Pulsed Field Ablation of Atrial Fibrillation: A Novel Technology for Safer and Faster Ablation
Abstract
:1. Introduction
2. Maintenance of Sinus Rhythm as the Target Therapeutic Goal
3. Catheter AF Ablation Needs to Be Safe, Effective and Quick
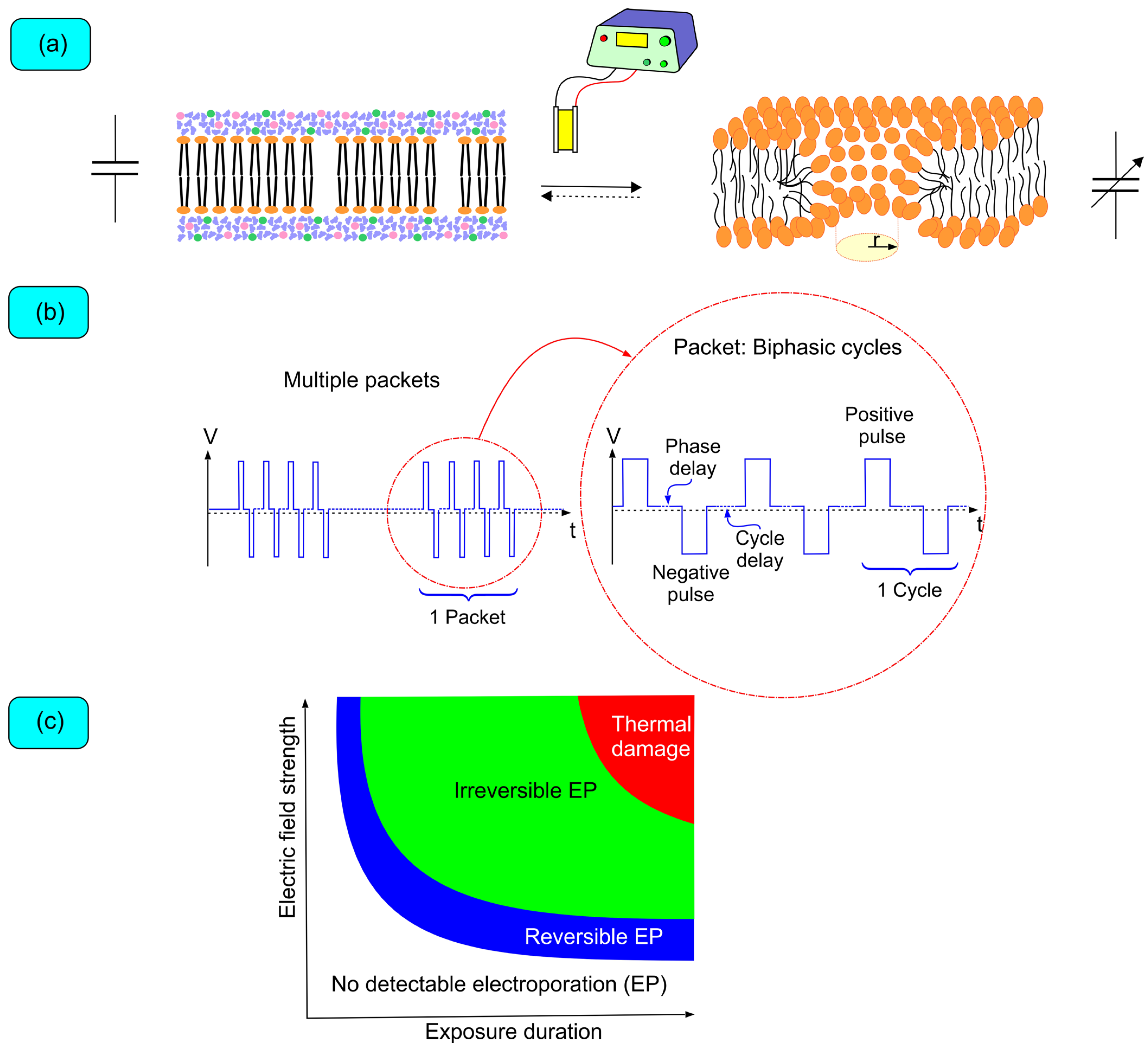
4. Biophysics of Pulsed Field Ablation
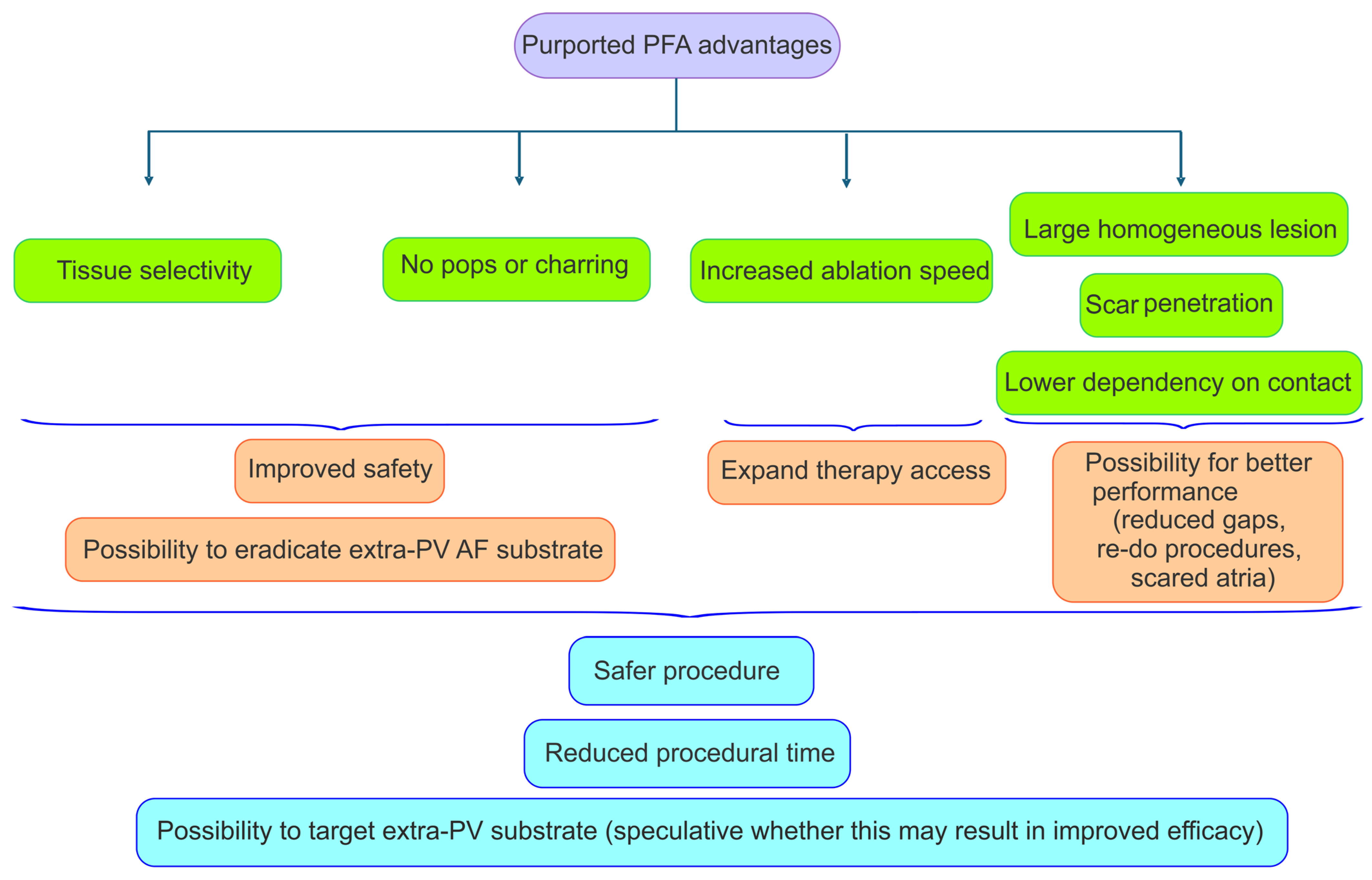
5. Advantages of PFA: Tissue Selectivity and Expeditious Energy Delivery
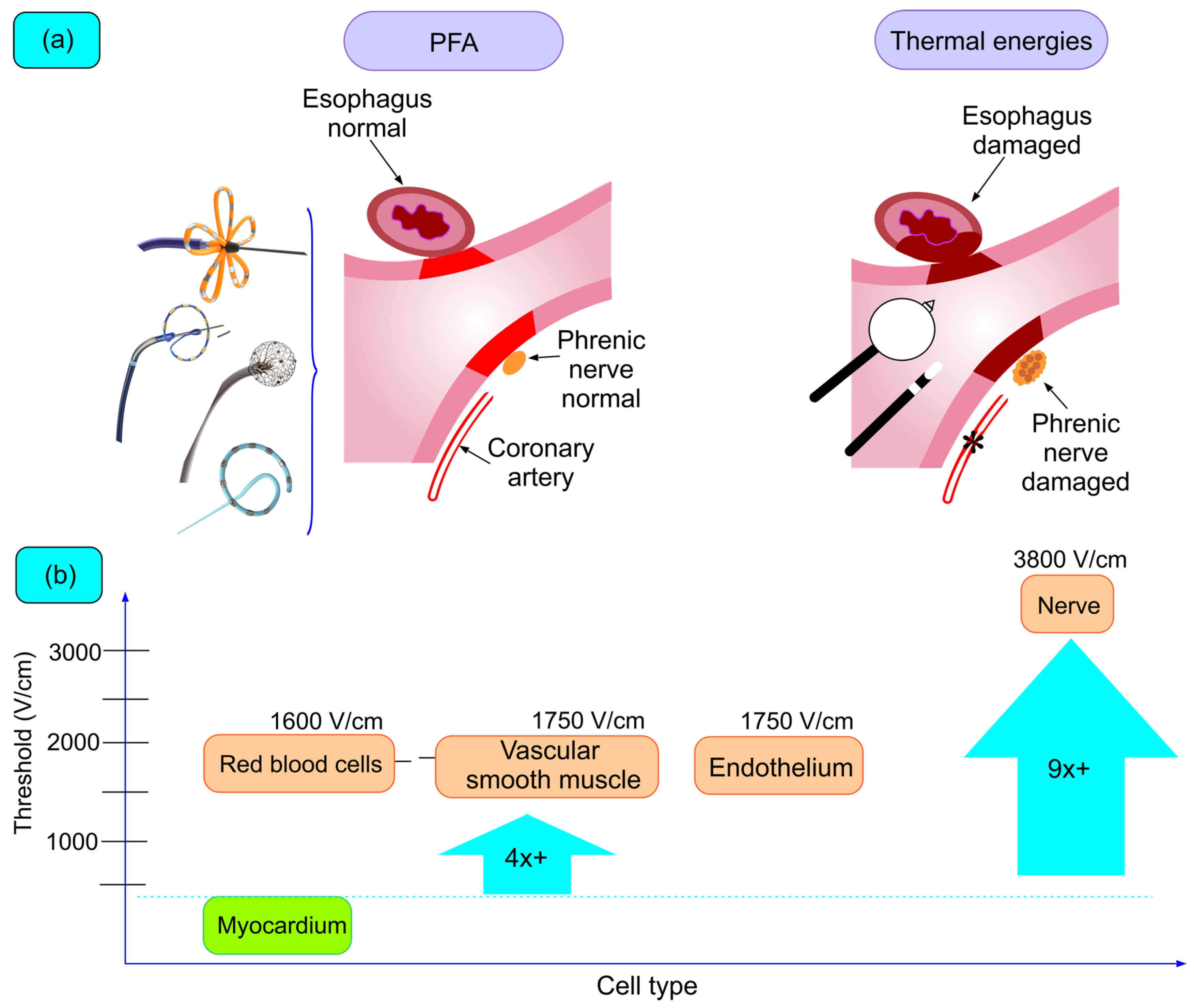
5.1. Tissue Selectivity
5.1.1. Esophageal Lesions
5.1.2. Nervous Lesions
5.1.3. Vascular Lesions (Pulmonary Veins and Coronary Arteries)
5.1.4. Cerebral Lesions
5.2. Procedural Technical Advantages
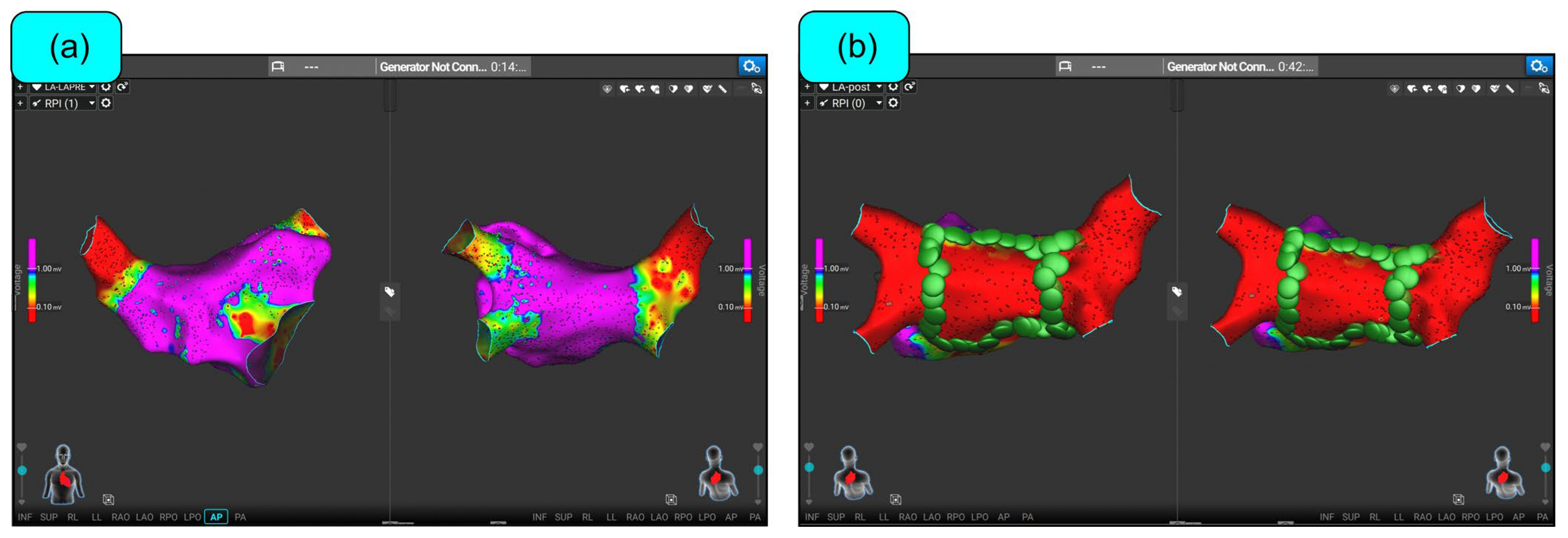
6. Clinical Experience
| Study | Catheter | N | Paroxysmal (%) | F/U PVI% | Clinical Efficacy | Complications | Anesthesia |
|---|---|---|---|---|---|---|---|
| 2018, Reddy [89] | Farawave | 15 | 100% | Not evaluated | Not evaluated | No acute complications. No long-term studies. | GA |
| 2020, Reddy [92] | Sphere-9 | 76 | 72% | Not evaluated | One groin hematoma. No AEF (60 pts with esophagoscopy), PVS (44 pts with CT) or PNP. 5/51 pts with silent cerebral lesions/events. | GA (100%) | |
| 2020, Reddy [90] | Farawave | 25 | 0% | 22 pts remapped at 3 mon. 86% of pts. *Posterior wall isolated in 100%. | Not evaluated | 1 effusion with RF-based remapping. No AEF (21 pts with endoscopic study), PVS (14 pts with CT) or PNP. | 80% conscious sedation; 20% GA |
| 2021, Reddy [91] | Farawave | 121 | 100% | 110 pts remapped 3 mon. 65% of pts. (84% with optimized waveform) | (excluding repeat procedure): 79%. Optimized waveform cohort (44 pts): 85% | 2 cardiac perforations and 1 vascular complication. No AEF (38 pts with endoscopic study), stroke (18 pts with MRI), PVS (74 pts with CT or MRI) or PNP. 2/18 pts evaluated with MRI had acute lesions. | GA for monophasic (15/15). Sedation for almost all biphasic (105/106) |
| 2021, Verma [44,45] | Pulsed-select | 38 | 92% | Not evaluated | No acute complications. No long-term studies. | GA (76%); conscious sedation | |
| 2023, Verma [93] | Pulsed-Select | 300 | 50% | Not evaluated | 70% paroxysmal, 62% persistent | 1 effusion and 1 cerebrovascular accident. No AEF, PVS (63 pts with MRI) or PNP. 4/45 pts evaluated with MRI had silent cerebral lesions. | GA or deep sedation in 95% of paroxysmal cohort and 92% persistent cohort |
| 2023, Reddy [94] | Sphere-9 | 178 | 39% | 122 pts remapped at 3 mon. 58% of patients (90% of pts. with optimized waveform) | 78% (for combined paroxysmal and persistent) | 1 inflammatory pericardial effusion medically treated. No AEF (124 pts with esophagoscopy), PVS (77 pts with CT) or PNP. 1 groin hematoma. 13/89 pts with silent cerebral lesions/events. | GA (100%) |
| 2024, Duytschaever [78] | Varipulse | 226 | 100% | Not evaluated | 76.9% and 78.9% for wave I and wave II | No AEF, PVS or PN injury. 8/39 pts with silent cerebral lesions/events; all resolved at 3 months post procedure. | Conscious sedation (27%) or GA (73%) |
7. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Meyre, P.; Blum, S.; Berger, S.; Aeschbacher, S.; Schoepfer, H.; Briel, M.; Osswald, S.; Conen, D. Risk of Hospital Admissions in Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. Can. J. Cardiol. 2019, 35, 1332–1343. [Google Scholar] [CrossRef]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Shamloo, A.S.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Europace 2024, 26, 5–8. [Google Scholar] [CrossRef]
- Camm, A.J.; Naccarelli, G.V.; Mittal, S.; Crijns, H.J.G.M.; Hohnloser, S.H.; Ma, C.S.; Natale, A.; Turakhia, M.P.; Kirchhof, P. The Increasing Role of Rhythm Control in Patients with Atrial Fibrillation: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 1932–1948. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Shantsila, E.; Choi, E.K.; Lane, D.A.; Joung, B.; Lip, G.Y.H. Atrial Fibrillation: Comorbidities, Lifestyle, and Patient Factors. The Lancet Reg. Health—Eur. 2024, 37, 100784. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Hagens, V.E.; Bosker, H.A.; Kingma, J.H.; Kamp, O.; Kingma, T.; Said, S.A.; Darmanata, J.I.; Timmermans, A.J.M.; Tijssen, J.G.P.; et al. A Comparison of Rate Control and Rhythm Control in Patients with Recurrent Persistent Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1834–1840. [Google Scholar] [CrossRef]
- Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [CrossRef]
- Carlsson, J.; Miketic, S.; Windeler, J.; Cuneo, A.; Haun, S.; Micus, S.; Walter, S.; Tebbe, U. Randomized Trial of Rate-Control versus Rhythm-Control in Persistent Atrial Fibrillation: The Strategies of Treatment of Atrial Fibrillation (STAF) Study. J. Am. Coll. Cardiol. 2003, 41, 1690–1696. [Google Scholar] [CrossRef]
- Roy, D.; Talajic, M.; Nattel, S.; Wyse, D.G.; Dorian, P.; Lee, K.L.; Bourassa, M.G.; Arnold, J.M.O.; Buxton, A.E.; Camm, A.J.; et al. Rhythm Control versus Rate Control for Atrial Fibrillation and Heart Failure. N. Engl. J. Med. 2008, 358, 2667–2677. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Kuck, K.H.; Lilienthal, J. Rhythm or Rate Control in Atrial Fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): A Randomised Trial. Lancet 2000, 356, 1789–1794. [Google Scholar] [CrossRef]
- Mont, L.; Bisbal, F.; Hernández-Madrid, A.; Pérez-Castellano, N.; Viñolas, X.; Arenal, A.; Arribas, F.; Fernández-Lozano, I.; Bodegas, A.; Cobos, A.; et al. Catheter Ablation vs. Antiarrhythmic Drug Treatment of Persistent Atrial Fibrillation: A Multicentre, Randomized, Controlled Trial (SARA Study). Eur. Heart J. 2014, 35, 501–507. [Google Scholar] [CrossRef]
- Cosedis Nielsen, J.; Johannessen, A.; Raatikainen, P.; Hindricks, G.; Walfridsson, H.; Kongstad, O.; Pehrson, S.; Englund, A.; Hartikainen, J.; Mortensen, L.S.; et al. Radiofrequency Ablation as Initial Therapy in Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2012, 367, 1587–1595. [Google Scholar] [CrossRef]
- Wazni, O.M.; Marrouche, N.F.; Martin, D.O.; Verma, A.; Bhargava, M.; Saliba, W.; Bash, D.; Schweikert, R.; Brachmann, J.; Gunther, J.; et al. Radiofrequency Ablation vs. Antiarrhythmic Drugs as First-Line Treatment of Symptomatic Atrial Fibrillation: A Randomized Trial. JAMA 2005, 293, 2634–2640. [Google Scholar] [CrossRef]
- Wilber, D.J.; Pappone, C.; Neuzil, P.; De Paola, A.; Marchlinski, F.; Natale, A.; Macle, L.; Daoud, E.G.; Calkins, H.; Hall, B.; et al. Comparison of Antiarrhythmic Drug Therapy and Radiofrequency Catheter Ablation in Patients with Paroxysmal Atrial Fibrillation: A Randomized Controlled Trial. JAMA 2010, 303, 333–340. [Google Scholar] [CrossRef]
- Forleo, G.B.; Mantica, M.; De Luca, L.; Leo, R.; Santini, L.; Panigada, S.; De Sanctis, V.; Pappalardo, A.; Laurenzi, F.; Avella, A.; et al. Catheter Ablation of Atrial Fibrillation in Patients with Diabetes Mellitus Type 2: Results from a Randomized Study Comparing Pulmonary Vein Isolation versus Antiarrhythmic Drug Therapy. J. Cardiovasc. Electrophysiol. 2009, 20, 22–28. [Google Scholar] [CrossRef]
- Packer, D.L.; Kowal, R.C.; Wheelan, K.R.; Irwin, J.M.; Champagne, J.; Guerra, P.G.; Dubuc, M.; Reddy, V.; Nelson, L.; Holcomb, R.G.; et al. Cryoballoon Ablation of Pulmonary Veins for Paroxysmal Atrial Fibrillation: First Results of the North American Arctic Front (STOP AF) Pivotal Trial. J. Am. Coll. Cardiol. 2013, 61, 1713–1723. [Google Scholar] [CrossRef]
- Jaïs, P.; Cauchemez, B.; Macle, L.; Daoud, E.; Khairy, P.; Subbiah, R.; Hocini, M.; Extramiana, F.; Sacher, F.; Bordachar, P.; et al. Catheter Ablation versus Antiarrhythmic Drugs for Atrial Fibrillation: The A4 Study. Circulation 2008, 118, 2498–2505. [Google Scholar] [CrossRef]
- Oral, H.; Pappone, C.; Chugh, A.; Good, E.; Bogun, F.; Pelosi, F.; Bates, E.R.; Lehmann, M.H.; Vicedomini, G.; Augello, G.; et al. Circumferential Pulmonary-Vein Ablation for Chronic Atrial Fibrillation. N. Engl. J. Med. 2006, 354, 934–941. [Google Scholar] [CrossRef]
- Pappone, C.; Augello, G.; Sala, S.; Gugliotta, F.; Vicedomini, G.; Gulletta, S.; Paglino, G.; Mazzone, P.; Sora, N.; Greiss, I.; et al. A Randomized Trial of Circumferential Pulmonary Vein Ablation versus Antiarrhythmic Drug Therapy in Paroxysmal Atrial Fibrillation: The APAF Study. J. Am. Coll. Cardiol. 2006, 48, 2340–2347. [Google Scholar] [CrossRef]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs. Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients with Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef]
- Nyong, J.; Amit, G.; Adler, A.J.; Owolabi, O.O.; Perel, P.; Prieto-Merino, D.; Lambiase, P.; Casas, J.P.; Morillo, C.A. Efficacy and Safety of Ablation for People with Non-Paroxysmal Atrial Fibrillation. Cochrane Database Syst. Rev. 2016, 2016, CD012088. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, E1–E156. [Google Scholar] [CrossRef]
- Kuck, K.H.; Lebedev, D.S.; Mikhaylov, E.N.; Romanov, A.; Geller, L.; Kalejs, O.; Neumann, T.; Davtyan, K.; On, Y.K.; Popov, S.; et al. Catheter Ablation or Medical Therapy to Delay Progression of Atrial Fibrillation: The Randomized Controlled Atrial Fibrillation Progression Trial (ATTEST). EP Eur. 2021, 23, 362–369a. [Google Scholar] [CrossRef]
- de With, R.R.; Erküner, Ö.; Rienstra, M.; Nguyen, B.O.; Körver, F.W.J.; Linz, D.; Ten, H.C.; Spronk, H.; Kroon, A.A.; Maass, A.H.; et al. Temporal Patterns and Short-Term Progression of Paroxysmal Atrial Fibrillation: Data from RACE V. Europace 2020, 22, 1162–1172. [Google Scholar] [CrossRef]
- Lu, Z.; Scherlag, B.J.; Lin, J.; Niu, G.; Fung, K.M.; Zhao, L.; Ghias, M.; Jackman, W.M.; Lazzara, R.; Jiang, H.; et al. Atrial Fibrillation Begets Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2008, 1, 184–192. [Google Scholar] [CrossRef]
- Yang, W.Y.; Du, X.; Fawzy, A.M.; He, L.; Li, H.W.; Dong, J.Z.; Lip, G.Y.H.; Ma, C.S.; Jiang, C.; Xia, S.J.; et al. Associations of Atrial Fibrillation Progression with Clinical Risk Factors and Clinical Prognosis: A Report from the Chinese Atrial Fibrillation Registry Study. J. Cardiovasc. Electrophysiol. 2021, 32, 333–341. [Google Scholar] [CrossRef]
- Pappone, C.; Radinovic, A.; Manguso, F.; Vicedomini, G.; Ciconte, G.; Sacchi, S.; Mazzone, P.; Paglino, G.; Gulletta, S.; Sala, S.; et al. Atrial Fibrillation Progression and Management: A 5-Year Prospective Follow-up Study. Heart Rhythm 2008, 5, 1501–1507. [Google Scholar] [CrossRef]
- Ríos-Muñoz, G.R.; Soto, N.; Ávila, P.; Carta, A.; Atienza, F.; Datino, T.; González-Torrecilla, E.; Fernández-Avilés, F.; Arenal, Á. Structural Remodeling and Rotational Activity in Persistent/Long-Lasting Atrial Fibrillation: Gender-Effect Differences and Impact on Post-Ablation Outcome. Front. Cardiovasc. Med. 2022, 9, 819429. [Google Scholar] [CrossRef]
- Wijffels, M.C.E.F.; Kirchhof, C.J.H.J.; Dorland, R.; Allessie, M.A. Atrial Fibrillation Begets Atrial Fibrillation. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef]
- Andrade, J.G.; Deyell, M.W.; Macle, L.; Wells, G.A.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; et al. Progression of Atrial Fibrillation after Cryoablation or Drug Therapy. N. Engl. J. Med. 2023, 388, 105–116. [Google Scholar] [CrossRef]
- Ganesan, A.N.; Chew, D.P.; Hartshorne, T.; Selvanayagam, J.B.; Aylward, P.E.; Sanders, P.; McGavigan, A.D. The Impact of Atrial Fibrillation Type on the Risk of Thromboembolism, Mortality, and Bleeding: A Systematic Review and Meta-Analysis. Eur. Heart J. 2016, 37, 1591–1602. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Hellkamp, A.S.; Lokhnygina, Y.; Patel, M.R.; Breithardt, G.; Hankey, G.J.; Becker, R.C.; Singer, D.E.; Halperin, J.L.; Hacke, W.; et al. Higher Risk of Death and Stroke in Patients with Persistent vs. Paroxysmal Atrial Fibrillation: Results from the ROCKET-AF Trial. Eur. Heart J. 2015, 36, 288–296. [Google Scholar] [CrossRef]
- Chiang, C.E.; Naditch-Brûlé, L.; Murin, J.; Goethals, M.; Inoue, H.; O-Neill, J.; Silva-Cardoso, J.; Zharinov, O.; Gamra, H.; Alam, S.; et al. Distribution and Risk Profile of Paroxysmal, Persistent, and Permanent Atrial Fibrillation in Routine Clinical Practice: Insight from the Real-Life Global Survey Evaluating Patients with Atrial Fibrillation International Registry. Circ. Arrhythmia Electrophysiol. 2012, 5, 632–639. [Google Scholar] [CrossRef]
- Brooks, A.G.; Stiles, M.K.; Laborderie, J.; Lau, D.H.; Kuklik, P.; Shipp, N.J.; Hsu, L.F.; Sanders, P. Outcomes of Long-Standing Persistent Atrial Fibrillation Ablation: A Systematic Review. Heart Rhythm 2010, 7, 835–846. [Google Scholar] [CrossRef]
- Chew, D.S.; Black-Maier, E.; Loring, Z.; Noseworthy, P.A.; Packer, D.L.; Exner, D.V.; Mark, D.B.; Piccini, J.P. Diagnosis-to-Ablation Time and Recurrence of Atrial Fibrillation Following Catheter Ablation: A Systematic Review and Meta-Analysis of Observational Studies. Circ. Arrhythmia Electrophysiol. 2020, 13, E008128. [Google Scholar] [CrossRef]
- Raatikainen, M.J.P.; Arnar, D.O.; Merkely, B.; Nielsen, J.C.; Hindricks, G.; Heidbuchel, H.; Camm, J. A Decade of Information on the Use of Cardiac Implantable Electronic Devices and Interventional Electrophysiological Procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. EP Eur. 2017, 19, ii1–ii90. [Google Scholar] [CrossRef]
- McCarthy, P.M.; Cox, J.L.; Kislitsina, O.N.; Kruse, J.; Churyla, A.; Malaisrie, S.C.; Mehta, C.K. Surgery and Catheter Ablation for Atrial Fibrillation: History, Current Practice, and Future Directions. J. Clin. Med. 2022, 11, 210. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Neuzil, P.; Koruth, J.S.; Petru, J.; Funosako, M.; Cochet, H.; Sediva, L.; Chovanec, M.; Dukkipati, S.R.; Jais, P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 315–326. [Google Scholar] [CrossRef]
- Kuck, K.H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. J. Cardiopulm. Rehabil. Prev. 2016, 36, 393–394. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Gerstenfeld, E.P.; Natale, A.; Whang, W.; Cuoco, F.A.; Patel, C.; Mountantonakis, S.E.; Gibson, D.N.; Harding, J.D.; Ellis, C.R.; et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1660–1671. [Google Scholar] [CrossRef]
- Hunter, R.J.; Baker, V.; Finlay, M.C.; Duncan, E.R.; Lovell, M.J.; Tayebjee, M.H.; Ullah, W.; Siddiqui, M.S.; McLEAN, A.; Richmond, L.; et al. Point-by-Point Radiofrequency Ablation Versus the Cryoballoon or a Novel Combined Approach: A Randomized Trial Comparing 3 Methods of Pulmonary Vein Isolation for Paroxysmal Atrial Fibrillation (The Cryo Versus RF Trial). J. Cardiovasc. Electrophysiol. 2015, 26, 1307–1314. [Google Scholar] [CrossRef]
- Sawhney, V.; Schilling, R.J.; Providencia, R.; Cadd, M.; Perera, D.; Chatha, S.; Mercer, B.; Finlay, M.; Halimi, F.; Pavin, D.; et al. Cryoablation for Persistent and Longstanding Persistent Atrial Fibrillation: Results from a Multicentre European Registry. Europace 2020, 22, 375–381. [Google Scholar] [CrossRef]
- Ferrero-De-Loma-Osorio, Á.; Cózar, R.; García-Alberola, A.; Valles, E.; Barrera, A.; Toquero, J.; Ormaetxe, J.M.; Sánchez, J.M.; Ruiz-Granell, R.; Amador, P.B.; et al. Primary Results of the Spanish Cryoballoon Ablation Registry: Acute and Long-Term Outcomes of the RECABA Study. Sci. Rep. 2021, 11, 17268. [Google Scholar] [CrossRef]
- Verma, A.; Boersma, L.; Haines, D.E.; Natale, A.; Marchlinski, F.E.; Sanders, P.; Calkins, H.; Packer, D.L.; Hummel, J.; Onal, B.; et al. First-in-Human Experience and Acute Procedural Outcomes Using a Novel Pulsed Field Ablation System: The PULSED AF Pilot Trial. Circ. Arrhythm. Electrophysiol. 2022, 15, E010168. [Google Scholar] [CrossRef]
- Di Biase, L.; Diaz, J.C.; Zhang, X.D.; Romero, J. Pulsed Field Catheter Ablation in Atrial Fibrillation. Trends Cardiovasc. Med. 2022, 32, 378–387. [Google Scholar] [CrossRef]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.H. Gene Transfer into Mouse Lyoma Cells by Electroporation in High Electric Fields. EMBO J. 1982, 1, 841. [Google Scholar] [CrossRef]
- Scuderi, M.; Dermol-Černe, J.; Amaral da Silva, C.; Muralidharan, A.; Boukany, P.E.; Rems, L. Models of Electroporation and the Associated Transmembrane Molecular Transport Should Be Revisited. Bioelectrochemistry 2022, 147, 108216. [Google Scholar] [CrossRef]
- Sugrue, A.; Maor, E.; Del-Carpio Munoz, F.; Killu, A.M.; Asirvatham, S.J. Cardiac Ablation with Pulsed Electric Fields: Principles and Biophysics. EP Eur. 2022, 24, 1213–1222. [Google Scholar] [CrossRef]
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef]
- Koruth, J.; Kuroki, K.; Iwasawa, J.; Enomoto, Y.; Viswanathan, R.; Brose, R.; Buck, E.D.; Speltz, M.; Dukkipati, S.R.; Reddy, V.Y. Preclinical Evaluation of Pulsed Field Ablation: Electrophysiological and Histological Assessment of Thoracic Vein Isolation. Circ. Arrhythm. Electrophysiol. 2019, 12, e007781. [Google Scholar] [CrossRef]
- Yavin, H.D.; Higuchi, K.; Sroubek, J.; Younis, A.; Zilberman, I.; Anter, E. Pulsed-Field Ablation in Ventricular Myocardium Using a Focal Catheter: The Impact of Application Repetition on Lesion Dimensions. Circ. Arrhythm. Electrophysiol. 2021, 14, E010375. [Google Scholar] [CrossRef]
- Baena-Montes, J.M.; O’Halloran, T.; Clarke, C.; Donaghey, K.; Dunne, E.; O’Halloran, M.; Quinlan, L.R. Electroporation Parameters for Human Cardiomyocyte Ablation In Vitro. J. Cardiovasc. Dev. Dis. 2022, 9, 240. [Google Scholar] [CrossRef]
- Tabaja, C.; Younis, A.; Hussein, A.A.; Taigen, T.L.; Nakagawa, H.; Saliba, W.I.; Sroubek, J.; Santangeli, P.; Wazni, O.M. Catheter-Based Electroporation: A Novel Technique for Catheter Ablation of Cardiac Arrhythmias. Clin. Electrophysiol. 2023, 9, 2008–2023. [Google Scholar] [CrossRef]
- García-Sánchez, T.; Amorós-Figueras, G.; Jorge, E.; Campos, M.C.; Maor, E.; Guerra, J.M.; Ivorra, A. Parametric Study of Pulsed Field Ablation with Biphasic Waveforms in an In Vivo Heart Model: The Role of Frequency. Circ. Arrhythmia Electrophysiol. 2022, 15, 693–705. [Google Scholar] [CrossRef]
- Amorós-Figueras, G.; Casabella-Ramon, S.; Moreno-Weidmann, Z.; Ivorra, A.; Guerra, J.M.; García-Sánchez, T. Dynamics of High-Density Unipolar Epicardial Electrograms During PFA. Circ. Arrhythmia Electrophysiol. 2023, 16, E011914. [Google Scholar] [CrossRef]
- Pliquett, U. Joule Heating during Solid Tissue Electroporation. Med. Biol. Eng. Comput. 2003, 41, 215–219. [Google Scholar] [CrossRef]
- Andrade, J.G.; Champagne, J.; Dubuc, M.; Deyell, M.W.; Verma, A.; Macle, L.; Leong-Sit, P.; Novak, P.; Badra-Verdu, M.; Sapp, J.; et al. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring: A Randomized Clinical Trial. Circulation 2019, 140, 1779–1788. [Google Scholar] [CrossRef]
- Neven, K.; Van Es, R.; Van Driel, V.; Van Wessel, H.; Fidder, H.; Vink, A.; Doevendans, P.; Wittkampf, F. Acute and Long-Term Effects of Full-Power Electroporation Ablation Directly on the Porcine Esophagus. Circ. Arrhythmia Electrophysiol. 2017, 10, e004672. [Google Scholar] [CrossRef]
- Howard, B.; Haines, D.E.; Verma, A.; Packer, D.; Kirchhof, N.; Barka, N.; Onal, B.; Fraasch, S.; Miklavčič, D.; Stewart, M.T. Reduction in Pulmonary Vein Stenosis and Collateral Damage with Pulsed Field Ablation Compared with Radiofrequency Ablation in a Canine Model. Circ. Arrhythmia Electrophysiol. 2020, 13, E008337. [Google Scholar] [CrossRef]
- Van Driel, V.J.H.M.; Neven, K.; Van Wessel, H.; Vink, A.; Doevendans, P.A.F.M.; Wittkampf, F.H.M. Low Vulnerability of the Right Phrenic Nerve to Electroporation Ablation. Heart Rhythm 2015, 12, 1838–1844. [Google Scholar] [CrossRef]
- Koruth, J.S.; Kuroki, K.; Kawamura, I.; Brose, R.; Viswanathan, R.; Buck, E.D.; Donskoy, E.; Neuzil, P.; Dukkipati, S.R.; Reddy, V.Y. Pulsed Field Ablation Versus Radiofrequency Ablation: Esophageal Injury in a Novel Porcine Model. Circ. Arrhythmia Electrophysiol. 2020, 13, E008303. [Google Scholar] [CrossRef]
- Kaminska, I.; Kotulska, M.; Stecka, A.; Saczko, J.; Drag-Zalesinska, M.; Wysocka, T.; Choromanska, A.; Skolucka, N.; Nowicki, R.; Marczak, J.; et al. Electroporation-Induced Changes in Normal Immature Rat Myoblasts (H9C2). Gen. Physiol. Biophys. 2012, 31, 19–25. [Google Scholar] [CrossRef]
- Stavrakis, S.; Nakagawa, H.; Po, S.S.; Scherlag, B.J.; Lazzara, R.; Jackman, W.M. The Role of the Autonomic Ganglia in Atrial Fibrillation. JACC Clin. Electrophysiol. 2015, 1, 1–13. [Google Scholar] [CrossRef]
- Katritsis, D.G.; Giazitzoglou, E.; Zografos, T.; Pokushalov, E.; Po, S.S.; Camm, A.J. Rapid Pulmonary Vein Isolation Combined with Autonomic Ganglia Modification: A Randomized Study. Heart Rhythm 2011, 8, 672–678. [Google Scholar] [CrossRef]
- Goff, Z.D.; Laczay, B.; Yenokyan, G.; Sivasambu, B.; Sinha, S.K.; Marine, J.E.; Ashikaga, H.; Berger, R.D.; Akhtar, T.; Spragg, D.D.; et al. Heart Rate Increase after Pulmonary Vein Isolation Predicts Freedom from Atrial Fibrillation at 1 Year. J. Cardiovasc. Electrophysiol. 2019, 30, 2818–2822. [Google Scholar] [CrossRef]
- Turagam, M.K.; Neuzil, P.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Safety and Effectiveness of Pulsed Field Ablation to Treat Atrial Fibrillation: One-Year Outcomes From the MANIFEST-PF Registry. Circulation 2023, 148, 35–46. [Google Scholar] [CrossRef]
- Schmidt, B.; Bordignon, S.; Neven, K.; Reichlin, T.; Blaauw, Y.; Hansen, J.; Adelino, R.; Ouss, A.; Füting, A.; Roten, L.; et al. EUropean Real-World Outcomes with Pulsed Field AblatiOn in Patients with Symptomatic AtRIAl Fibrillation: Lessons from the Multi-Centre EU-PORIA Registry. EP Eur. 2023, 25, 1–11. [Google Scholar] [CrossRef]
- Mansour, M.; Gerstenfeld, E.P.; Patel, C.; Natale, A.; Whang, W.; Cuoco, F.A.; Mountantonakis, S.E.; Gibson, D.N.; Harding, J.D.; Holland, S.K.; et al. Pulmonary Vein Narrowing after Pulsed Field versus Thermal Ablation. Europace 2024, 26, euad185. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Petru, J.; Funasako, M.; Kopriva, K.; Hala, P.; Chovanec, M.; Janotka, M.; Kralovec, S.; Neuzil, P. Coronary Arterial Spasm during Pulsed Field Ablation to Treat Atrial Fibrillation. Circulation 2022, 146, 1808–1819. [Google Scholar] [CrossRef]
- Gunawardene, M.A.; Schaeffer, B.N.; Jularic, M.; Eickholt, C.; Maurer, T.; Akbulak, R.; Flindt, M.; Anwar, O.; Hartmann, J.; Willems, S. Coronary Spasm During Pulsed Field Ablation of the Mitral Isthmus Line. Clin. Electrophysiol. 2021, 7, 1618–1620. [Google Scholar] [CrossRef]
- Zhang, C.; Neuzil, P.; Petru, J.; Funasako, M.; Hala, P.; Kopriva, K.; Koruth, J.S.; Dukkipati, S.R.; Reddy, V.Y. Coronary Artery Spasm During Pulsed Field vs. Radiofrequency Catheter Ablation of the Mitral Isthmus. JAMA Cardiol. 2024, 9, 72–77. [Google Scholar] [CrossRef]
- Malyshev, Y.; Neuzil, P.; Petru, J.; Funasako, M.; Hala, P.; Kopriva, K.; Schneider, C.; Achyutha, A.; Vanderper, A.; Musikantow, D.; et al. Nitroglycerin to Ameliorate Coronary Artery Spasm During Focal Pulsed-Field Ablation for Atrial Fibrillation. JACC Clin. Electrophysiol. 2024, 10, 885–896. [Google Scholar] [CrossRef]
- Reinsch, N.; Füting, A.; Höwel, D.; Bell, J.; Lin, Y.; Neven, K. Cerebral Safety after Pulsed Field Ablation for Paroxysmal Atrial Fibrillation. Heart Rhythm 2022, 19, 1813–1818. [Google Scholar] [CrossRef]
- Ladejobi, A.; Christopoulos, G.; Tan, N.; Ladas, T.P.; Tri, J.; Van Zyl, M.; Yasin, O.; Sugrue, A.; Khabsa, M.; Uecker, D.R.; et al. Effects of Pulsed Electric Fields on the Coronary Arteries in Swine. Circ. Arrhythmia Electrophysiol. 2022, 15, 641–647. [Google Scholar] [CrossRef]
- Neven, K.; Füting, A.; Byrd, I.; Heil, R.W.; Fish, J.M.; Feeney, D.A.; Donskoy, E.; Jensen, J.A. Absence of (Sub-)Acute Cerebral Events or Lesions after Electroporation Ablation in the Left-Sided Canine Heart. Heart Rhythm 2021, 18, 1004–1011. [Google Scholar] [CrossRef]
- Calvert, P.; Kollias, G.; Pürerfellner, H.; Narasimhan, C.; Osorio, J.; Lip, G.Y.H.; Gupta, D. Silent Cerebral Lesions Following Catheter Ablation for Atrial Fibrillation: A State-of-the-Art Review. Europace 2023, 25, euad151. [Google Scholar] [CrossRef]
- Ekanem, E.; Reddy, V.Y.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Multi-National Survey on the Methods, Efficacy, and Safety on the Post-Approval Clinical Use of Pulsed Field Ablation (MANIFEST-PF). Europace 2022, 24, 1256–1266. [Google Scholar] [CrossRef]
- Duytschaever, M.; De Potter, T.; Grimaldi, M.; Anic, A.; Vijgen, J.; Neuzil, P.; Van Herendael, H.; Verma, A.; Skanes, A.; Scherr, D.; et al. Paroxysmal Atrial Fibrillation Ablation Using a Novel Variable-Loop Biphasic Pulsed Field Ablation Catheter Integrated with a 3-Dimensional Mapping System: 1-Year Outcomes of the Multicenter InspIRE Study. Circ. Arrhythmia Electrophysiol. 2023, 16, E011780. [Google Scholar] [CrossRef]
- Chaumont, C.; Hayoun, C.; Savoure, A.; Al Hamoud, R.; Auquier, N.; McDonnell, E.; Eltchaninoff, H.; Anselme, F. Pentaspline Pulsed Field Ablation Catheter Versus Cryoballoon for Atrial Fibrillation Ablation: Results from a Prospective Comparative Study. J. Am. Heart Assoc. 2024, 13, 33146. [Google Scholar] [CrossRef]
- Urbanek, L.; Bordignon, S.; Schaack, D.; Chen, S.; Tohoku, S.; Efe, T.H.; Ebrahimi, R.; Pansera, F.; Hirokami, J.; Plank, K.; et al. Pulsed Field Versus Cryoballoon Pulmonary Vein Isolation for Atrial Fibrillation: Efficacy, Safety, and Long-Term Follow-Up in a 400-Patient Cohort. Circ. Arrhythmia Electrophysiol. 2023, 16, 389–398. [Google Scholar] [CrossRef]
- Nakagawa, H.; Farshchi-Heydari, S.; Maffre, J.; Sharma, T.; Govari, A.; Beeckler, C.T.; Altmann, A.; Ikeda, A.; Sugawara, M.; Jackman, W.M.; et al. Evaluation of Ablation Parameters to Predict Irreversible Lesion Size During Pulsed Field Ablation. Circ. Arrhythmia Electrophysiol. 2024, 17, e012814. [Google Scholar] [CrossRef]
- Nakagawa, H.; Castellvi, Q.; Neal, R.; Girouard, S.; Laughner, J.; Ikeda, A.; Sugawara, M.; An, Y.; Hussein, A.A.; Nakhla, S.; et al. Effects of Contact Force on Lesion Size During Pulsed Field Catheter Ablation: Histochemical Characterization of Ventricular Lesion Boundaries. Circ. Arrhythmia Electrophysiol. 2024, 17, e012026. [Google Scholar] [CrossRef]
- Venier, S.; Vaxelaire, N.; Jacon, P.; Carabelli, A.; Desbiolles, A.; Garban, F.; Defaye, P. Severe Acute Kidney Injury Related to Haemolysis after Pulsed Field Ablation for Atrial Fibrillation. Europace 2023, 26, euad371. [Google Scholar] [CrossRef]
- Nies, M.; Koruth, J.S.; Mlček, M.; Watanabe, K.; Tibenská, V.C.; Královec, Š.; Tejkl, L.; Neuzil, P.; Reddy, V.Y. Hemolysis After Pulsed Field Ablation: Impact of Lesion Number and Catheter-Tissue Contact. Circ. Arrhythmia Electrophysiol. 2024, 17, e012765. [Google Scholar] [CrossRef]
- Ekanem, E.; Neuzil, P.; Reichlin, T.; Kautzner, J.; van der Voort, P.; Jais, P.; Chierchia, G.B.; Bulava, A.; Blaauw, Y.; Skala, T.; et al. Safety of Pulsed Field Ablation in More than 17,000 Patients with Atrial Fibrillation in the MANIFEST-17K Study. Nat. Med. 2024, 30, 2020–2029. [Google Scholar] [CrossRef]
- Younis, A.; Zilberman, I.; Krywanczyk, A.; Higuchi, K.; Yavin, H.D.; Sroubek, J.; Anter, E. Effect of Pulsed-Field and Radiofrequency Ablation on Heterogeneous Ventricular Scar in a Swine Model of Healed Myocardial Infarction. Circ. Arrhythmia Electrophysiol. 2022, 15, 683–692. [Google Scholar] [CrossRef]
- Im, S.I.; Higuchi, S.; Lee, A.; Stillson, C.; Buck, E.; Morrow, B.; Schenider, K.; Speltz, M.; Gerstenfeld, E.P. Pulsed Field Ablation of Left Ventricular Myocardium in a Swine Infarct Model. JACC Clin. Electrophysiol. 2022, 8, 722–731. [Google Scholar] [CrossRef]
- Wittkampf, F.H.; Van Driel, V.J.; Van Wessel, H.; Vink, A.; Hof, I.E.; GrÜndeman, P.F.; Hauer, R.N.; Loh, P. Feasibility of Electroporation for the Creation of Pulmonary Vein Ostial Lesions. J. Cardiovasc. Electrophysiol. 2011, 22, 302–309. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Koruth, J.; Jais, P.; Petru, J.; Timko, F.; Skalsky, I.; Hebeler, R.; Labrousse, L.; Barandon, L.; Kralovec, S.; et al. Ablation of Atrial Fibrillation with Pulsed Electric Fields: An Ultra-Rapid, Tissue-Selective Modality for Cardiac Ablation. JACC Clin. Electrophysiol. 2018, 4, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Anic, A.; Koruth, J.; Petru, J.; Funasako, M.; Minami, K.; Breskovic, T.; Sikiric, I.; Dukkipati, S.R.; Kawamura, I.; et al. Pulsed Field Ablation in Patients with Persistent Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 1068–1080. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Dukkipati, S.R.; Neuzil, P.; Anic, A.; Petru, J.; Funasako, M.; Cochet, H.; Minami, K.; Breskovic, T.; Sikiric, I.; et al. Pulsed Field Ablation of Paroxysmal Atrial Fibrillation: 1-Year Outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin. Electrophysiol. 2021, 7, 614–627. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Anter, E.; Rackauskas, G.; Peichl, P.; Koruth, J.S.; Petru, J.; Funasako, M.; Minami, K.; Natale, A.; Jais, P.; et al. Lattice-Tip Focal Ablation Catheter That Toggles Between Radiofrequency and Pulsed Field Energy to Treat Atrial Fibrillation: A First-in-Human Trial. Circ. Arrhythmia Electrophysiol. 2020, 13, E008718. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Haines, D.E.; Boersma, L.V.; Sood, N.; Natale, A.; Marchlinski, F.E.; Calkins, H.; Sanders, P.; Packer, D.L.; Kuck, K.H.; et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation 2023, 147, 1422–1432. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Peichl, P.; Anter, E.; Rackauskas, G.; Petru, J.; Funasako, M.; Minami, K.; Koruth, J.S.; Natale, A.; Jais, P.; et al. A Focal Ablation Catheter Toggling Between Radiofrequency and Pulsed Field Energy to Treat Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 1786–1801. [Google Scholar] [CrossRef]
- Anter, E.; Mansour, M.; Nair, D.G.; Sharma, D.; Taigen, T.L.; Neuzil, P.; Kiehl, E.L.; Kautzner, J.; Osorio, J.; Mountantonakis, S.; et al. Dual-Energy Lattice-Tip Ablation System for Persistent Atrial Fibrillation: A Randomized Trial. Nat. Med. 2024, 30, 2303–2310. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carta-Bergaz, A.; Ríos-Muñoz, G.R.; Ávila, P.; Atienza, F.; González-Torrecilla, E.; Arenal, Á. Pulsed Field Ablation of Atrial Fibrillation: A Novel Technology for Safer and Faster Ablation. Biomedicines 2024, 12, 2232. https://doi.org/10.3390/biomedicines12102232
Carta-Bergaz A, Ríos-Muñoz GR, Ávila P, Atienza F, González-Torrecilla E, Arenal Á. Pulsed Field Ablation of Atrial Fibrillation: A Novel Technology for Safer and Faster Ablation. Biomedicines. 2024; 12(10):2232. https://doi.org/10.3390/biomedicines12102232
Chicago/Turabian StyleCarta-Bergaz, Alejandro, Gonzalo R. Ríos-Muñoz, Pablo Ávila, Felipe Atienza, Esteban González-Torrecilla, and Ángel Arenal. 2024. "Pulsed Field Ablation of Atrial Fibrillation: A Novel Technology for Safer and Faster Ablation" Biomedicines 12, no. 10: 2232. https://doi.org/10.3390/biomedicines12102232






