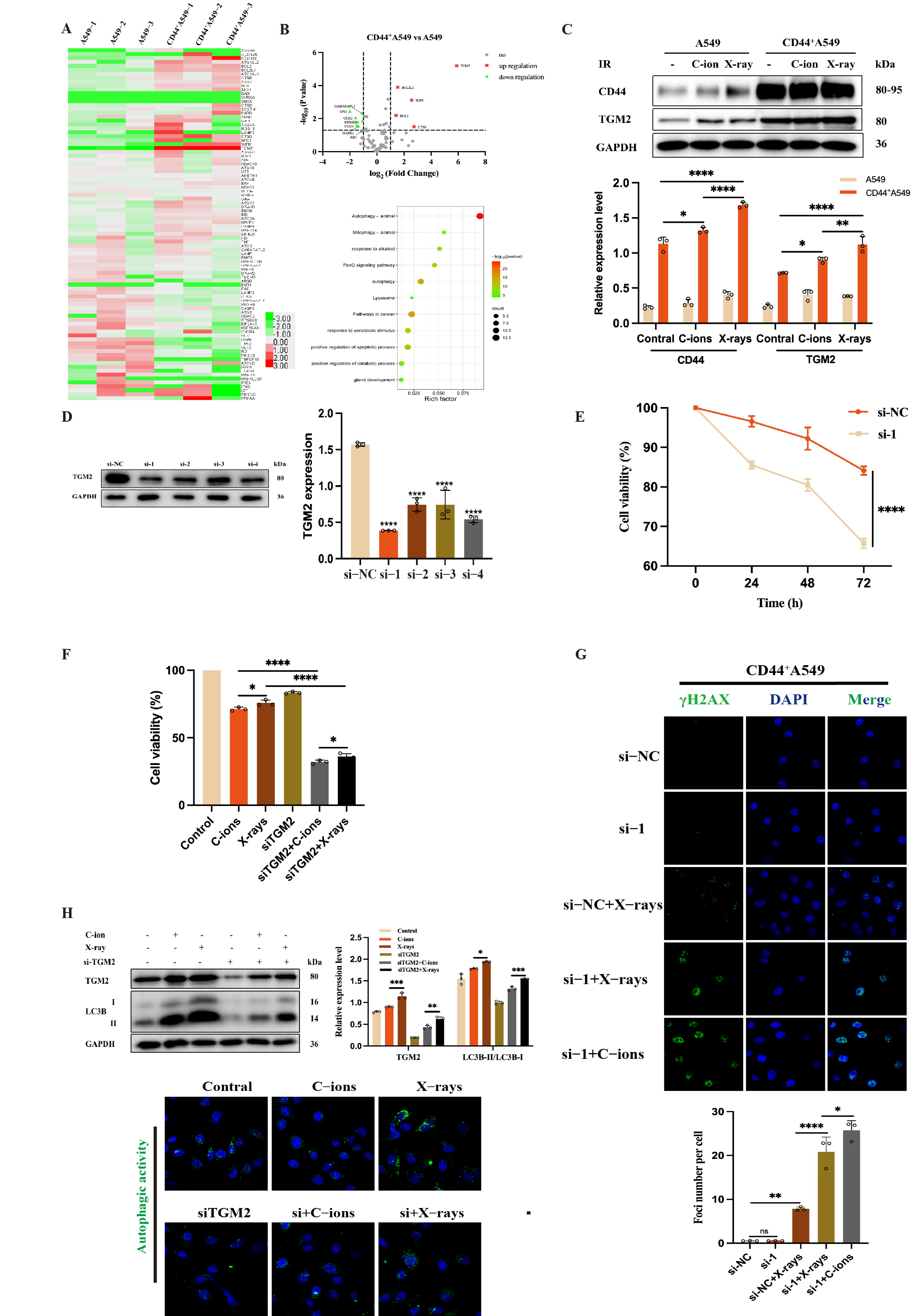
3.4. TGM2 Is Up-Regulated in CD44+A549 Cells, CD44+A549 Cell Regulates Radiation Sensitivity by TGM2
3.5. TGM2 Regulates Autophagy by Interacting with LC3B within CD44+A549 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.; Daley, A.; Begh, R.; Aveyard, P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: Systematic review of observational studies with meta-analysis. BMJ 2010, 340, 251. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Chen, S.; Zhang, K.; Zhang, K.; Wang, M.; Wang, P. Single-cell transcriptomic sequencing data reveal aberrant DNA methylation in SMAD3 promoter region in tumor-associated fibroblasts affecting molecular mechanism of radiosensitivity in non-small cell lung cancer. J. Transl. Med. 2024, 22, 288. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The Cancer Stem Cell Niche: How Essential Is the Niche in Regulating Stemness of Tumor Cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Nazio, F.; Bordi, M.; Cianfanelli, V.; Locatelli, F.; Cecconi, F. Autophagy and cancer stem cells: Molecular mechanisms and therapeutic applications. Cell Death Differ. 2019, 26, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Colak, S.; Medema, P.J. Cancer stem cells—Important players in tumor therapy resistance. FEBS J. 2015, 281, 4779–4791. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; White, J.; Zhang, L. Lung cancer stem cells and implications for future therapeutics. Cell Biochem. Biophys. 2014, 69, 389–398. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, R.; Zhang, Q.; Luo, H.; Wu, X.; Du, T.; Chen, Y.; Tan, M.; Liu, Z.; Sun, S.; et al. Biological effects of cancer stem cells irradiated by charged particle: A systematic review of in vitro studies. J. Cancer Res. Clin. Oncol. 2023, 149, 6625–6638. [Google Scholar] [CrossRef]
- Tang, D.G.; Patrawala, L.; Calhoun, T.; Bhatia, B.; Choy, G.; Schneider-Broussard, R.; Jeter, C. Prostate cancer stem/progenitor cells: Identifcation, characterization, and implica-tions. Mol. Carcinog. 2007, 46, 1–14. [Google Scholar] [CrossRef]
- Wu, C.; Alman, B.A. Side population cells in human cancers. Cancer Lett. 2008, 268, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Podberezin, M.; Wen, J.; Chang, C.-C.J. Cancer stem cells: A review of potential clinical applications. Arch. Pathol. Lab. Med. 2013, 137, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar] [PubMed]
- Vermeulen, L.; De Melo, F.D.S.; Richel, D.J.; Medema, J.P. The developing cancer stem-cell model: Clinical challenges and opportunities. Lancet Oncol. 2012, 13, e83–e89. [Google Scholar] [CrossRef] [PubMed]
- Jaggupilli, A.; Elkord, E. Significance of CD44 and CD24 as Cancer Stem Cell Markers: An Enduring Ambiguity. Clin. Dev. Immunol. 2012, 2012, 708036. [Google Scholar] [CrossRef] [PubMed]
- Pustovalova, M.; Blokhina, T.; Alhaddad, L.; Chigasova, A.; Chuprov-Netochin, R.; Veviorskiy, A.; Filkov, G.; Osipov, A.N.; Leonov, S. CD44+ and CD133+ Non-Small Cell Lung Cancer Cells Exhibit DNA Damage Response Pathways and Dormant Polyploid Giant Cancer Cell Enrichment Relating to Their p53 Status. Int. J. Mol. Sci. 2022, 23, 4922. [Google Scholar] [CrossRef]
- Gomez-Casal, R.; Bhattacharya, C.; Ganesh, N.; Bailey, L.; Basse, P.; Gibson, M.; Epperly, M.; Levina, V. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol. Cancer 2013, 12, 94. [Google Scholar] [CrossRef]
- Du, L.; Wang, H.; He, L.; Zhang, J.; Ni, B.; Wang, X.; Jin, H.; Cahuzac, N.; Mehrpour, M.; Lu, Y.; et al. CD44 is of functional importance for colorectal cancer stem cells. Clin. Cancer Res. 2008, 14, 6751–6760. [Google Scholar] [CrossRef]
- Naor, D.; Wallach-Dayan, S.B.; Zahalka, M.A.; Sionov, R.V. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin. Cancer Biol. 2008, 18, 260–267. [Google Scholar] [CrossRef]
- Roy, A.; Bera, S.; Saso, L.; Dwarakanath, B.S. Role of autophagy in tumor response to radiation: Implications for im-proving radiotherapy. Front. Oncol. 2022, 12, 957373. [Google Scholar] [CrossRef]
- Sudo, M.; Tsutsui, H.; Hayashi, S.; Yasuda, K.; Mitani, K.; Iwami, N.; Anzai, M.; Tsubouchi, T.; Ishida, M.; Satoi, S.; et al. Autophagy Inhibition Increased Sensitivity of Pancreatic Cancer Cells to Carbon Ion Radiotherapy. Cell Physiol. Biochem. 2023, 57, 212–225. [Google Scholar]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2018, 24, 605–616. [Google Scholar] [CrossRef]
- Denton, D.; Xu, T.; Kumar, S. Autophagy as a pro-death pathway. Immunol. Cell Biol. 2015, 93, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Jeong, H.; Yu, S.W. Autophagy as a decisive process for cell death. Exp. Mol. Med. 2020, 52, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lu, F.; Yan, J.; Wang, R.; Xia, Y.; Wang, L.; Li, L.; Chang, L.; Li, W. The role of radiotherapy-related autophagy genes in the prognosis and immune infiltration in lung adenocarcinoma. Front. Immunol. 2022, 13, 992626. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Song, X.; Yang, Z.; Mao, Y.; Zhang, K.; Wang, Y.; Su, B.; Li, Q.; Chen, H.; Li, Y. Upregulation of KCNQ1OT1 promotes resistance to stereotactic body radiotherapy in lung adenocarcinoma by inducing ATG5/ATG12-mediated autophagy via miR-372-3p. Cell Death Dis. 2020, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Lijun, W.; Hang, Y.; Jun, W. ROS/Autophagy/Nrf2 Pathway Mediated Low-Dose Radiation Induced Radio-Resistance in Human Lung Adenocarcinoma A549 Cell. Int. J. Biol. Sci. 2015, 11, 833–844. [Google Scholar]
- Shrestha, R.; Tatsukawa, H.; Ishibashi, N.; Matsuura, T.; Kagechika, H.; Kose, S.; Hitomi, K.; Imamoto, N.; Kojima, S. Molecular mechanism by which acyclic retinoid induces nuclear localization of transglutaminase 2 in human hepatocellular carcinoma cells. Cell Death Dis. 2015, 6, e2002. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Choi, S.S.; Ha, K.-S. Transglutaminase 2: A multi-functional protein in multiple subcellular compartments. Amino Acids 2010, 39, 619–631. [Google Scholar] [CrossRef]
- Mehta, K.; Kumar, A.; Kim, H.I. Transglutaminase 2: A multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem. Pharmacol. 2010, 80, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yu, D.; Zhou, J.; Zhuang, S.; Jiang, T. TGM2 interference regulates the angiogenesis and apoptosis of colorectal cancer via Wnt/beta-catenin pathway. Cell Cycle 2019, 18, 1122–1134. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Itahana, Y.; Guo, A.K.; Han, R.; Iwamoto, K.; Nguyen, H.T.; Bao, Y.; Kleiber, K.; Wu, Y.J.; Bay, B.H.; et al. Transglutaminase 2 contributes to a TP53-induced autophagy program to prevent oncogenic transformation. Elife 2016, 5, e07101. [Google Scholar] [CrossRef]
- Lee, F.T.; Mountain, A.J.; Kelly, M.P.; Hall, C.; Rigopoulos, A.; Johns, T.G.; Smyth, F.E.; Brechbiel, M.W.; Nice, E.C.; Burgess, A.W.; et al. Enhanced efficacy of radioimmunotherapy with 90Y-CHX-A-DTPA-hu3S193 by inhibition of epidermal growth factor receptor (EGFR) signaling with EGFR tyrosine kinase inhibitor AG1478. Clin. Cancer Res. 2005, 11 Pt 2, 7080s–7086s. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 2021, 21, 396. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, Y.; Tian, M.; Yue, J.; Xia, Y.; Hu, S.; Wu, K. The Role of TGM2 on Cell Proliferation, Migration and Invasion of BGC-823 Cell in Gastric Cancer. Prog. Mod. Biomed. 2018, 18, 1816. [Google Scholar]
- Lu, K; Zimmermann, M.; Görg, B.; Bidmon, H.J.; Biermann, B.; Klöcker, N.; Häussinger, D.; Reichert, A.S. Hepatic encephalopathy is linked to alterations of autophagic flux in astrocytes. EBioMedicine 2019, 48, 539–553. [CrossRef] [PubMed]
- Kang, S.; Oh, S.C.; Min, B.W.; Lee, D.-H. Transglutaminase 2 Regulates Self-renewal and Stem Cell Marker of Human Colorectal Cancer Stem Cells. Anticancer. Res. 2018, 38, 787–794. [Google Scholar] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Nishino, M.; Ozaki, M.; Hegab, A.E.; Hamamoto, J.; Kagawa, S.; Arai, D.; Yasuda, H.; Naoki, K.; Soejima, K.; Saya, H.; et al. Variant CD44 expression is enriching for a cell population with cancer stem cell-like characteristics in human lung adenocarcinoma. J. Cancer 2017, 8, 1774–1785. [Google Scholar] [CrossRef]
- Liao, C.; Wang, Q.; An, J.; Chen, J.; Li, X.; Long, Q.; Xiao, L.; Guan, X.; Liu, J. CD44 Glycosylation as a Therapeutic Target in Oncology. Front. Oncol. 2022, 12, 883831. [Google Scholar] [CrossRef] [PubMed]
- Pustovalova, M.; Alhaddad, L.; Blokhina, T.; Smetanina, N.; Chigasova, A.; Chuprov-Netochin, R.; Eremin, P.; Gilmutdinova, I.; Osipov, A.N.; Leonov, S. The CD44high Subpopulation of Multifraction Irradiation-Surviving NSCLC Cells Exhibits Partial EMT-Program Activation and DNA Damage Response Depending on Their p53 Status. Int. J. Mol. Sci. 2021, 22, 2369. [Google Scholar] [CrossRef]
- Sai, S.; Kim, E.H.; Koom, W.S.; Vares, G.; Suzuki, M.; Yamada, S.; Hayashi, M. Carbon-Ion Beam Irradiation and the miR-200c Mimic Effectively Eradicate Pancreatic Cancer Stem Cells Under in vitro and in vivo Conditions. OncoTargets Ther. 2021, 14, 4749–4760. [Google Scholar] [CrossRef] [PubMed]
- Yazal, T.; Bailleul, J.; Ruan, Y.; Sung, D.; Chu, F.I.; Palomera, D.; Dao, A.; Sehgal, A.; Gurunathan, V.; Aryan, L.; et al. Radiosensitizing Pancreatic Cancer via Effective Autophagy Inhibition. Mol. Cancer Ther. 2021, 21, 79–88. [Google Scholar] [CrossRef]
- Chaurasia, M.; Bhatt, A.N.; Das, B.A.; Dwarakanath, B.S.; Sharma, K. Radiation-induced autophagy: Mechanisms and consequences. Free Radic. Res. 2016, 50, 273–290. [Google Scholar] [CrossRef]
- Zhang, H.; McCarty, N. Tampering with cancer chemoresistance by targeting the TGM2-IL6-autophagy regulatory network. Autophagy 2017, 13, 627–628. [Google Scholar] [CrossRef]
- Yin, J.; Oh, Y.T.; Kim, S.S.; Choi, E.; Kim, T.H.; Hong, J.H.; Chang, N.; Cho, H.J.; Sa, J.K.; Kim, J.C.; et al. Transglutaminase 2 Inhibition Reverses Mesenchymal Transdifferentiation of Glioma Stem Cells by Regulating C/EBPβ Signaling. Cancer Res. 2017, 77, 4973–4984. [Google Scholar] [CrossRef]
- Dymova, M.A.; Vasileva, N.S.; Kuligina, E.V.; Savinovskaya, Y.I.; Zinchenko, N.D.; Ageenko, A.B.; Mishinov, S.V.; Stepanov, G.A.; Richter, V.A.; Semenov, D.V. MicroRNA and mRNA Expression Changes in Glioblastoma Cells Cultivated under Conditions of Neurosphere Formation. Curr. Issues Mol. Biol. 2022, 44, 5294–5311. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, Q.; Liu, H.; Zeng, L.; Zhou, Y.; Liu, X.; Bai, Y.; Zhang, J.; Pan, Y.; Shao, C. SDC1-dependent TGM2 determines radiosensitivity in glioblastoma by coordinating EPG5-mediated fusion of autophagosomes with lysosomes. Autophagy 2023, 19, 839–857. [Google Scholar] [CrossRef]




| Gene Name | CD44+A549/A549 FC | p Value | Difference Labeling |
|---|---|---|---|
| AKT1 | 1.916798987 | 0.031498127 | * |
| ARSB | 0.540021603 | 0.007457067 | ** |
| ATG4B | 1.178883886 | 0.024688472 | * |
| ATG7 | 1.606276736 | 0.019764521 | * |
| ATG9A | 1.563050332 | 0.002539375 | ** |
| BCL2 | 2.661168151 | 0.006385583 | ** |
| BCL2L1 | 2.900310928 | 0.000126638 | *** |
| BECN1 | 1.411060457 | 0.023430197 | * |
| CLN3 | 0.568644846 | 0.042009251 | * |
| CTSS | 6.700339499 | 0.031175413 | * |
| DAPK1 | 0.386402211 | 0.028667488 | * |
| GABARAPL1 | 0.468758375 | 0.004627373 | ** |
| HGS | 1.76961699 | 0.000679948 | *** |
| PIK3R4 | 0.589085589 | 0.007750301 | ** |
| PTEN | 0.353429337 | 0.025497525 | * |
| RB1 | 0.485138608 | 0.044292159 | * |
| RPS6KB1 | 0.47761233 | 0.013796305 | * |
| TGM2 | 59.24354241 | 6.42 × 10−6 | *** |
| TP53 | 0.251151782 | 0.003567178 | ** |
| ULK2 | 0.334679544 | 0.008775852 | ** |
| WIPI1 | 5.937977239 | 0.000775029 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, Q.; Wang, X.; Luo, H.; Du, T.; Wu, L.; Tan, M.; Chen, Y.; Wu, X.; Sun, S.; et al. TGM2-Mediated Autophagy Contributes to the Radio-Resistance of Non-Small Cell Lung Cancer Stem-like Cells. Biomedicines 2024, 12, 2231. https://doi.org/10.3390/biomedicines12102231
Wang Q, Zhang Q, Wang X, Luo H, Du T, Wu L, Tan M, Chen Y, Wu X, Sun S, et al. TGM2-Mediated Autophagy Contributes to the Radio-Resistance of Non-Small Cell Lung Cancer Stem-like Cells. Biomedicines. 2024; 12(10):2231. https://doi.org/10.3390/biomedicines12102231
Chicago/Turabian StyleWang, Qian, Qiuning Zhang, Xiaohu Wang, Hongtao Luo, Tianqi Du, Luyao Wu, Mingyu Tan, Yanliang Chen, Xun Wu, Shilong Sun, and et al. 2024. "TGM2-Mediated Autophagy Contributes to the Radio-Resistance of Non-Small Cell Lung Cancer Stem-like Cells" Biomedicines 12, no. 10: 2231. https://doi.org/10.3390/biomedicines12102231
APA StyleWang, Q., Zhang, Q., Wang, X., Luo, H., Du, T., Wu, L., Tan, M., Chen, Y., Wu, X., Sun, S., Liu, Z., Xie, Y., & Yuan, W. (2024). TGM2-Mediated Autophagy Contributes to the Radio-Resistance of Non-Small Cell Lung Cancer Stem-like Cells. Biomedicines, 12(10), 2231. https://doi.org/10.3390/biomedicines12102231







