Abstract
Background/Objectives: We aimed to investigate the genetic loci related to disc hemorrhage (DH) and the relationship of causation between DH and primary open-angle glaucoma (POAG) using a genome-wide association study (GWAS) in East Asian individuals. Methods: The GWAS included 8488 Koreans who underwent ocular examination including fundus photography to determine the presence of DH and POAG. We performed a GWAS to identify significant single-nucleotide polymorphisms (SNPs) associated with DH and analyzed the heritability of DH and genetic correlation between DH and POAG. The identified SNPs were utilized as instrumental variables (IVs) for two-sample Mendelian randomization (MR) analysis. The POAG outcome dataset was adopted from Biobank Japan data (n = 179,351). Results: We found that the rs62463744 (TMEM270;ELN), rs11658281 (CCDC42), and rs77127203 (PDE10A;LINC00473) SNPs were associated with DH. The SNP heritability of DH was estimated to be 6.7%, with an absence of a genetic correlation with POAG. MR analysis did not reveal a causal association between DH and POAG for East Asian individuals. Conclusions: The novel loci underlying DH in the Korean cohort revealed SNPs in the ELN, CCDC41, and LINC00473 genes. The absence of a causal association between DH and POAG implies that DH is a shared risk factor, rather than an independent culprit factor, and warrants further investigation.
1. Introduction
Glaucoma is a chronic progressive optic neuropathy that results in a typical optic nerve head (ONH) appearance and accompanying visual field (VF) loss [1]. Posterior displacement of the lamina cribrosa (LC) with blockade of axoplasmic flow are the putative features of primary open-angle glaucoma (POAG) [2,3,4,5,6,7,8]; however, its precise pathogenesis remains unknown. Elevated intraocular pressure (IOP) is a significant risk factor for POAG, demonstrated by the fact that lowering IOP is the only effective treatment for glaucoma [9]. Although glaucoma progression can occur despite a significant reduction in IOP [10,11,12], the IOP-related mechanism does not completely explain glaucoma pathogenesis [13]. Moreover, previous studies have demonstrated that various factors beyond IOP are implicated in the pathophysiology of glaucoma, disc hemorrhage (DH) being one such factor [14,15,16,17].
DH is a characteristic of glaucomatous optic neuropathy, which is commonly observed in low-pressure glaucoma and is uncommon in normal eyes [18,19]. Previous studies have reported DH in approximately 7.1–20% of the cases with POAG and have found it to be a precursor of glaucomatous disc changes and associated VF defects [19,20]. Additionally, a recent randomized clinical trial confirmed that DH is a risk factor for the development and progression of glaucoma [21]. Prior studies have shown that eyes with DH had greater VF deterioration than those without DH [22,23] and that recurrent DH is associated with VF deterioration [24]. Moreover, eyes with DH had a higher rate of retinal nerve fiber layer (RNFL) atrophy and conversion to first VF loss [25]. Furthermore, the Cox hazard models evaluating the risk of exposure during a particular time period support the status of DH as a risk factor for glaucoma [26]. Nonetheless, the causal association between DH and glaucoma remains ambiguous. DH is detected in early ONH changes and VF damage, and IOP-lowering medication is effective in preventing or delaying progressive VF loss. In contrast, a recent report from the Early Manifest Glaucoma Trial revealed the lack of a significant association between IOP-lowering treatment and the occurrence of DH [27]. Conversely, the identification of RNFL loss or progression prior to DH detection serves as compelling evidence of an associated finding for DH [28,29]. However, a recent study on VF showed that DH was associated with the presence and progression of central VF defect [30].
Given that the pathogenesis of DH has not been completely elucidated, identifying risk factors associated with DH may contribute to our understanding of the mechanisms involved in its occurrence. Previous studies have indicated a potential correlation between mechanical damage to the blood vessels at the LC tissue or the edge of the RNFL defect and the development of DH [31,32]. In addition, features of DH, including the retro-bulbar mechanism [33,34,35], should be investigated with respect to a causal association between DH and POAG.
In recent years, the integration of bioinformatics and statistical methodologies in the analysis of genetic data, alongside epidemiological data, has facilitated the emergence of Mendelian randomization (MR) analysis [36]. This strategy of genetic epidemiology employs genetic variants that are linked to risk factors as instrumental variables (IVs) to investigate their causal impact on diseases and outcomes [37,38,39,40,41,42,43,44,45,46]. Thus, it is possible to investigate whether DH is a causal factor of POAG, using MR analysis. Notably, the Primary Open-Angle African American Glaucoma Genetics study did not associate the LMX1B gene with DH, according to the few studies that have looked into gene-related DH [47]. Consequently, we aimed to conduct a genome-wide association study (GWAS) in a Korean population cohort to identify unknown DH-related SNPs. Additionally, a two-sample MR analysis was performed to assess DH’s effect on POAG using the DH-related SNPs identified in our study as exposures, along with a GWAS summary of Biobank Japan data (n = 179,351) as the outcome dataset [48].
2. Materials and Methods
2.1. Study Design
The research protocol was approved by the Seoul National University Hospital (SNUH) Institutional Review Board (IRB No. H-1505-047-671 and IRB No. H-1804-039-935) and the Veterans Health Service Medical Center (IRB No. 2022-03-034) and conducted in accordance with the tenets of the Declaration of Helsinki. Schematic plots of the analytical study design are illustrated in Figure 1. Data were obtained from the GENIE (Gene-Environmental Interaction and Phenotype; Seoul National University Hospital Healthcare System Gangnam Center, n = 10,579) cohort [49,50,51]. A case group which included patients with DH was identified in the GENIE cohort by an ophthalmologist (HJC) using fundus photography. Since DH is transient, lasting for about 3 months, and POAG can occur independent of DH, DH detection is not always feasible. Given the high correlation between POAG and DH, it is necessary to define a control group with precision. Therefore, a control group was established wherein the absence of DH was detected using fundus photography and there was no evidence of POAG. Moreover, POAG was defined as the presence of glaucomatous optic disc changes and RNFL defect [51]. Glaucomatous optic disc changes were defined as vertical cup-to-disc ratio (C/D) > 0.7, neuroretinal rim thinning (superior or inferior rim width <0.1 times disc diameter), notching, or excavation. The baseline IOP value was defined as the mean of at least two measurements before the initiation of IOP-lowering treatment. Patients with missing genotype data or missing fundus photography images, with RNFL defects and non-glaucomatous optic disc changes (n = 152), and participants with uveitis history or diseases affecting the VF (stroke, Alzheimer’s diseases, and dementia) were excluded from this study. Participants with a diagnosis or history of any secondary glaucoma, a history of ocular trauma, a history of systemic or ocular infection, or a history of systemic or ocular use of glucocorticoids were also excluded.
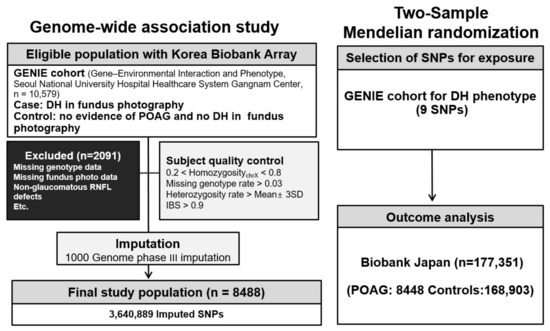
Figure 1.
Diagram presentation of the study design. POAG, primary open-angle glaucoma; DH, disc hemorrhage; GENIE cohort, Gene-Environmental Interaction and Phenotype; SNP, single-nucleotide polymorphism.
2.2. Genotyping
We utilized genome-wide variants genotyped with the Korea Biobank array (KoreanChip), which was developed by the Center for Genome Science at the Korea National Institutes of Health using the Affymetrix Axiom® Array (Affymetrix, Santa Clara, CA, USA). Additionally, we used SHAPEIT2 v2.r904 and IMPUTE2 version 2.3.2 for haplotype phasing and imputation, respectively [52,53]. The 1000 Genomes Phase III data were used as a reference panel for imputation. Any variant with genotype call rates < 95%, minor allele frequency (MAF) values < 0.05, or in violation of the Hardy-Weinberg equilibrium (p < 1 × ) was removed. Only SNPs with quality scores > 0.5 were retained, resulting in 3,640,889 SNPs in the GENIE cohort. The National Center for Biotechnology Information Human Genome Build 37 (hg19) was used to confirm gene locations (Figure 1).
2.3. Genome-Wide Association Study
The GWAS for DH was conducted using logistic regression with an additive model, PLINK 1.9. Age, sex, and ten principal component scores were included as covariates. The quantile-quantile (Q-Q) and Manhattan plots and the regional plot with LocusZoom software version 1.3 were generated for GWAS results [54].
2.4. Selection of the Genetic Instrumental Variables for Mendelian Randomization
SNPs associated with DH at the threshold value (p < 1.0 × ) were utilized as IVs. SNPs were clumped using linkage disequilibrium (LD) with r2 < 0.001 within 10,000 kb to ensure the independence of IVs. The East Asian dataset from 1000 genomes phase III was utilized as the reference panel for computing LD for the clumping process. F-values were utilized to evaluate the strengths of genetic IVs using the formula F = R2(n − 2)/(1 − R2), where n was the sample size and R2 was the proportion of variance in exposure by the genetic variants [55]. F values > 10 were regarded as ‘no evidence of weak instrument bias’ [56].
2.5. Statistics for Mendelian Randomization
The MR study was based on the following assumptions for IVs: (1) they ought to reveal an essential connection in relation to exposure, (2) they must have no association with the confounds of the exposure-outcome connection, and (3) they should only impact results via exposure, indicating that there is no directional horizontal pleiotropy effect. We employed inverse-variance weighted (IVW) MR with multiplicative random effects as the main method [45,56,57]. This approach is most efficient when all genetic variants meet the three conditions for IVs [58]. However, if one or more of the variants are not valid, the estimate of the IVW analysis may be skewed [59]. Additionally, the weighted median [59], MR-Egger (with or without adjustment via the Simulation Extrapolation [SIMEX] method) regression [60,61], and the MR pleiotropy residual sum and outlier (MR-PRESSO) [62] were utilized. The weighted median method generates precise calculations of causality even when half of the instruments are erroneous [59]. The MR-Egger technique permits a suitable calculation of causal impacts regardless of a setting of pleiotropic effects, allowing for a non-zero intercept which clearly displays the average horizontal pleiotropic effects [60]. The MR-Egger with SIMEX can be utilized to rectify the bias when the presumption of no measurement error is violated [61]. The MR-PRESSO test, which detects outliers, adjusts the IVW analysis results for horizontal pleiotropy by eliminating the outliers [62]. Cochran’s Q and Rücker’s Q′ statistics were employed to evaluate the heterogeneity of IVW and MR-Egger [57,63]. Directional horizontal pleiotropy was evaluated via the MR-PRESSO global test. Hence, the results were interpreted according to the appropriate MR analysis method [64]. p < 0.05 for Cochran’s Q statistic, Rücker’s Q′ statistic, and MR–PRESSO global test indicated potential pleiotropy in the genetic variations. All analyses were conducted using the ‘TwoSampleMR’ and ‘Simex’ packages in R version 3.6.3 (R Core Team, Vienna, Austria).
3. Results
3.1. Characteristics of the Study Participants
Demographic data are presented in Table 1. The median age of the study population was 54.0 years in the DH group and 52.0 years in the control group, with no significant differences (p = 0.304, Table 1). The IOPs in the DH group were significantly higher than those in the control group (p < 0.05). However, the weight, body mass index, systolic blood pressure, and diastolic blood pressure were not significantly different between the DH and control groups (all p > 0.05). In addition, no discernible disparity was noted in the frequencies of comorbidities such as diabetes and hypertension and laboratory blood examinations such as hemoglobin A1c, fasting glucose level, and lipid level (all p > 0.05, Table 1).

Table 1.
Baseline characteristics of enrolled patients.
3.2. Genome-Wide Association Study
The Q-Q plot showed no inflation (Figure 2A). While no SNPs reached genome-wide significance in the GWAS, SNPs with p-values below the threshold of 1 × 10−5 are listed in Table 2. SNPs such as rs62463744 (TMEM270;ELN), rs11658281(CCDC42), rs77127203 (PDE10A;LINC00473), and rs7589033 (THADA) were linked to DH (Table 2 and Figure 2). In addition, Figure 3 presents the regional association plots for the top four SNPs, showing the locations of all candidate genes within the region. The heritability of the SNPs was 6.7% with GIF = 0.993 (Table 3); however, no genetic correlation was observed between POAG and DH (p = 0.373).
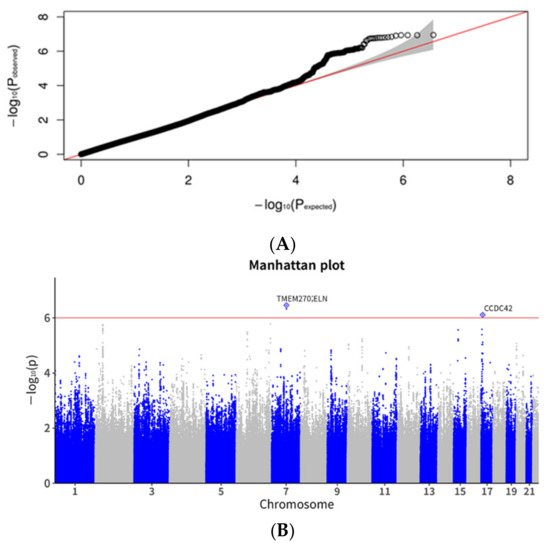
Figure 2.
Quantile-quantile and Manhattan plots for disc hemorrhage in the genome-wide association study. (A). Quantile-quantile (Q-Q) plot. The expected line is shown in red and confidence bands are shown in gray. (B). Manhattan plot. The red line indicates the preset threshold of p = 1.0 × 10−6.

Table 2.
SNPs reaching suggestive significance at p < 1 × 10−5 in GWAS.
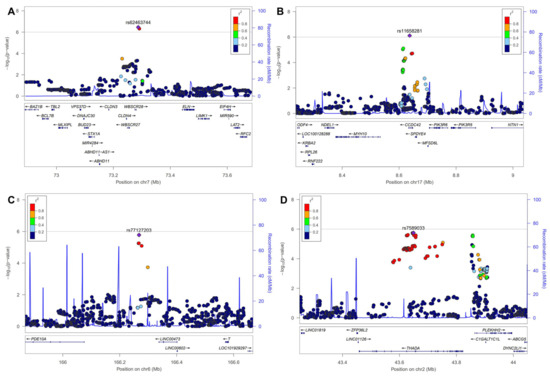
Figure 3.
Regional association plots for top 4 SNPs. SNP, single-nucleotide polymorphism. (A): rs62463744, (B): rs11658281, (C): rs77127203, (D): rs7589033.

Table 3.
Estimated heritability and genetic correlation.
3.3. Mendelian Randomization
Nine SNPs with significance level p < 1.0 × were selected as DH-related IVs (Figure 4 and Supplementary Table S1). The mean F statistics for DH (21.73) used for MR were >10, displaying that there was a low opportunity of fragile instrument bias (Table 4).
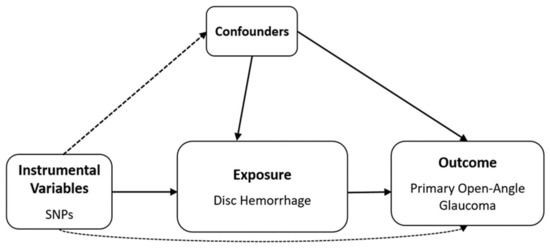
Figure 4.
Schematic design of Mendelian randomization analysis. SNP, single-nucleotide polymorphism. Solid lines indicate the presence of an association, dashed lines indicate the absence of an association.

Table 4.
Heterogeneity and horizontal pleiotropy of the instrumental variables.
Detailed information on the IVs used is listed in Supplementary Table S1. IVW was used as primary method because the IVs for DH were not heterogeneous in Cochran’s Q test p > 0.05) (Table 4). In addition, Rücker’s Q′ test from MR–Egger revealed no heterogeneity between IVs, and the MR–Egger regression intercepts showed no horizontal pleiotropic effect before (p > 0.05) and after SIMEX adjustment (p > 0.05), indicating the absence of a pleiotropic effect (Table 4). Additionally, the MR–PRESSO global test demonstrated no horizontal pleiotropy (p > 0.05; Table 4). DH did not show a significant causal association with POAG in all MR analyses for the East Asian population [IVW MR OR = 1.00, 95% confidence intervals (CIs): 0.99–1.01, p = 0.625, weighted median MR OR = 1.00, 95% CI: 0.98–1.02, p = 0.766, MR–Egger MR OR = 1.09, 95% CI: 0.87–1.35, p = 0.476, and MR–Egger (SIMEX) MR OR = 1.26, 95% CI: 0.93–1.70, p = 0.182, Figure 5].
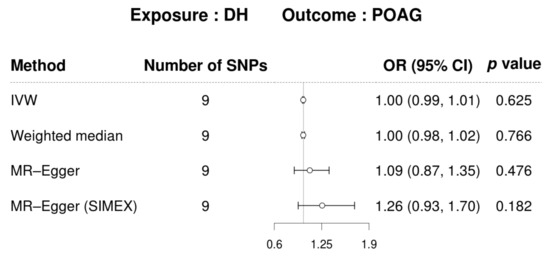
Figure 5.
MR visualizations of the effect of DH on POAG. CI, confidence interval; DH, disc hemorrhage; POAG, primary open-angle glaucoma; OR, odds ratio; SIMEX, simulation extrapolation; SNP, single-nucleotide polymorphism.
A scatter plot displays the genetic associations of DH against genetic associations with POAG for each SNP (Figure 6).
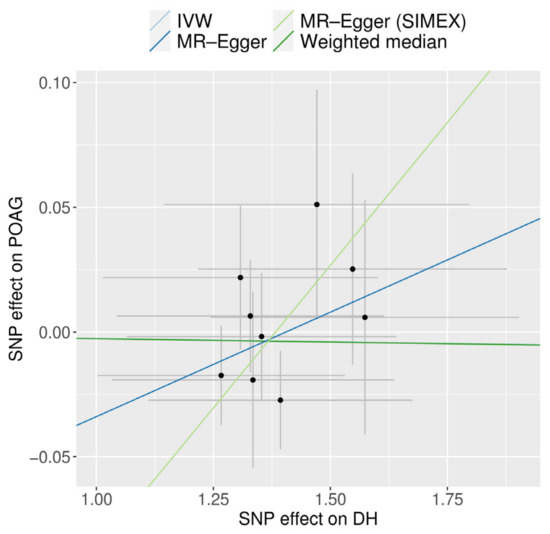
Figure 6.
Scatter plot of MR analyses using different MR methods evaluating the impact of the existence of DH on POAG. Light blue, dark blue, light green, and dark green regression lines represent IVW, MR-Egger, MR-Egger (SIMEX), and weighted median estimate, respectively. SNP, single-nucleotide polymorphism; DH, disc hemorrhage; POAG, primary open-angle glaucoma; MR, Mendelian randomization; SIMEX, simulation extrapolation.
4. Discussion
In the present study, we performed a GWAS to assess the effect of DH on POAG in the Korean population and identified several novel candidate loci (rs62463744 in TMEM270;ELN, rs11658281 in CCDC42, rs77127203 in PDE10A;LINC00473, rs7589033 in THADA) related to DH. The heritability of DH was estimated to 6.7%, with an absence of a genetic correlation with POAG. Additionally, the MR analysis showed that a causal association between DH and POAG was not present in the East Asian population.
Several studies have suggested that DH is a risk factor for POAG, since it has a strong association with glaucoma. However, a “causal” risk factor with further supporting data is necessary to establish a significant association between an undocumented exposure and an outcome and is known as a correlate of the outcome or a risk marker [35]. As microinfarctions and ischemic changes can make capillaries more vulnerable to rupture [65], DH is considered a sign of ischemic optic nerve damage. In addition, systemic hypertension or hypotension, diabetes, migraine, or medication, including platelet aggregation inhibitors, have been suggested to be associated with DH [66]. Stretching of the vessels from posterior migration of the LC [2] and vessel damage due to mechanical collapse have also been suggested as possible causes. Additionally, the hypotheses that 1) the presence of DH at the affected LC is not consistently observed in nearby regions [67] and 2) the detection of DH occurs after to the advancement of glaucoma [35] raise concerns regarding the potential causal relationship between DH and glaucoma. Therefore, further investigation is necessary to address these hypotheses.
GWAS is a powerful tool that allows us to scan the genome comprehensively to identify genetic variants associated with traits or diseases [68]. Thus, we identified specific genetic markers that contribute to the risk of DH, providing a genetic foundation which not only enhances our understanding of the biological pathways involved but also helps in identifying potential targets for therapeutic intervention. MR uses genetic variants as IVs to estimate the causal effect of an exposure (DH) on an outcome (POAG) while minimizing confounding and bias, which are typical in observational studies. Consequently, MR allows us to infer causality by mimicking the conditions of a randomized controlled trial by leveraging the genetic variants associated with DH identified from our GWAS as tools. This approach is particularly useful in understanding whether the pathways leading to DH contribute causally to the development of POAG, rather than simply being associated with it. To date, several GWAS studies have identified various genetic loci and risk factors that are associated with glaucoma [69,70]. DH is often observed in the ONH in patients with POAG and is considered an important clinical sign of glaucoma progression. While GWAS studies have explored the genetics of glaucoma, limited studies have specifically evaluated the genetics of DH in patients with glaucoma. This may be due to the challenge of obtaining large enough sample sizes of patients with DH, as it is a relatively rare event in patients with glaucoma, as well as a limited phenotype, since a large genetic cohort does not contain phenotypes such as DH. Notably, the GWAS for DH highlighted the involvement of rs62463744 (TMEM270;ELN), rs11658281 (CCDC42), rs77127203 (PDE10A;LINC00473), and rs7589033 (THADA) as SNPs linked to DH. The identification of these genetic markers underscores the multifactorial nature of DH and suggests a genetic predisposition that may contribute to its pathogenesis.
The rs62463744 SNP is present in the TMEM270 and ELN (Elastin) genes. The ELN gene encodes a protein elastin fiber which is present in the extracellular matrix and provides elasticity to tissues, including the heart, skin, and blood vessels [71]. Additionally, it provides recoil tissue for vascular elasticity [72]. Consequently, genetic mutations in the ELN gene that reduce elastin protein levels are associated with focal arterial stenosis or narrowing of the arterial lumen. Although the genetic analysis of DH is limited, the presence of a specific variant of the ELN gene has been found to indicate a heightened vulnerability among to the development of intracranial aneurysms in individuals of East Asian descent [73,74]. According to previous studies showing abnormal elastin synthesis in an experimental model glaucomatous optic neuropathy in monkeys, this variant is specific to elevated IOP and not secondary to axonal loss [75]. These abnormalities in elastin may be related to the development and progression of glaucoma in patients with DH. However, as one previous study reported a lack of association of polymorphisms in elastin with pseudo exfoliation syndrome and glaucoma [76], these results suggest that ELN may be associated with DH as vascular factor. In addition, the TMEM270 gene (Transmembrane Protein 270) is related to hemorrhage and aortic measurement. The rs11658281 SNP is present in the CCDC42 (Coiled-coil domain-containing protein 42) gene and is related to sex hormones, type 2 diabetes, and vision disorders. The rs77127203 SNP is related to PDE10A (Phosphodiesterase 10A) and LINC00473 (Long Intergenic Non-Protein Coding RNA 473) genes. PDE10A plays a role in signal transduction by regulating the intracellular concentration of cyclic nucleotides, which is associated with platelet count, blood pressure, thyroid function, refractive errors, and cataract. In addition, the LINC00473 gene, affiliated with the lncRNA class, is related to some phenotypes, such as mean arterial pressure, systolic blood pressure, and platelet count. The rs7589033 SNP is in the THADA (THADA Armadillo Repeat Containing) gene and encodes a protein which is likely involved in the death receptor pathway and apoptosis. The THADA gene is related to phenotypes such as platelet count and type 2 diabetes. According to a previous study [77], the THADA gene is related with IOP in glaucoma GWASs.
These (TMEM270, ELN, CCDC42, PDE10A, and LINC00473) genes associated with DH are involved in various cellular processes, including vascular regulation, extracellular matrix maintenance, and intracellular signaling pathways. Therefore, elucidating how variations in these genes contribute to the vulnerability of the optic nerve head and retinal vasculature could enhance our understanding of the mechanistic links between DH and glaucoma. Moreover, the genetic analysis of DH and glaucoma has the potential to inform personalized medicine or targeted intervention. Vascular management intervention in addition to IOP-lowering treatment may be considered when this customized treatment has more evidence and a higher propensity to be associated with vascular factors. Thus, identifying individuals with a genetic predisposition to DH and glaucoma may enable earlier intervention and more targeted treatment strategies. Additionally, it may facilitate the development of novel therapeutic interventions aimed at mitigating the genetic risk factors associated with these conditions.
One of the primary features of our work is the comprehensive characterization of a DH phenotype, which was achieved through the analysis of phenotypes acquired from a standardized fundus examination undertaken at a single institution by an ophthalmologist. Unvalidated participants were not included as the control group, and the phenotype was defined strictly. Furthermore, given that Koreans are classified as a homogeneous ethnic group and that the MR analysis was conducted using Japanese data, it is anticipated that the impact of racial disparities would be minimal for East Asian individuals. Nevertheless, a notable constraint lies in the limited scope of the replication cohort due to the absence of comparable investigations. Additionally, our study exclusively involved East Asian individuals; therefore, the genetic variants identified may have different allele frequencies or effects in other populations due to genetic diversity and environmental factors. Although this focus allows for a clearer understanding of genetic predispositions within this demographic, it indeed limits the immediate applicability of our findings to other ethnic groups. Such population-specific results might not capture genetic variants influential in other ethnicities, potentially missing broader genetic insights related to DH and POAG. Therefore, we suggest that further studies be conducted in diverse populations to either confirm or refine our findings. Additionally, cross-population studies could be employed to explore the genetic architecture across different ethnic groups, enhancing the understanding of how these findings can be applied globally. Furthermore, it is important to note that the proof of causality is constrained by the restricted availability of significant IVs. However, given the absence of prior GWAS research on DH and causation verification of DH and POAG, the anticipated outcome of this study on DH will contribute to the fundamental understanding of the risk variables associated with POAG, albeit with certain limitations.
5. Conclusions
We identified candidate novel genetic loci that were related to DH. This genetic analysis of DH and glaucoma provides valuable insights into the complex interplay of genetic factors in the pathogenesis of these ocular disorders. In addition, the non-significant causal association between DH and POAG implies that the role of DH as an indicator of glaucoma-related phenomenon rather than a risk factor for glaucoma, supported by researchers and increasing evidence. Several studies have reported that DH is a risk factor for glaucoma and glaucoma progression [21,78]; however, the results of our study suggest that DH is a biomarker for glaucoma as a shared risk factor rather than an independent culprit factor. However, further research is warranted to validate these genetic associations, unravel the molecular mechanisms involved, and translate these findings into clinical applications for more effective management and personalized care in individuals at risk for DH and glaucoma. In addition, covariate analysis of factors related to DH is necessary to determine whether it is a fundamental risk factor for glaucoma.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines12102253/s1, Supplementary Table S1. Instrumental variables for Mendelian randomization for disc hemorrhage and glaucoma.
Author Contributions
Conceptualization, Y.L., H.J.C. and J.H.S.; methodology, Y.L.; software, Y.L.; validation, Y.L., H.J.C. and J.H.S.; formal analysis, Y.L.; investigation, Y.L., H.J.C. and J.H.S.; resources, Y.L., H.J.C. and J.H.S.; data curation, Y.L., H.J.C. and J.H.S.; writing—original draft preparation, Y.L., H.J.C. and J.H.S.; writing—review and editing, Y.L., H.J.C. and J.H.S.; visualization, Y.L.; supervision, H.J.C.; funding acquisition, Y.L. and J.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (Ministry of Science and ICT) (No. 2022R1C1C1002929) and a Veterans Health Service Medical Center Research Grant (grant no.: VHSMC24002). The funders had no role in the study design, collection, analyses, interpretation of data, writing of the manuscript, or decision to publish the results.
Institutional Review Board Statement
This study protocol was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-1505-047-671, 29 June 2015) for the primary GENIE cohort. An informed consent waiver (IRB No. H-1804-039-935, 9 April 2018) was approved since this study was performed in a retrospective manner. This study was conducted in compliance with the Helsinki Declaration. The institutional review board of the Veterans Health Service Medical Center approved the study protocol (IRB No. 2022-03-034, 21 April, 2022).
Informed Consent Statement
A written informed consent form was obtained by each patient before starting this study (IRB No. 1612-093-815) for the primary GENIE cohort. The Institutional Review Board of Seoul National University Hospital approved an additional informed consent waiver (IRB No. H-1804-039-935) since this study was performed in a retrospective manner. This study was conducted in compliance with the Helsinki Declaration.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from Biobank Japan (BBJ; https://pheweb.jp/) [43]. The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
Biobank Japan (BBJ https://pheweb.jp/, accessed on 27 October 2022) [43]; GWAS catalogue (https://www.ebi.ac.uk/gwas/summary-statistics, accessed on 31 October 2022).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Addicks, E.M.; Green, W.R.; Maumenee, A.E. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Hohman, R.M.; Addicks, E.M.; Massof, R.W.; Green, W.R. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am. J. Ophthalmol. 1983, 95, 673–691. [Google Scholar] [CrossRef]
- Jonas, J.B.; Berenshtein, E.; Holbach, L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5189–5195. [Google Scholar] [CrossRef]
- Jonas, J.B.; Berenshtein, E.; Holbach, L. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2660–2665. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Kim, T.W.; Weinreb, R.N. Lamina cribrosa depth in healthy eyes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Strouthidis, N.G.; Fortune, B.; Yang, H.; Sigal, I.A.; Burgoyne, C.F. Effect of acute intraocular pressure elevation on the monkey optic nerve head as detected by spectral domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9431–9437. [Google Scholar] [CrossRef] [PubMed]
- Fatehee, N.; Yu, P.K.; Morgan, W.H.; Cringle, S.J.; Yu, D.Y. The impact of acutely elevated intraocular pressure on the porcine optic nerve head. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6192–6198. [Google Scholar] [CrossRef]
- Musch, D.C.; Gillespie, B.W.; Lichter, P.R.; Niziol, L.M.; Janz, N.K.; Investigators, C.S. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology 2009, 116, 200–207. [Google Scholar] [CrossRef]
- Leske, M.C. Ocular perfusion pressure and glaucoma: Clinical trial and epidemiologic findings. Curr. Opin. Ophthalmol. 2009, 20, 73–78. [Google Scholar] [CrossRef]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000, 130, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1998, 126, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.S.; Suelves, A.M.; Baheti, U.; Foster, C.S. Glaucoma and uveitis. Surv. Ophthalmol. 2013, 58, 1–10. [Google Scholar] [CrossRef]
- Drance, S.M. Disc hemorrhages in the glaucomas. Surv. Ophthalmol. 1989, 33, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C.; Heijl, A.; Hussein, M.; Bengtsson, B.; Hyman, L.; Komaroff, E.; Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch. Ophthalmol. 2003, 121, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Budenz, D.L.; Anderson, D.R.; Feuer, W.J.; Beiser, J.A.; Schiffman, J.; Parrish, R.K., II; Piltz-Seymour, J.R.; Gordon, M.O.; Kass, M.A.; Ocular Hypertension Treatment Study Group. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology 2006, 113, 2137–2143. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Alencar, L.M.; Sample, P.A.; Zangwill, L.M.; Susanna, R., Jr.; Weinreb, R.N. The relationship between intraocular pressure reduction and rates of progressive visual field loss in eyes with optic disc hemorrhage. Ophthalmology 2010, 117, 2061–2066. [Google Scholar] [CrossRef]
- Drance, S.M.; Begg, I.S. Sector haemorrhage—A probable acute ischaemic disc change in chronic simple glaucoma. Can. J. Ophthalmol. 1970, 5, 137–141. [Google Scholar]
- Sonnsjo, B.; Dokmo, Y.; Krakau, T. Disc haemorrhages, precursors of open angle glaucoma. Prog. Retin. Eye Res. 2002, 21, 35–56. [Google Scholar] [CrossRef]
- Ozturker, Z.K.; Munro, K.; Gupta, N. Optic disc hemorrhages in glaucoma and common clinical features. Can. J. Ophthalmol. 2017, 52, 583–591. [Google Scholar] [CrossRef]
- Suh, M.H.; Park, K.H. Pathogenesis and clinical implications of optic disk hemorrhage in glaucoma. Surv. Ophthalmol. 2014, 59, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Shihab, Z.M.; Lee, P.F.; Hay, P. The significance of disc hemorrhage in open-angle glaucoma. Ophthalmology 1982, 89, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Drance, S.; Anderson, D.R.; Schulzer, M.; Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am. J. Ophthalmol. 2001, 131, 699–708. [Google Scholar] [CrossRef] [PubMed]
- An, D.; House, P.; Barry, C.; Turpin, A.; McKendrick, A.M.; Chauhan, B.C.; Manners, S.; Graham, S.; Yu, D.Y.; Morgan, W.H. Recurrent Optic Disc Hemorrhage and Its Association with Visual Field Deterioration in Glaucoma. Ophthalmol. Glaucoma 2020, 3, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Diehl, D.L.; Quigley, H.A.; Miller, N.R.; Sommer, A.; Burney, E.N. Prevalence and significance of optic disc hemorrhage in a longitudinal study of glaucoma. Arch. Ophthalmol. 1990, 108, 545–550. [Google Scholar] [CrossRef]
- Leske, M.C.; Heijl, A.; Hyman, L.; Bengtsson, B.; Dong, L.; Yang, Z.; EMGT Group. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007, 114, 1965–1972. [Google Scholar] [CrossRef]
- Bengtsson, B.; Leske, M.C.; Yang, Z.; Heijl, A.; EMGT Group. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology 2008, 115, 2044–2048. [Google Scholar] [CrossRef]
- de Beaufort, H.C.; De Moraes, C.G.; Teng, C.C.; Prata, T.S.; Tello, C.; Ritch, R.; Liebmann, J.M. Recurrent disc hemorrhage does not increase the rate of visual field progression. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 839–844. [Google Scholar] [CrossRef]
- Furlanetto, R.L.; De Moraes, C.G.; Teng, C.C.; Liebmann, J.M.; Greenfield, D.S.; Gardiner, S.K.; Ritch, R.; Krupin, T.; Low-Pressure Glaucoma Treatment Study Group. Risk factors for optic disc hemorrhage in the low-pressure glaucoma treatment study. Am. J. Ophthalmol. 2014, 157, 945–952. [Google Scholar] [CrossRef]
- Shukla, A.G.; Sirinek, P.E.; De Moraes, C.G.; Blumberg, D.M.; Cioffi, G.A.; Skaat, A.; Girkin, C.A.; Weinreb, R.N.; Zangwill, L.M.; Hood, D.C.; et al. Disc Hemorrhages Are Associated with the Presence and Progression of Glaucomatous Central Visual Field Defects. J. Glaucoma 2020, 29, 429–434. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, K.H. Lamina cribrosa defects in eyes with glaucomatous disc haemorrhage. Acta Ophthalmol. 2016, 94, e468–e473. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Lee, J.; Jung, Y.; Park, C.K. Optic Disc Hemorrhage and Lamina Cribrosa Defects in Glaucoma Progression. Sci. Rep. 2017, 7, 3489. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Kim, T.W.; Weinreb, R.N.; Kim, Y.A.; Kim, M. Relationship of intraocular pressure and frequency of spontaneous retinal venous pulsation in primary open-angle glaucoma. Ophthalmology 2012, 119, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, T.W.; Weinreb, R.N.; Lee, E.J.; Seo, J.H. Spontaneous retinal venous pulsation and disc hemorrhage in open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2822–2826. [Google Scholar] [CrossRef][Green Version]
- Lee, E.J.; Kee, H.J.; Han, J.C.; Kee, C. Evidence-based understanding of disc hemorrhage in glaucoma. Surv. Ophthalmol. 2021, 66, 412–422. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 2015, 181, 251–260. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, Y. Causal Association between Iritis or Uveitis and Glaucoma: A Two-Sample Mendelian Randomisation Study. Genes 2023, 14, 642. [Google Scholar] [CrossRef]
- Hanyuda, A.; Goto, A.; Nakatochi, M.; Sutoh, Y.; Narita, A.; Nakano, S.; Katagiri, R.; Wakai, K.; Takashima, N.; Koyama, T.; et al. Association Between Glycemic Traits and Primary Open-Angle Glaucoma: A Mendelian Randomization Study in the Japanese Population. Am. J. Ophthalmol. 2022, 245, 193–201. [Google Scholar] [CrossRef]
- Kim, J.; Aschard, H.; Kang, J.H.; Lentjes, M.A.H.; Do, R.; Wiggs, J.L.; Khawaja, A.P.; Pasquale, L.R.; Modifiable Risk Factors for Glaucoma Collaboration. Intraocular Pressure, Glaucoma, and Dietary Caffeine Consumption: A Gene-Diet Interaction Study from the UK Biobank. Ophthalmology 2021, 128, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, F.; Liu, X.; Lin, X.; Yin, H.; Tang, Q.; Jiang, L.; Yao, K. Appraising the Effects of Metabolic Traits on the Risk of Glaucoma: A Mendelian Randomization Study. Metabolites 2023, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhong, Z.; Wang, Q.; Su, G.; Cao, Q.; Kijlstra, A.; Yang, P. Genetically predicted fasting blood glucose level plays a causal role in intraocular pressure: A Mendelian randomisation study. Clin. Exp. Ophthalmol. 2022, 50, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, D.; Huang, Y.; Khawaja, A.P.; Foster, P.J.; Zhu, Z.; Guggenheim, J.A.; He, M. High Blood Pressure and Intraocular Pressure: A Mendelian Randomization Study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 29. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, Y.A.; Seo, J.H. Causal Association of Obesity and Dyslipidemia with Type 2 Diabetes: A Two-Sample Mendelian Randomization Study. Genes 2022, 13, 2407. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, Y. Possible Causal Association between Type 2 Diabetes and Glycaemic Traits in Primary Open-Angle Glaucoma: A Two-Sample Mendelian Randomisation Study. Biomedicines 2024, 12, 866. [Google Scholar] [CrossRef]
- Meer, E.; Qin, V.L.; Gudiseva, H.V.; McGeehan, B.; Salowe, R.; Pistilli, M.; He, J.; Daniel, E.; Ying, G.S.; Chavali, V.R.M.; et al. LMX1B Locus Associated with Low-Risk Baseline Glaucomatous Features in the POAAGG Study. Genes 2021, 12, 1252. [Google Scholar] [CrossRef]
- Sakaue, S.; Kanai, M.; Tanigawa, Y.; Karjalainen, J.; Kurki, M.; Koshiba, S.; Narita, A.; Konuma, T.; Yamamoto, K.; Akiyama, M.; et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021, 53, 1415–1424. [Google Scholar] [CrossRef]
- Lee, C.; Choe, E.K.; Choi, J.M.; Hwang, Y.; Lee, Y.; Park, B.; Chung, S.J.; Kwak, M.S.; Lee, J.E.; Kim, J.S.; et al. Health and Prevention Enhancement (H-PEACE): A retrospective, population-based cohort study conducted at the Seoul National University Hospital Gangnam Center, Korea. BMJ Open 2018, 8, e019327. [Google Scholar] [CrossRef]
- Kim, Y.A.; Yoon, J.W.; Lee, Y.; Choi, H.J.; Yun, J.W.; Bae, E.; Kwon, S.H.; Ahn, S.E.; Do, A.R.; Jin, H.; et al. Unveiling Genetic Variants Underlying Vitamin D Deficiency in Multiple Korean Cohorts by a Genome-Wide Association Study. Endocrinol. Metab. 2021, 36, 1189–1200. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, Y.H.; Kim, J.S.; Lee, J.; Kim, Y.J.; Cheong, H.S.; Kim, S.H.; Park, K.H.; Kim, D.M.; Choi, H.J.; et al. Genetic analysis of primary open-angle glaucoma-related risk alleles in a Korean population: The GLAU-GENDISK study. Br. J. Ophthalmol. 2021, 105, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Delaneau, O.; Marchini, J.; Zagury, J.F. A linear complexity phasing method for thousands of genomes. Nat. Methods 2011, 9, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Marchini, J.; Howie, B.; Myers, S.; McVean, G.; Donnelly, P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 2007, 39, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Pruim, R.J.; Welch, R.P.; Sanna, S.; Teslovich, T.M.; Chines, P.S.; Gliedt, T.P.; Boehnke, M.; Abecasis, G.R.; Willer, C.J. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics 2010, 26, 2336–2337. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G.; Crp Chd Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Holmes, M.V.; Minelli, C.; Relton, C.L.; et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.A.; Thompson, J.R. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 2016, 45, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Publisher Correction: Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 1196. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.F.; Minelli, C.; Sheehan, N.A.; Thompson, J.R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 2015, 34, 2926–2940. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lee, S.; Won, S. Causal Evaluation of Laboratory Markers in Type 2 Diabetes on Cancer and Vascular Diseases Using Various Mendelian Randomization Tools. Front. Genet. 2020, 11, 597420. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.E.; Bonnet, F.; Oldfield, M.; Jandeleit-Dahm, K. Mechanisms of diabetic vasculopathy: An overview. Am. J. Hypertens. 2001, 14, 475–486. [Google Scholar] [CrossRef]
- Lee, E.J.; Han, J.C.; Kee, C. A novel hypothesis for the pathogenesis of glaucomatous disc hemorrhage. Prog. Retin. Eye Res. 2017, 60, 20–43. [Google Scholar] [CrossRef]
- Rasker, M.T.; van den Enden, A.; Bakker, D.; Hoyng, P.F. Deterioration of visual fields in patients with glaucoma with and without optic disc hemorrhages. Arch. Ophthalmol. 1997, 115, 1257–1262. [Google Scholar] [CrossRef]
- Lichou, F.; Trynka, G. Functional studies of GWAS variants are gaining momentum. Nat. Commun. 2020, 11, 6283. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segre, A.V.; Rouhana, J.M.; et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat. Commun. 2021, 12, 1258. [Google Scholar] [CrossRef]
- Wang, Z.; Wiggs, J.L.; Aung, T.; Khawaja, A.P.; Khor, C.C. The genetic basis for adult onset glaucoma: Recent advances and future directions. Prog. Retin. Eye Res. 2022, 90, 101066. [Google Scholar] [CrossRef]
- Procknow, S.S.; Kozel, B.A. Emerging mechanisms of elastin transcriptional regulation. Am. J. Physiol. Cell Physiol. 2022, 323, C666–C677. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Cocciolone, A.J.; Wagenseil, J.E. Elastin, arterial mechanics, and stenosis. Am. J. Physiol. Cell Physiol. 2022, 322, C875–C886. [Google Scholar] [CrossRef] [PubMed]
- Paterakis, K.; Koutsias, S.; Doxani, C.; Xanthopoulou, P.; Kokkali, C.; Mpoulimari, I.; Tziastoudi, M.; Karampelas, I.; Dardiotis, E.; Hadjigeorgiou, G.; et al. Variants of the elastin (ELN) gene and susceptibility to intracranial aneurysm: A synthesis of genetic association studies using a genetic model-free approach. Int. J. Neurosci. 2017, 127, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Narita, A.; Nakaoka, H.; Cui, T.; Takahashi, T.; Yasuno, K.; Tajima, A.; Krischek, B.; Yamamoto, K.; Kasuya, H.; et al. Genome-wide association study to identify genetic variants present in Japanese patients harboring intracranial aneurysms. J. Hum. Genet. 2010, 55, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Pena, J.D.; Agapova, O.; Gabelt, B.T.; Levin, L.A.; Lucarelli, M.J.; Kaufman, P.L.; Hernandez, M.R. Increased elastin expression in astrocytes of the lamina cribrosa in response to elevated intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2303–2314. [Google Scholar]
- Fan, B.J.; Figuieredo Sena, D.R.; Pasquale, L.R.; Grosskreutz, C.L.; Rhee, D.J.; Chen, T.C.; Delbono, E.A.; Haines, J.L.; Wiggs, J.L. Lack of association of polymorphisms in elastin with pseudoexfoliation syndrome and glaucoma. J. Glaucoma 2010, 19, 432–436. [Google Scholar] [CrossRef]
- MacGregor, S.; Ong, J.S.; An, J.; Han, X.; Zhou, T.; Siggs, O.M.; Law, M.H.; Souzeau, E.; Sharma, S.; Lynn, D.J.; et al. Genome-wide association study of intraocular pressure uncovers new pathways to glaucoma. Nat. Genet. 2018, 50, 1067–1071. [Google Scholar] [CrossRef]
- Kim, K.E.; Park, K.H. Optic disc hemorrhage in glaucoma: Pathophysiology and prognostic significance. Curr. Opin. Ophthalmol. 2017, 28, 105–112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).