Linking Microbiota Profiles to Disease Characterization in Common Variable Immunodeficiency: The Case of Granulomatous–Lymphocytic Interstitial Lung Disease
Abstract
:1. Introduction
2. Materials and Methods
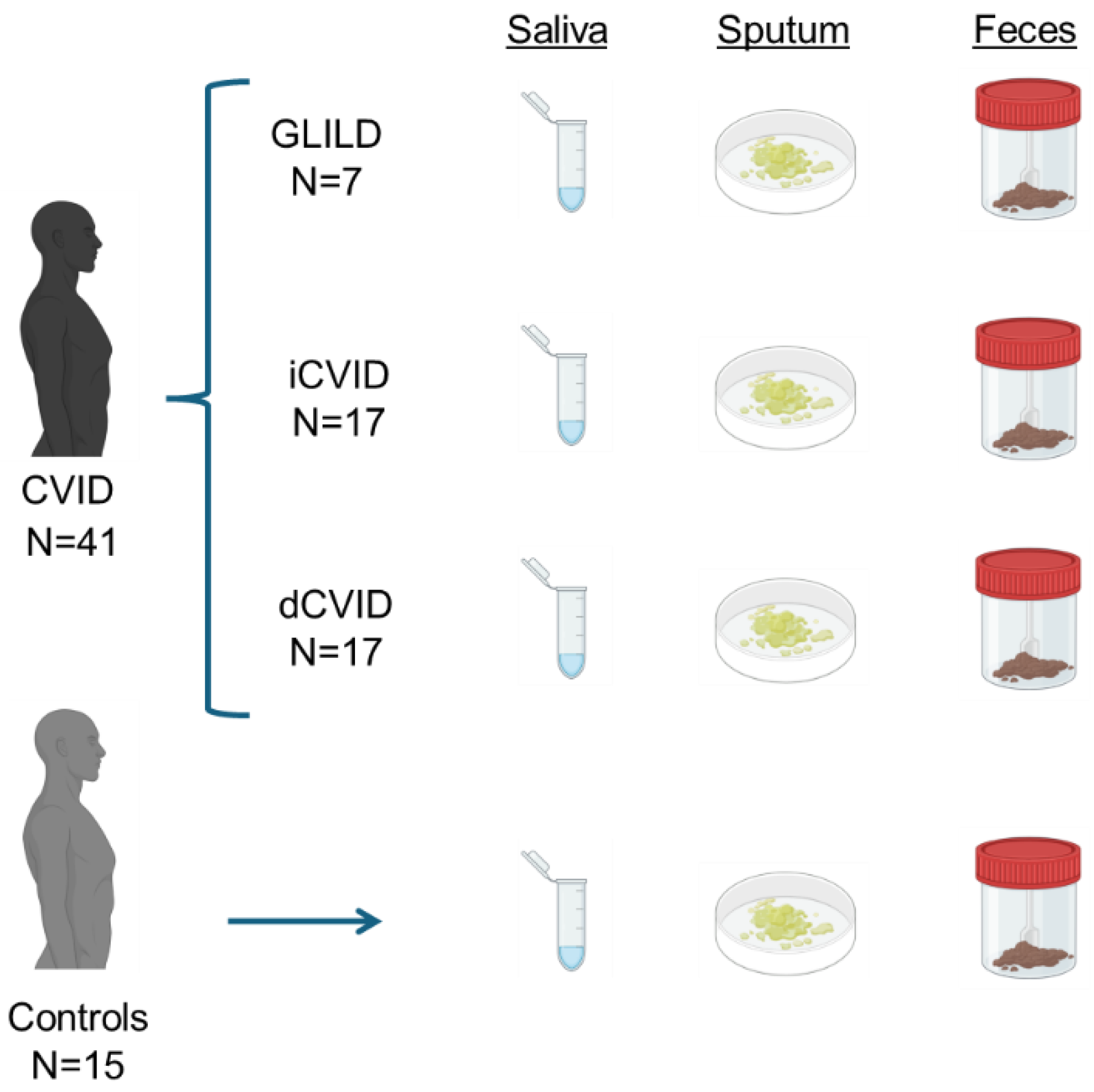
2.1. Patients
2.2. Sampling and DNA Extraction
2.3. Bioinformatic Analysis and Statistics
3. Results
3.1. Population Characteristics
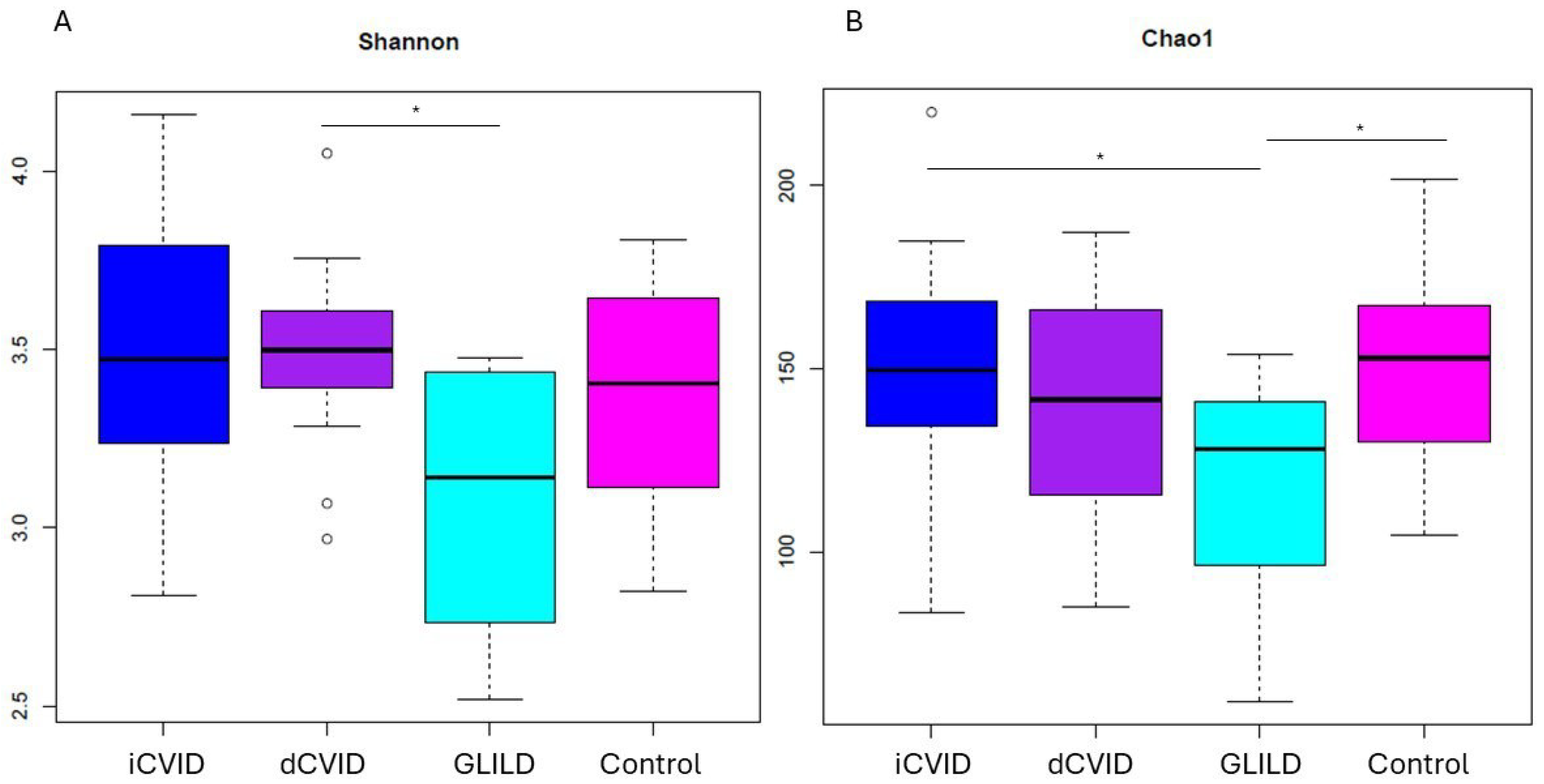
3.2. Global Microbiome Biodiversity Indicators
3.3. Microbiological Differences in GLILD Patients, CVID Subgroups, and Healthy Controls
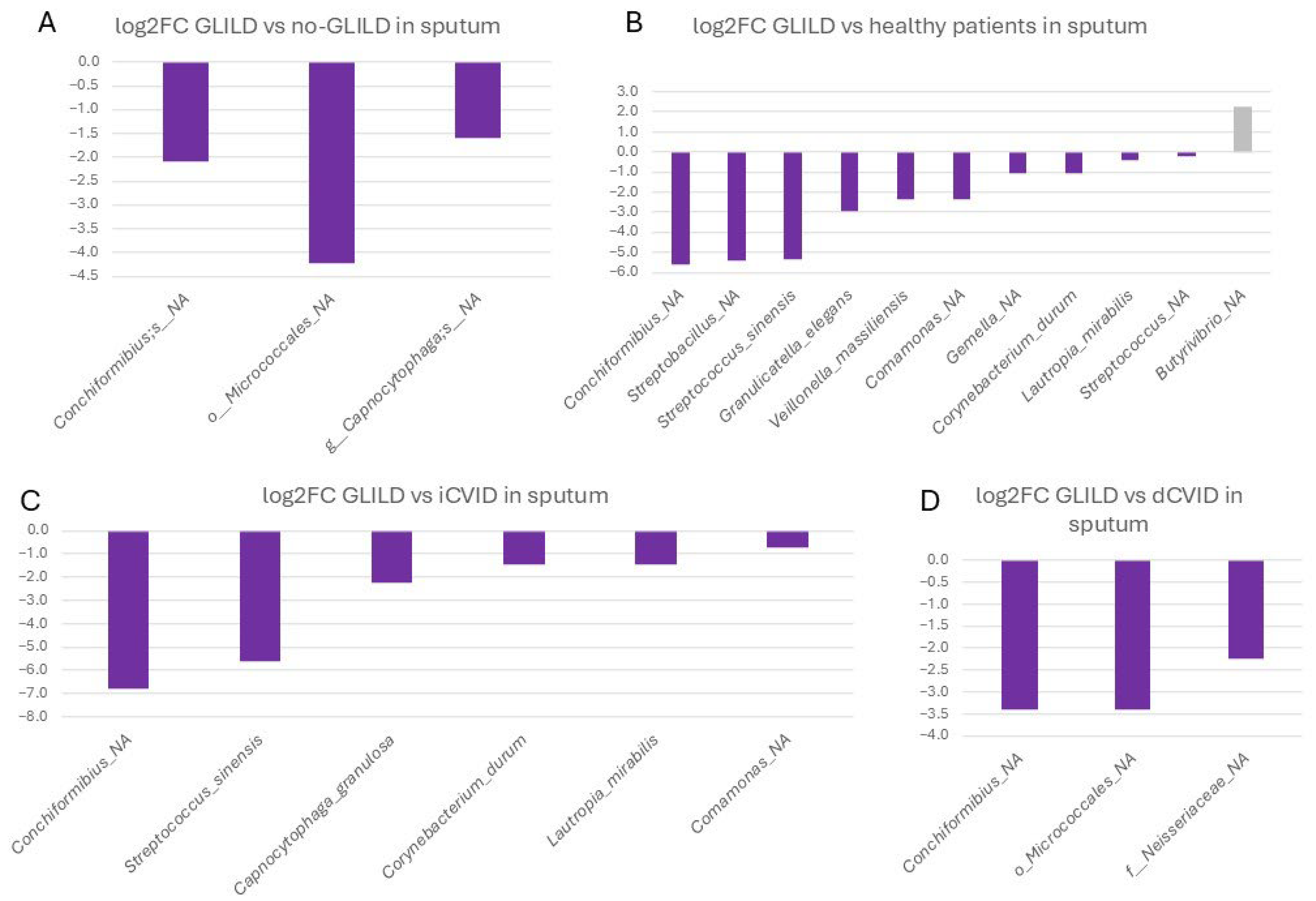
3.3.1. Sputum
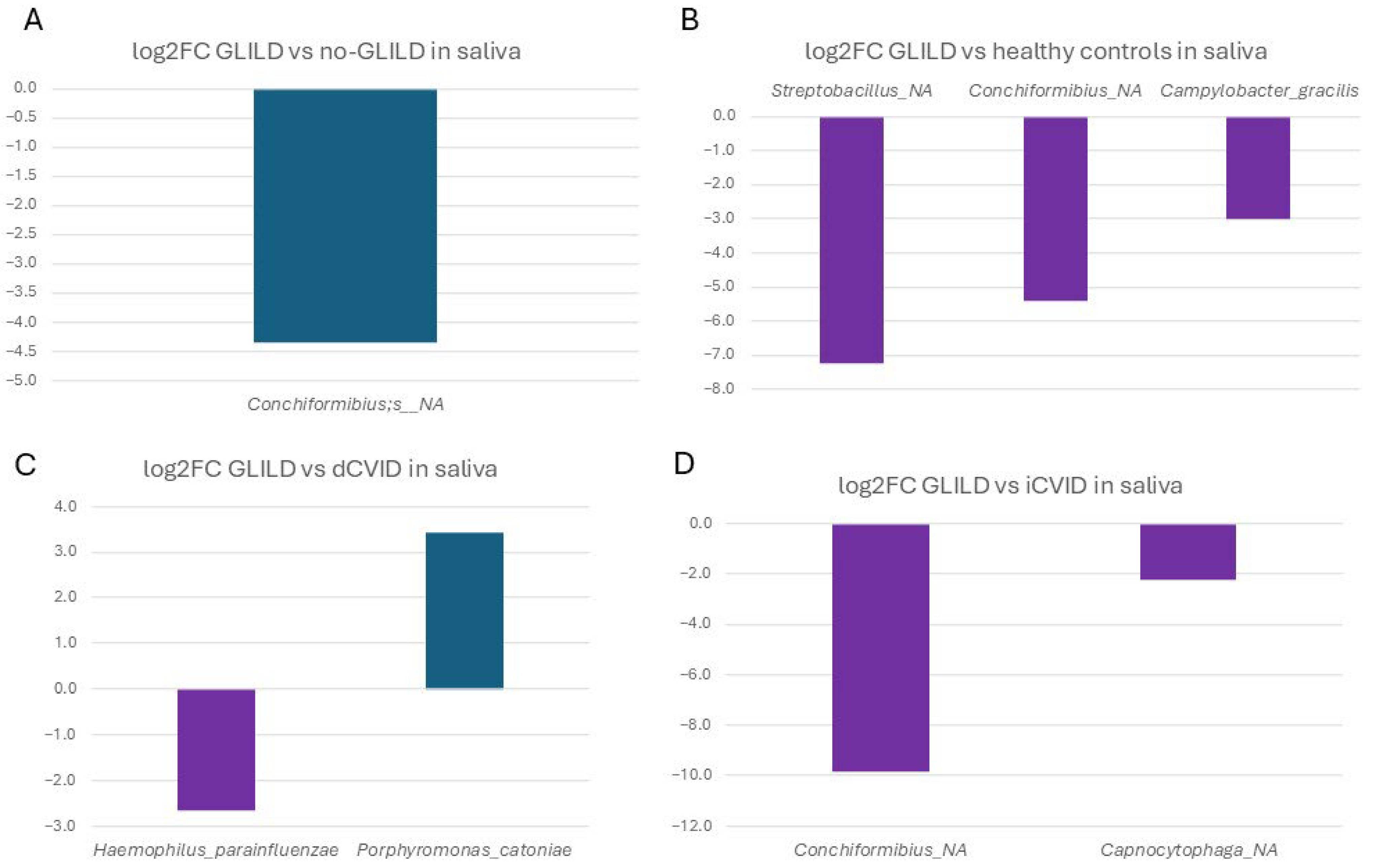
3.3.2. Saliva
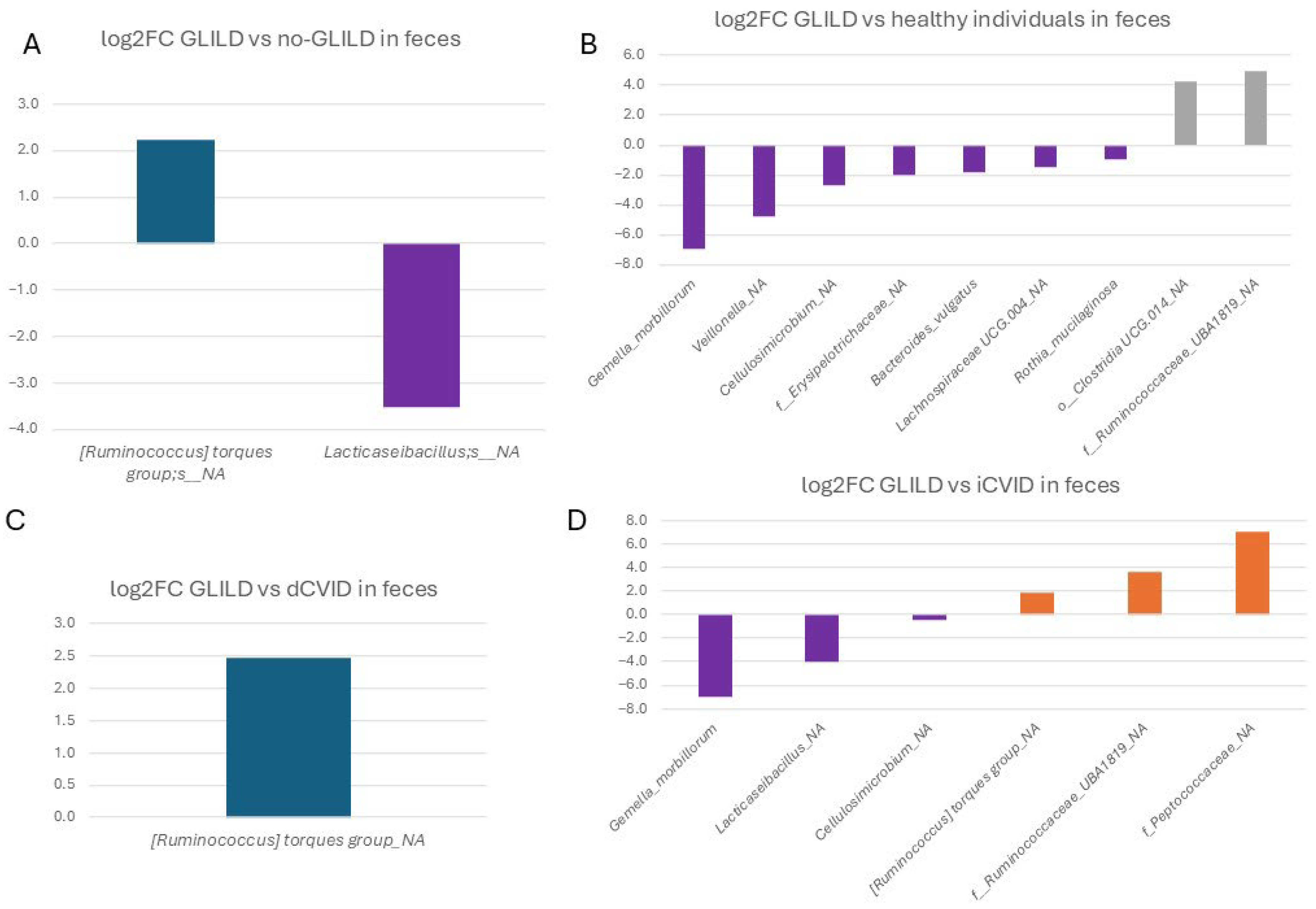
3.3.3. Feces
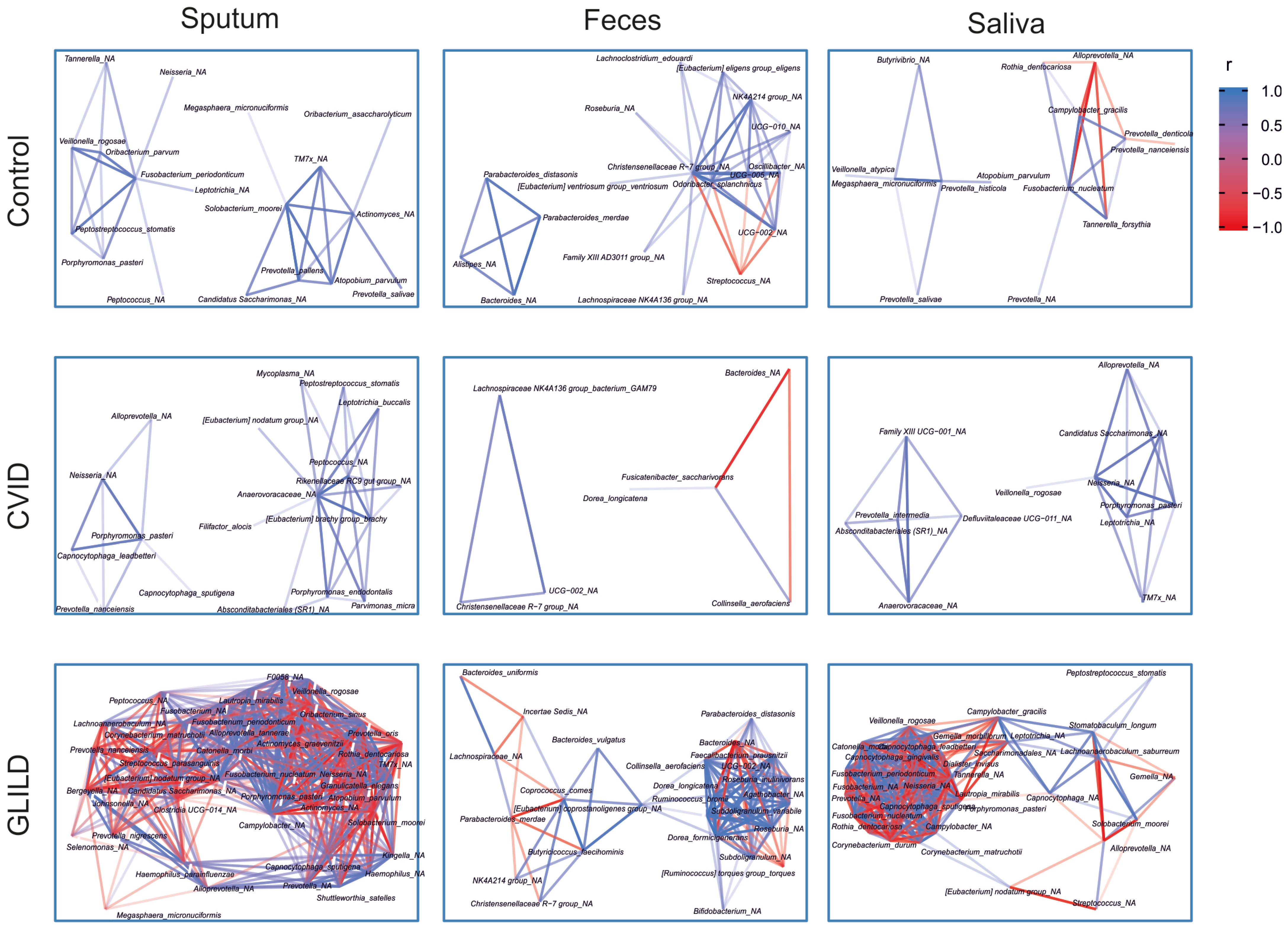
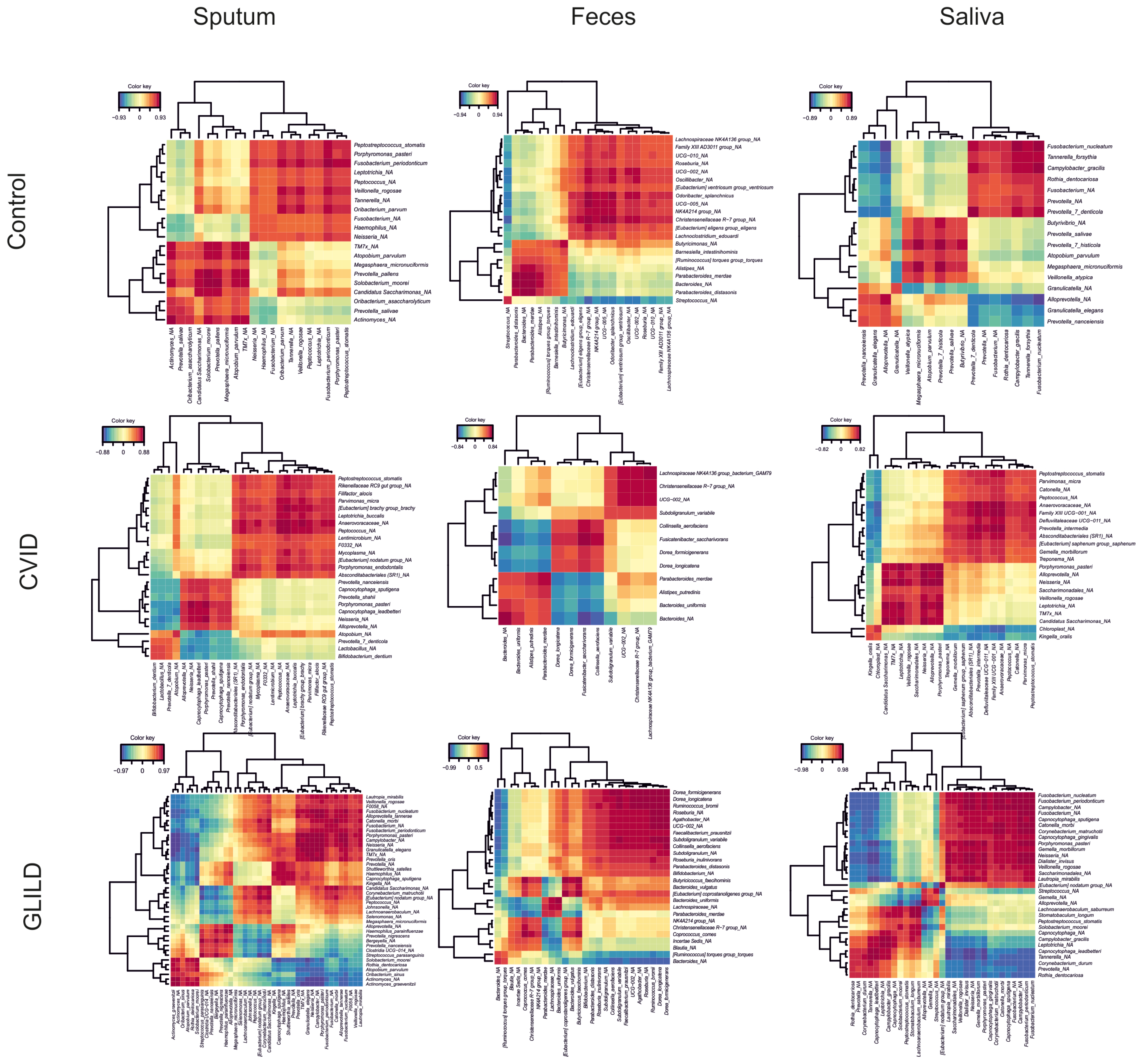
3.3.4. Pathobionts, Patterns, and Species Associated with GLILD in the Different Niches
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seidel, M.G.; Kindle, G.; Gathmann, B.; Quinti, I.; Buckland, M.; van Montfrans, J.; Scheible, R.; Rusch, S.; Gasteiger, L.M.; Grimbacher, B.; et al. ESID Registry Working Party and collaborators. The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J. Allergy Clin. Immunol. Pract. 2019, 7, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S.; Grossman, W.J.; Navickis, R.J.; Wilkes, M.M. Impact of Trough IgG on Pneumonia Incidence in Primary Immunodeficiency: A Meta-Analysis of Clinical Studies. Clin. Immunol. 2010, 137, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Resnick, E.S.; Moshier, E.L.; Godbold, J.H.; Cunningham-Rundles, C. Morbidity and Mortality in Common Variable Immune Deficiency Over 4 Decades. Blood 2012, 119, 1650–1657. [Google Scholar] [CrossRef]
- Gathmann, B.; Mahlaoui, N.; Gérard, L.; Oksenhendler, E.; Warnatz, K.; Schulze, I.; Kindle, G.; Kuijpers, T.W.; van Beem, R.T.; Guzman, D.; et al. European Society for Immunodeficiencies Registry Working Party. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J. Allergy Clin. Immunol. 2014, 134, 116–126. [Google Scholar] [CrossRef]
- Chapel, H.; Lucas, M.; Lee, M.; Bjorkander, J.; Webster, D.; Grimbacher, B.; Fieschi, C.; Thon, V.; Abedi, M.R.; Hammarstrom, L. Common variable immunodeficiency disorders: Division into distinct clinical phenotypes. Blood 2008, 112, 277–286. [Google Scholar] [CrossRef]
- Cabañero-Navalon, M.D.; Garcia-Bustos, V.; Nuñez-Beltran, M.; Císcar Fernández, P.; Mateu, L.; Solanich, X.; Carrillo-Linares, J.L.; Robles-Marhuenda, Á.; Puchades-Gimeno, F.; Pelaez Ballesta, A.; et al. Current clinical spectrum of common variable immunodeficiency in Spain: The multicentric nationwide GTEM-SEMI-CVID registry. Front. Immunol. 2022, 13, 1033666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bates, C.A.; Ellison, M.C.; Lynch, D.A.; Cool, C.D.; Brown, K.K.; Routes, J.M. Granulomatous-Lymphocytic Lung Disease Shortens Survival in Common Variable Immunodeficiency. J. Allergy Clin. Immunol. 2004, 114, 415–421. [Google Scholar] [CrossRef]
- Hurst, J.R.; Verma, N.; Lowe, D.; Baxendale, H.E.; Jolles, S.; Kelleher, P.; Longhurst, H.J.; Patel, S.Y.; Renzoni, E.A.; Sander, C.R.; et al. British Lung Foundation/United Kingdom Primary Immunodeficiency Network Consensus Statement on the Definition, Diagnosis, and Management of Granulomatous-Lymphocytic Interstitial Lung Disease in Common Variable Immunodeficiency Disorders. J. Allergy Clin. Immunol. Pract. 2017, 5, 938–945. [Google Scholar] [CrossRef]
- Verma, N.; Grimbacher, B.; Hurst, J.R. Lung Disease in Primary Antibody Deficiency. Lancet Respir. Med. 2015, 3, 651–660. [Google Scholar] [CrossRef]
- Gereige, J.D.; Maglione, P.J. Current Understanding and Recent Developments in Common Variable Immunodeficiency Associated Autoimmunity. Front. Immunol. 2019, 10, 2753. [Google Scholar] [CrossRef]
- Jørgensen, S.F.; Trøseid, M.; Kummen, M.; Anmarkrud, J.A.; Michelsen, A.E.; Osnes, L.T.; Holm, K.; Høivik, M.L.; Rashidi, A.; Dahl, C.P.; et al. Altered gut microbiota profile in common variable immunodeficiency associates with levels of lipopolysaccharide and markers of systemic immune activation. Mucosal Immunol. 2016, 9, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.F.; Fevang, B.; Aukrust, P. Autoimmunity and Inflammation in CVID: A Possible Crosstalk between Immune Activation, Gut Microbiota, and Epigenetic Modifications. J. Clin. Immunol. 2019, 39, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Pagliuca, A.; Vermi, M.; Pizzirusso, D.; Innammorato, M.; Sglavo, R.; Scarso, F.; Salemi, S.; Laganà, B.; Di Rosa, R.; et al. The Role of Lung Colonization in Connective Tissue Disease-Associated Interstitial Lung Disease. Microorganisms 2021, 9, 932. [Google Scholar] [CrossRef] [PubMed]
- Inaoka, P.T.; Shono, M.; Kamada, M.; Espinoza, J.L. Host-microbe interactions in the pathogenesis and clinical course of sarcoidosis. J. Biomed. Sci. 2019, 26, 45. [Google Scholar] [CrossRef]
- Young, R.P.; Hopkins, R.J.; Marsland, B. The Gut-Liver-Lung Axis. Modulation of the Innate Immune Response and Its Possible Role in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 161–169. [Google Scholar] [CrossRef]
- Varricchi, G.; Poto, R.; Ianiro, G.; Punziano, A.; Marone, G.; Gasbarrini, A.; Spadaro, G. Gut Microbiome and Common Variable Immunodeficiency: Few Certainties and Many Outstanding Questions. Front. Immunol. 2021, 12, 712915. [Google Scholar] [CrossRef]
- Berbers, R.M.; Mohamed Hoesein, F.A.A.; Ellerbroek, P.M.; van Montfrans, J.M.; Dalm, V.A.S.H.; van Hagen, P.M.; Paganelli, F.L.; Viveen, M.C.; Rogers, M.R.; de Jong, P.A.; et al. Low IgA Associated with Oropharyngeal Microbiota Changes and Lung Disease in Primary Antibody Deficiency. Front. Immunol. 2020, 11, 1245. [Google Scholar] [CrossRef]
- Hand, T.W. The Role of the Microbiota in Shaping Infectious Immunity. Trends Immunol. 2016, 37, 647–658. [Google Scholar] [CrossRef]
- Cabanero-Navalon, M.D.; Garcia-Bustos, V.; Mira, A.; Moral Moral, P.; Salavert-Lleti, M.; Forner Giner, M.J.; Núñez Beltrán, M.; Todolí Parra, J.; Bracke, C.; Carda-Diéguez, M. Dysimmunity in common variable immunodeficiency is associated with alterations in oral, respiratory, and intestinal microbiota. Clin. Immunol. 2023, 256, 109796. [Google Scholar] [CrossRef]
- Elvers, K.T.; Wilson, V.J.; Hammond, A.; Duncan, L.; Huntley, A.L.; Hay, A.D.; van der Werf, E.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: A systematic review. BMJ Open 2020, 10, e035677. [Google Scholar] [CrossRef]
- Rosier, B.T.; Buetas, E.; Moya-Gonzalvez, E.M.; Artacho, A.; Mira, A. Nitrate as a potential prebiotic for the oral microbiome. Sci. Rep. 2020, 10, 12895. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, M.; Collado, M.C.; Abrahamsson, T.; Artacho, A.; Stensson, M.; Jenmalm, M.C.; Mira, A. Oral microbiome development during childhood: An ecological succession influenced by postnatal factors and associated with tooth decay. ISME J. 2018, 9, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, O.; O’Hara, P.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.6-6.1; The Comprehensive R Archive Network: Vienna, Austria, 2024. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 10 June 2024).
- Lin, H.; Peddada, S.D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Macpherson, M.E.; Skarpengland, T.; Hov, J.R.; Ranheim, T.; Vestad, B.; Dahl, T.B.; Fraz, M.S.A.; Michelsen, A.E.; Holven, K.B.; Fevang, B.; et al. Increased Plasma Levels of Triglyceride-Enriched Lipoproteins Associate with Systemic Inflammation, Lipopolysaccharides, and Gut Dysbiosis in Common Variable Immunodeficiency. J. Clin. Immunol. 2023, 43, 1229–1240. [Google Scholar] [CrossRef]
- Pulvirenti, F.; Giufrè, M.; Pentimalli, T.M.; Camilli, R.; Milito, C.; Villa, A.; Sculco, E.; Cerquetti, M.; Pantosti, A.; Quinti, I. Oropharyngeal microbial ecosystem perturbations influence the risk for acute respiratory infections in common variable immunodeficiency. Front. Immunol. 2024, 15, 1371118. [Google Scholar] [CrossRef]
- Fiedorová, K.; Radvanský, M.; Bosák, J.; Grombiříková, H.; Němcová, E.; Králíčková, P.; Černochová, M.; Kotásková, I.; Lexa, M.; Litzman, J.; et al. Bacterial but Not Fungal Gut Microbiota Alterations Are Associated With Common Variable Immunodeficiency (CVID) Phenotype. Front. Immunol. 2019, 10, 1914. [Google Scholar] [CrossRef]
- Jorgensen, S.F.; Macpherson, M.E.; Skarpengland, T.; Berge, R.K.; Fevang, B.; Halvorsen, B.; Aukrust, P. Disturbed lipid profile in common variable immunodeficiency—A pathogenic loop of inflammation and metabolic disturbances. Front. Immunol. 2023, 14, 1199727. [Google Scholar] [CrossRef]
- Ho, H.E.; Radigan, L.; Bongers, G.; El-Shamy, A.; Cunningham-Rundles, C. Circulating bioactive bacterial DNA is associated with immune activation and complications in common variable immunodeficiency. J. Clin. Investig. 2021, 6, e144777. [Google Scholar] [CrossRef] [PubMed]
- Nöltner, C.; Bulashevska, A.; Hübscher, K.; Haberstroh, H.; Grimbacher, B.; Proietti, M. Fecal Immunoglobulin Levels as a Modifier of the Gut Microbiome in Patients with Common Variable Immunodeficiency. . Clin. Immunol. 2023, 43, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Franco-Esquivias, A.P.; Peña, C.G.; Torres-Lozano, C.; Vaca-Paniagua, F.; Díaz-Velásquez, C.; Ortega-Cisneros, M.; Quintero-Ramos, A. Gut microbiota in Mexican patients with common variable immunodeficiency. Gac. Med. Mex. 2019, 155, 447–452. [Google Scholar] [CrossRef]
- Bosák, J.; Lexa, M.; Fiedorová, K.; Gadara, D.C.; Micenková, L.; Spacil, Z.; Litzman, J.; Freiberger, T.; Šmajs, D. Patients With Common Variable Immunodeficiency (CVID) Show Higher Gut Bacterial Diversity and Levels of Low-Abundance Genes Than the Healthy Housemates. Front. Immunol. 2021, 12, 671239. [Google Scholar] [CrossRef]
- Chang, H.W.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmak, D.; Lee, K.; Afifi, L.; Fadrosh, D.; Leech, J.; et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018, 6, 154. [Google Scholar] [CrossRef]
- Roels, E.; Taminiau, B.; Darnis, E.; Neveu, F.; Daube, G.; Clercx, C. Comparative analysis of the respiratory microbiota of healthy dogs and dogs with canine idiopathic pulmonary fibrosis. In Proceedings of the 3rd FARAH Day 2016, Liège, Belgium, 21 October 2016; Faculté de Médecine vétérinaire de l’Université de Liège: Liège, Belgium, 2016. [Google Scholar]
- Gupta, S.; Shariff, M.; Chaturvedi, G.; Sharma, A.; Goel, N.; Yadav, M.; Mortensen, M.S.; Sørensen, S.J.; Mukerji, M.; Chauhan, N.S. Comparative analysis of the alveolar microbiome in COPD, ECOPD, Sarcoidosis, and ILD patients to identify respiratory illnesses specific microbial signatures. Sci. Rep. 2021, 11, 3963. [Google Scholar] [CrossRef]
- Puiu, R.; Motoc, N.S.; Lucaciu, S.; Ruta, M.V.; Rajnoveanu, R.M.; Todea, D.A.; Man, M.A. The Role of Lung Microbiome in Fibrotic Interstitial Lung Disease-A Systematic Review. Biomolecules 2024, 14, 247. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Moodley, Y.; Yagnik, L.; Birnie, D.; Dwivedi, G. The role of the host-microbiome and metabolomics in sarcoidosis. Am. J. Physiol. Cell Physiol. 2023, 325, C1336–C1353. [Google Scholar] [CrossRef]
- Man, M.A.; Ungur, R.A.; Motoc, N.S.; Pop, L.A.; Berindan-Neagoe, I.; Ruta, V.M. Lung Microbiota in Idiopathic Pulmonary Fibrosis, Hypersensitivity Pneumonitis, and Unclassified Interstitial Lung Diseases: A Preliminary Pilot Study. Diagnostics 2023, 13, 3157. [Google Scholar] [CrossRef]
- Goleva, E.; Jackson, L.P.; Harris, J.K.; Robertson, C.E.; Sutherland, E.R.; Hall, C.F.; Good JTJr Gelfand, E.W.; Martin, R.J.; Leung, D.Y. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1193–1201. [Google Scholar] [CrossRef]
- Versi, A.; Ivan, F.X.; Abdel-Aziz, M.I.; Bates, S.; Riley, J.; Baribaud, F.; Kermani, N.Z.; Montuschi, P.; Dahlen, S.E.; Djukanovic, R.; et al. U-BIOPRED consortium. Haemophilus influenzae and Moraxella catarrhalis in sputum of severe asthma with inflammasome and neutrophil activation. Allergy 2023, 78, 2906–2920. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, A.; Kerschbaum, J.; Mayer, G. The Influence and Role of Microbial Factors in Autoimmune Kidney Diseases: A Systematic Review. Immunol. Res. 2015, 2015, 858027. [Google Scholar] [CrossRef] [PubMed]
- Ó Cuív, P.; de Wouters, T.; Giri, R.; Mondot, S.; Smith, W.J.; Blottière, H.M.; Begun, J.; Morrison, M. The gut bacterium and pathobiont Bacteroides vulgatus activates NF-κB in a human gut epithelial cell line in a strain and growth phase dependent manner. Anaerobe 2017, 47, 209–217. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- English, J.; Patrick, S.; Stewart, L.D. The potential role of molecular mimicry by the anaerobic microbiota in the aetiology of autoimmune disease. Anaerobe 2023, 80, 102721. [Google Scholar] [CrossRef]
- Cabanero-Navalon, M.D.; Garcia-Bustos, V.; Forero-Naranjo, L.F.; Baettig-Arriagada, E.J.; Núñez-Beltrán, M.; Cañada-Martínez, A.J.; Forner Giner, M.J.; Catalán-Cáceres, N.; Martínez Francés, M.; Moral Moral, P. Integrating Clinics, Laboratory, and Imaging for the Diagnosis of Common Variable Immunodeficiency-Related Granulomatous-Lymphocytic Interstitial Lung Disease. Front. Immunol. 2022, 13, 813491. [Google Scholar] [CrossRef]
- Hartono, S.; Motosue, M.S.; Khan, S.; Rodriguez, V.; Iyer, V.N.; Divekar, R.; Joshi, A.Y. Predictors of granulomatous lymphocytic interstitial lung disease in common variable immunodeficiency. Ann. Allergy Asthma Immunol. 2017, 118, 614–620. [Google Scholar] [CrossRef]
- Mannina, A.; Chung, J.H.; Swigris, J.J.; Solomon, J.J.; Huie, T.J.; Yunt, Z.X.; Truong, T.Q.; Brown, K.K.; Achcar, R.D.; Olson, A.L.; et al. Clinical Predictors of a Diagnosis of Common Variable Immunodeficiency-related Granulomatous-Lymphocytic Interstitial Lung Disease. Ann. Am. Thorac. Soc. 2016, 13, 1042–1049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabanero-Navalon, M.D.; Carda-Diéguez, M.; Moral Moral, P.; Mira, A.; Balastegui-Martin, H.; Salavert-Lletí, M.; Garcia-Bustos, V. Linking Microbiota Profiles to Disease Characterization in Common Variable Immunodeficiency: The Case of Granulomatous–Lymphocytic Interstitial Lung Disease. Biomedicines 2024, 12, 2239. https://doi.org/10.3390/biomedicines12102239
Cabanero-Navalon MD, Carda-Diéguez M, Moral Moral P, Mira A, Balastegui-Martin H, Salavert-Lletí M, Garcia-Bustos V. Linking Microbiota Profiles to Disease Characterization in Common Variable Immunodeficiency: The Case of Granulomatous–Lymphocytic Interstitial Lung Disease. Biomedicines. 2024; 12(10):2239. https://doi.org/10.3390/biomedicines12102239
Chicago/Turabian StyleCabanero-Navalon, Marta Dafne, Miguel Carda-Diéguez, Pedro Moral Moral, Alex Mira, Héctor Balastegui-Martin, Miguel Salavert-Lletí, and Victor Garcia-Bustos. 2024. "Linking Microbiota Profiles to Disease Characterization in Common Variable Immunodeficiency: The Case of Granulomatous–Lymphocytic Interstitial Lung Disease" Biomedicines 12, no. 10: 2239. https://doi.org/10.3390/biomedicines12102239




