Transitions in Immunoassay Leading to Next-Generation Lateral Flow Assays and Future Prospects
Abstract
:1. Introduction
2. Basic Principle and Structure of LFA
3. Pros and Cons of the LFA
4. Efforts towards the Development of New LFA: Development of High-Performance Antibodies
4.1. Efforts towards New LFA Development—About New Detection Methods
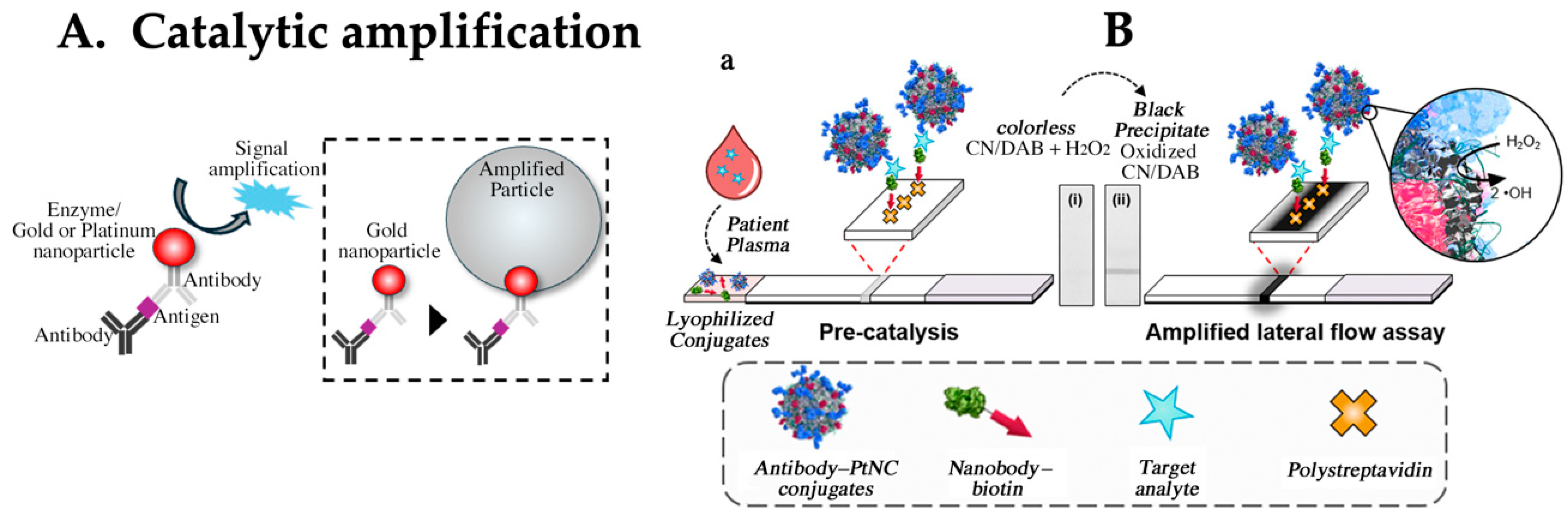
4.2. Enhancing Sensitivity via Catalytic Detection Principle and Amplification Reactions
4.3. LFA Using Raman Scattering Phenomenon
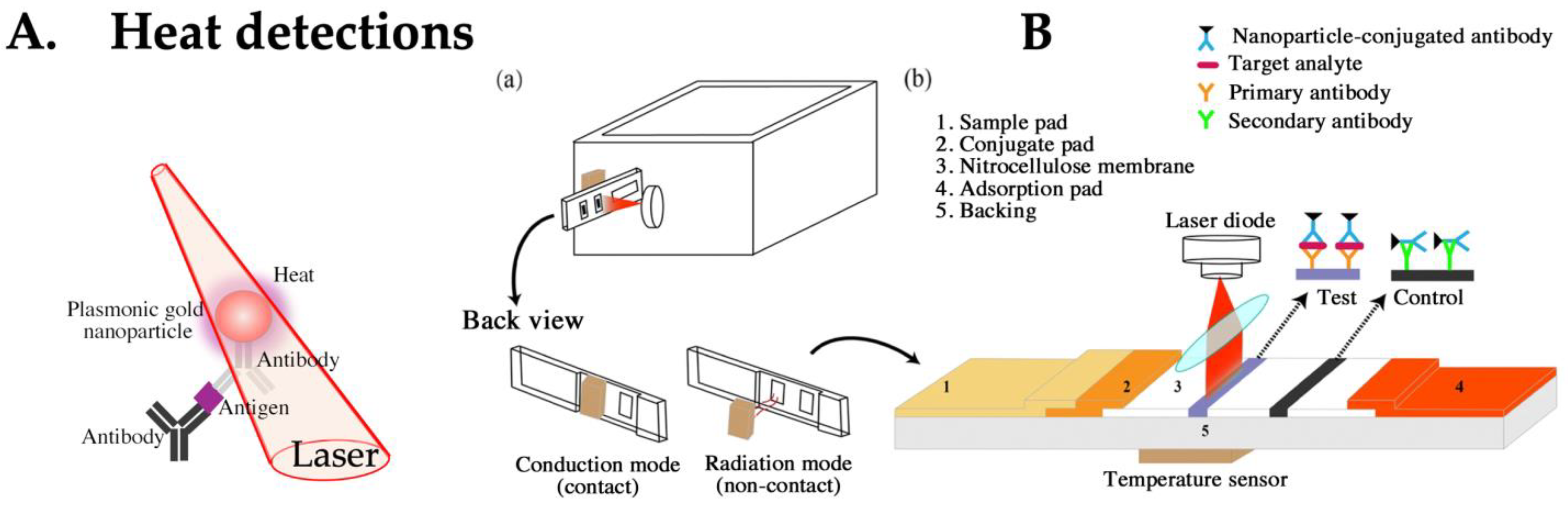
4.4. LFA Based on Heat Detection
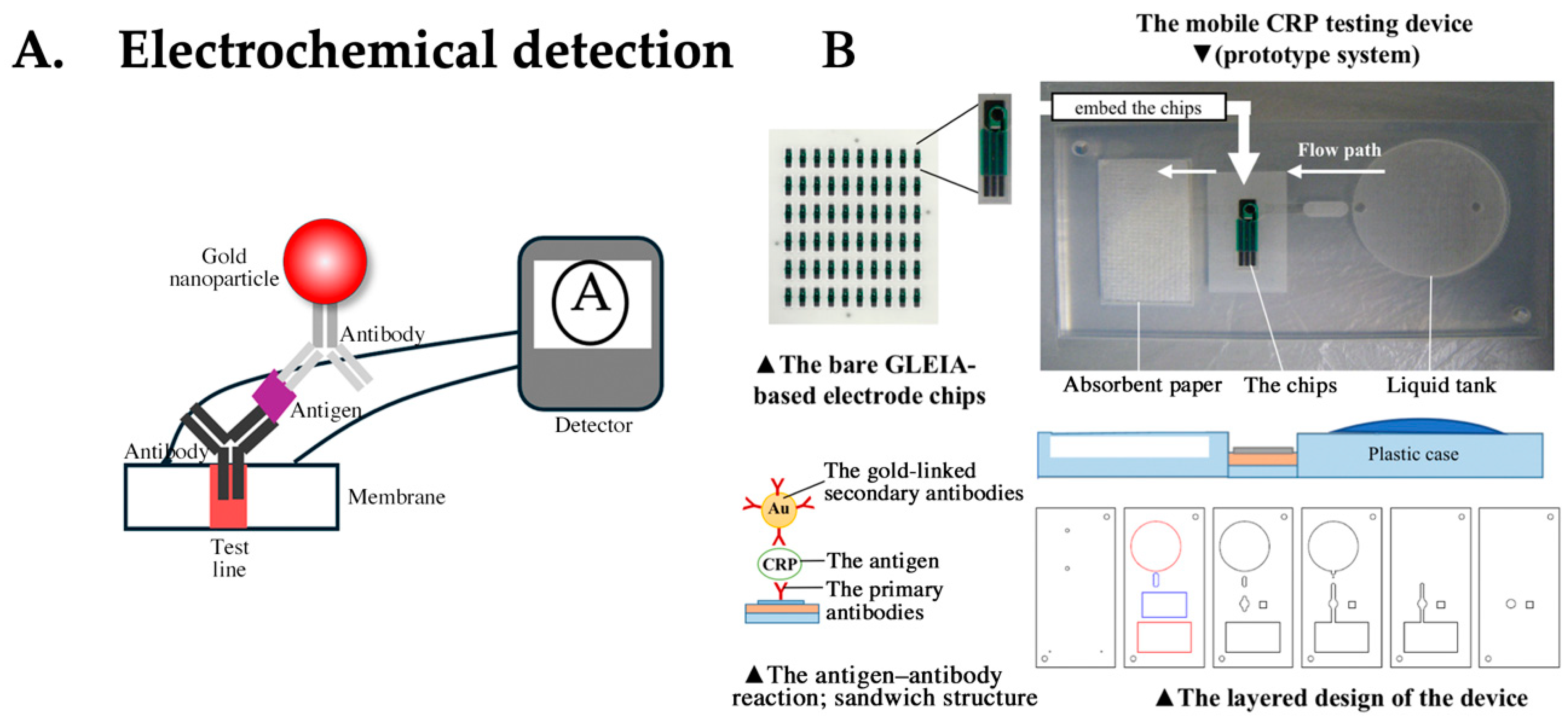
4.5. LFA Based on Electrochemical Detection
4.6. LFA Using Magnetic Particles

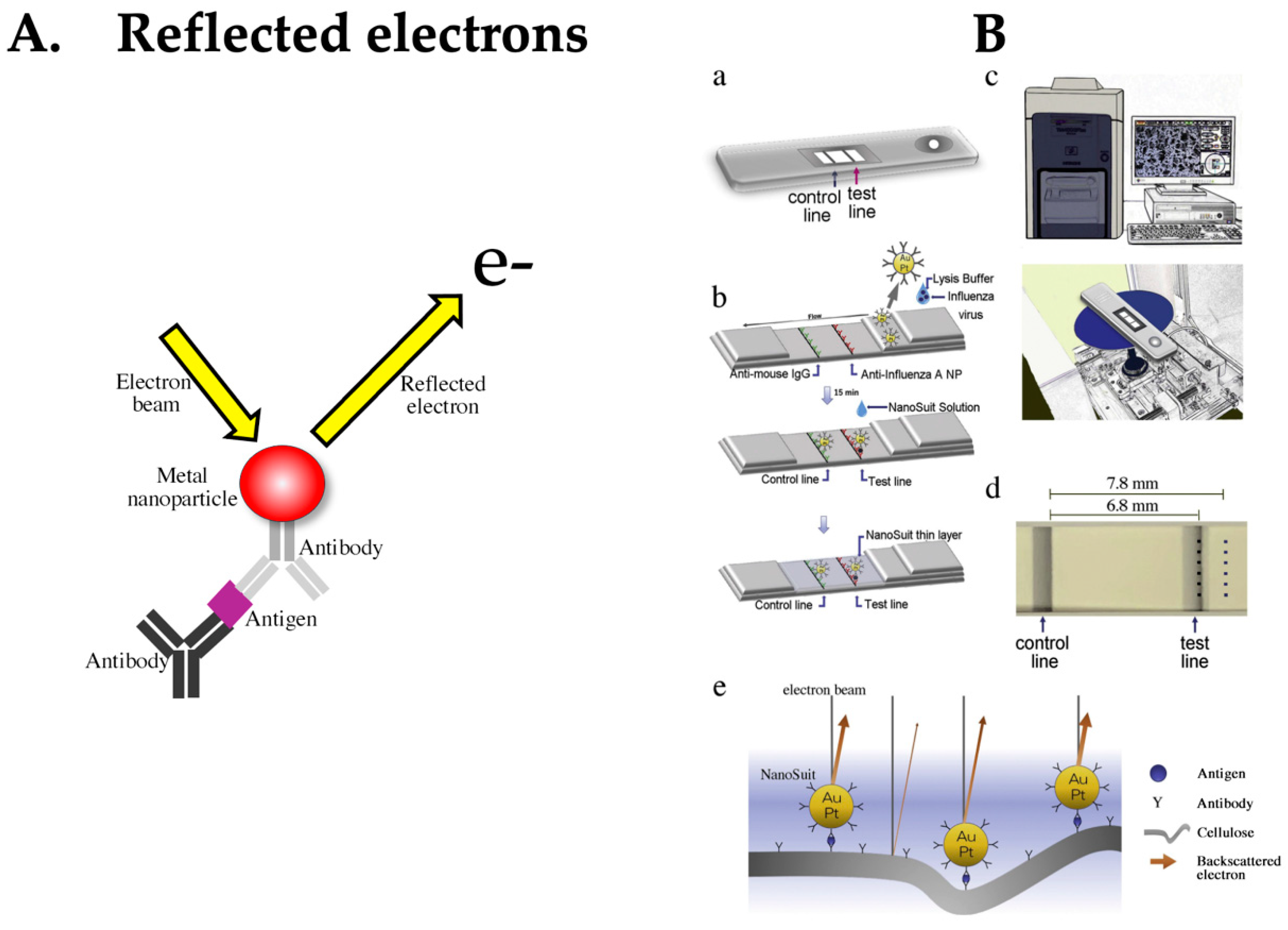
4.7. LFA Based on Detection of Reflected Electrons
5. Conclusions
- Improvement of positive concordance rate using two antibodies. This approach involves incorporating antibodies that specifically react with two or more different antigens expressed by the target into the immunoassay [101]. By utilizing specific antibodies against antigens expressed by different subtypes of a pathogen, this strategy is expected to facilitate the detection of pathogens with multiple subtypes. Additionally, as multiple antigens become the detection targets, the overall target amount for detection is increased (although the antigen amount itself cannot be amplified).
- Bispecific monoclonal antibodies were proposed as the second generation of monoclonal antibodies, with initial reports from Suresh et al. in the 1980s [102]. These bispecific antibodies independently recognize two different antigens. Given the current advancements in recombinant technology, early application of these antibodies is anticipated. The first advantage is that a single antibody can target twice the number of detection targets. The second advantage lies in using two independent paratopes: while one binds to the antigen, the other binds to a signal amplification substance, such as an enzyme. This method may provide high sensitivity through a secondary reaction with enzyme amplification when particle-based detection alone is insufficient [103].
- Sensitivity enhancement using secondary antibodies. Typically, the antibody paired in a sandwich assay is labeled with a marker. Instead of this labeled antibody, sensitivity improvements can sometimes be attempted by using highly binding substances, like protein G/A or streptavidin. Additionally, although the reproducibility may be lower, polyclonal antibodies or other multivalent antibodies may improve the sensitivity, as opposed to using monoclonal antibodies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briggs, C.; Guthrie, D.; Hyde, K.; Mackie, I.; Parker, N.; Popek, M.; Porter, N.; Stephens, C.; British Committee for Standards in Haematology General Haematology Task Force. Guidelines for point-of-care testing: Haematology. Br. J. Haematol. 2008, 142, 904–915. [Google Scholar] [CrossRef]
- Beyette, F.R.; Gaydos, C.A.; Kost, G.J.; Weigl, B.H. Point-of-care technologies for health care. IEEE Trans. Bio Med. Eng. 2011, 58, 732–735. [Google Scholar] [CrossRef]
- Price, C.P. Point of care testing. BMJ 2001, 322, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Kost, G.J. Guidelines for point-of-care testing. Improving patient outcomes. Am. J. Clin. Pathol. 1995, 104 (Suppl. 1), S111–S127. [Google Scholar]
- Rajan, A.; Glorikian, H. Point-of-care diagnostics: Market trends and growth drivers. Expert. Opin. Med. Diagn. 2009, 3, 1–4. [Google Scholar] [CrossRef]
- Krienitz, D.; Little, J. How accelerated regulation will affect point-of-care testing. MLO Med. Lab. Obs. 1991, 23, 47–51. [Google Scholar] [PubMed]
- Hughes, M. Market trends in point-of-care testing. Point Care J. Near Patient Test. Technol. 2002, 1, 84–94. [Google Scholar] [CrossRef]
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of care diagnostics: Status and future. Anal. Chem. 2012, 84, 487–515. [Google Scholar] [CrossRef]
- Park, H.D. Current status of clinical application of point-of-care testing. Arch. Pathol. Lab. Med. 2021, 145, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Wan, X.; Sohan, A.S.M.M.; Lin, X. Microfluidics-based POCT for SARS-CoV-2 diagnostics. Micromachines 2022, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Mina, M.J.; Parker, R.; Larremore, D.B. Rethinking Covid-19 test sensitivity—A strategy for containment. N. Engl. J. Med. 2020, 383, e120. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Porte, L.; Legarraga, P.; Vollrath, V.; Aguilera, X.; Munita, J.M.; Araos, R.; Pizarro, G.; Vial, P.; Iruretagoyena, M.; Dittrich, S.; et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020, 99, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Biby, A.; Wang, X.; Liu, X.; Roberson, O.; Henry, A.; Xia, X. Rapid testing for coronavirus disease 2019 (COVID-19). MRS Commun 2022, 12, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, M.; Kendall, F.E.; Teorell, T. Quantitative studies on the precipitin reaction: Effect of salts on the reaction. J. Exp. Med. 1936, 63, 819–826. [Google Scholar] [CrossRef]
- Yalow, R.S.; Berson, S.A. Assay of plasma insulin in human subjects by immunological methods. Nature 1959, 184 (Suppl. 21), 1648–1649. [Google Scholar] [CrossRef]
- Avrameas, S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry 1969, 6, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Van Weemen, B.K.; Schuurs, A.H. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971, 15, 232–236. [Google Scholar] [CrossRef]
- Weeks, I.; Beheshti, I.; McCapra, F.; Campbell, A.K.; Woodhead, J.S. Acridinium esters as high-specific-activity labels in immunoassay. Clin. Chem. 1983, 29, 1474–1479. [Google Scholar] [CrossRef]
- Campbell, R.L.; Wagner, D.B.; O’Connell, J.P. Solid Phase Assay with Visual Readout. U.S. Patent 4,703,017, 1987. [Google Scholar]
- Yamazaki, M.; Mitamura, K.; Ichikawa, M.; Kimura, K.; Komiyama, O.; Shimizu, H.; Kawakami, C.; Watanabe, S.; Imai, M.; Cho, H.; et al. Evaluation of flow-through immunoassay for rapid detection of influenza A and B viruses. Kansenshogaku Zasshi 2004, 78, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.V.; Dantzler, J.L.; Weigl, B.H. Analytical tools to improve optimization procedures for lateral flow assays. Diagnostics 2017, 7, 29. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Adachi, K.; Handharyani, E.; Sari, D.K.; Takama, K.; Fukuda, K.; Endo, I.; Yamamoto, R.; Sawa, M.; Tanaka, M.; Konishi, I.; et al. Development of neutralization antibodies against highly pathogenic H5N1 avian influenza virus using ostrich (Struthio camelus) yolk. Mol. Med. Rep. 2008, 1, 203–209. [Google Scholar] [PubMed]
- Greenberg, A.S.; Avila, D.; Hughes, M.; Hughes, A.; McKinney, E.C.; Flajnik, M.F. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature 1995, 374, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Cheong, W.S.; Leow, C.Y.; Abdul Majeed, A.B.; Leow, C.H. Diagnostic and therapeutic potential of shark variable new antigen receptor (VNAR) single domain antibody. Int. J. Biol. Macromol. 2020, 147, 369–375. [Google Scholar] [CrossRef]
- Tao, G.Z.; Nakamichi, I.; Ku, N.O.; Wang, J.; Frolkis, M.; Gong, X.; Zhu, W.; Pytela, R.; Omary, M.B. Bispecific and human disease-related anti-keratin rabbit monoclonal antibodies. Exp. Cell Res. 2006, 312, 411–422. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, P.; Ke, Y.; Zhang, Y.; Chen, J.; Dai, J.; Li, M.; Zhu, W.; Yu, G.L. Development of humanized rabbit monoclonal antibodies against vascular endothelial growth factor receptor 2 with potential antitumor effects. Biochem. Biophys. Res. Commun. 2013, 436, 543–550. [Google Scholar] [CrossRef]
- Henderson, K.A.; Streltsov, V.A.; Coley, A.M.; Dolezal, O.; Hudson, P.J.; Batchelor, A.H.; Gupta, A.; Bai, T.; Murphy, V.J.; Anders, R.F.; et al. Structure of an IgNAR-AMA1 complex: Targeting a conserved hydrophobic cleft broadens malarial strain recognition. Structure 2007, 15, 1452–1466. [Google Scholar] [CrossRef] [PubMed]
- Rumfelt, L.L.; Avila, D.; Diaz, M.; Bartl, S.; McKinney, E.C.; Flajnik, M.F. A shark antibody heavy chain encoded by a nonsomatically rearranged VDJ is preferentially expressed in early development and is convergent with mammalian IgG. Proc. Natl. Acad. Sci. USA 2001, 98, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Pinto Torres, J.E.; Goossens, J.; Ding, J.; Li, Z.; Lu, S.; Vertommen, D.; Naniima, P.; Chen, R.; Muyldermans, S.; Sterckx, Y.G.J.; et al. Development of a Nanobody-based lateral flow assay to detect active Trypanosoma congolense infections. Sci. Rep. 2018, 8, 9019. [Google Scholar] [CrossRef]
- Doerflinger, S.Y.; Tabatabai, J.; Schnitzler, P.; Farah, C.; Rameil, S.; Sander, P.; Koromyslova, A.; Hansman, G.S. Development of a nanobody-based lateral flow immunoassay for detection of human Norovirus. mSphere 2016, 1, e00219-16. [Google Scholar] [CrossRef]
- Qin, X.; Duan, M.; Pei, D.; Lin, J.; Wang, L.; Zhou, P.; Yao, W.; Guo, Y.; Li, X.; Tao, L.; et al. Development of novel-nanobody-based lateral-flow immunochromatographic strip test for rapid detection of recombinant human interferon α2b. J. Pharm. Anal. 2022, 12, 308–316. [Google Scholar] [CrossRef]
- Zubler, R.H.; Erard, F.; Lees, R.K.; Van Laer, M.; Mingari, C.; Moretta, L.; MacDonald, H.R. Mutant EL-4 thymoma cells polyclonally activate murine and human B cells via direct cell interaction. J. Immunol. 1985, 134, 3662–3668. [Google Scholar] [CrossRef] [PubMed]
- Spieker-Polet, H.; Sethupathi, P.; Yam, P.C.; Knight, K.L. Rabbit monoclonal antibodies: Generating a fusion partner to produce rabbit-rabbit hybridomas. Proc. Natl. Acad. Sci. USA 1995, 92, 9348–9352. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, N.; Yoshioka, M.; Fujimoto, R.; Yamagishi, F.; Isobe, M. Rapid production of antigen-specific monoclonal antibodies from a variety of animals. BMC Biol. 2012, 10, 80. [Google Scholar] [CrossRef]
- Seeber, S.; Ros, F.; Thorey, I.; Tiefenthaler, G.; Kaluza, K.; Lifke, V.; Fischer, J.A.A.; Klostermann, S.; Endl, J.; Kopetzki, E.; et al. A robust high throughput platform to generate functional recombinant monoclonal antibodies using rabbit B cells from peripheral blood. PLoS ONE 2014, 9, e86184. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, T.; Fujino, Y.; Ueda, T. PURE ribosome display and its application in antibody technology. Biochim. Biophys. Acta 2014, 1844, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, C.; Amstutz, P.; Plückthun, A. Ribosome display: Selecting and evolving proteins in vitro that specifically bind to a target. Nat. Methods 2007, 4, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.A.; Wittrup, K.D. Yeast surface display for protein engineering and characterization. Curr. Opin. Struct. Biol. 2007, 17, 467–473. [Google Scholar] [CrossRef]
- Saito, Y.; Oikawa, M.; Nakazawa, H.; Niide, T.; Kameda, T.; Tsuda, K.; Umetsu, M. Machine-learning-guided mutagenesis for directed evolution of fluorescent proteins. ACS Synth. Biol. 2018, 7, 2014–2022. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.K.; Wu, Z.; Arnold, F.H. Machine-learning-guided directed evolution for protein engineering. Nat. Methods 2019, 16, 687–694. [Google Scholar] [CrossRef]
- Lopez, E.; Scott, N.E.; Wines, B.D.; Hogarth, P.M.; Wheatley, A.K.; Kent, S.J.; Chung, A.W. Low pH exposure during immunoglobulin G purification methods results in aggregates that avidly bind Fcγ receptors: Implications for measuring Fc dependent antibody functions. Front. Immunol. 2019, 10, 2415. [Google Scholar] [CrossRef]
- Peltomaa, R.; Barderas, R.; Benito-Peña, E.; Moreno-Bondi, M.C. Recombinant antibodies and their use for food immunoanalysis. Anal. Bioanal. Chem. 2022, 414, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Satake, A.; Matsumoto, M.; Kido, Y.; Tsuji, A.; Ito, K.; Maeda, M. Monoclonal-based enzyme-linked immunosorbent assay and immunochromatographic rapid assay for monensin. Analyst 1998, 123, 2573–2578. [Google Scholar] [CrossRef]
- Du, D.; Wang, J.; Wang, L.; Lu, D.; Lin, Y. Integrated lateral flow test strip with electrochemical sensor for quantification of phosphorylated cholinesterase: Biomarker of exposure to organophosphorus agents. Anal. Chem. 2012, 84, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, G.L.; Nocini, R.; Gianfilippi, G.; Fiorio, G.; Pighi, L.; De Nitto, S.; Cominziolli, A.; Henry, B.M.; Lippi, G. Performance of Fujirebio Espline SARS-CoV-2 rapid antigen test for identifying potentially infectious individuals. Diagnosis 2021, 9, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Samsonova, J.V.; Safronova, V.A.; Osipov, A.P. Pretreatment-free lateral flow enzyme immunoassay for progesterone detection in whole cows’ milk. Talanta 2015, 132, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Kawamura, M.; Arao, S.; Nariuchi, H. A highly sensitive quantitative immunochromatography assay for antigen-specific IgE. J. Immunol. Methods 2003, 272, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Wang, S. Development of multianalyte flow-through and lateral-flow assays using gold particles and horseradish peroxidase as tracers for the rapid determination of carbaryl and endosulfan in agricultural products. J. Agric. Food Chem. 2006, 5, 2502–2507. [Google Scholar] [CrossRef]
- Cho, I.H.; Irudayaraj, J. Lateral-flow enzyme immunoconcentration for rapid detection of Listeria monocytogenes. Anal. Bioanal. Chem. 2013, 405, 3313–3319. [Google Scholar] [CrossRef] [PubMed]
- Panferov, V.G.; Safenkova, I.V.; Varitsev, Y.A.; Zherdev, A.V.; Dzantiev, B.B. Enhancement of lateral flow immunoassay by alkaline phosphatase: A simple and highly sensitive test for potato virus X. Mikrochim. Acta 2017, 185, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, B.; Xiao, S.; Liu, X. Engineering a hierarchically micro-/nanostructured Si@Au-based artificial enzyme with improved accessibility of active sites for enhanced catalysis. RSC Adv. 2024, 14, 2697–2703. [Google Scholar] [CrossRef] [PubMed]
- Bezuneh, T.T.; Fereja, T.H.; Kitte, S.A.; Li, H.; Jin, Y. Gold nanoparticle-based signal amplified electrochemiluminescence for biosensing applications. Talanta 2022, 248, 123611. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Q.; Qiu, W.; Li, K.; Qian, L.; Zhang, X.; Liu, G. Gold-platinum nanoflowers as a label and as an enzyme mimic for use in highly sensitive lateral flow immunoassays: Application to detection of rabbit IgG. Mikrochim. Acta 2019, 186, 357. [Google Scholar] [CrossRef] [PubMed]
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum nanocatalyst amplification: Redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.; Liang, T.; Houghtaling, J.; Ramachandran, S.; Ramsey, S.A.; Lutz, B.; Yager, P. Enhanced sensitivity of lateral flow tests using a two-dimensional paper network format. Anal. Chem. 2011, 83, 7941–7946. [Google Scholar] [CrossRef]
- Bu, T.; Huang, Q.; Yan, L.; Huang, L.; Zhang, M.; Yang, Q.; Yang, B.; Wang, J.; Zhang, D. Ultra technically simple and sensitive detection for Salmonella enteritidis by immunochromatographic assay based on gold growth. Food Control 2018, 84, 536–543. [Google Scholar] [CrossRef]
- Cho, I.H.; Seo, S.M.; Paek, E.H.; Paek, S.H. Immunogold-silver staining-on-a-chip biosensor based on cross-flow chromatography. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2010, 878, 271–277. [Google Scholar] [CrossRef]
- Peng, T.; Jiao, X.; Liang, Z.; Zhao, H.; Zhao, Y.; Xie, J.; Jiang, Y.; Yu, X.; Fang, X.; Dai, X. Lateral flow immunoassay coupled with copper enhancement for rapid and sensitive SARS-CoV-2 nucleocapsid protein detection. Biosensors 2021, 12, 13. [Google Scholar] [CrossRef]
- Rahbar, M.; Wu, Y.; Subramony, J.A.; Liu, G. Sensitive colorimetric detection of interleukin-6 via Lateral Flow Assay Incorporated Silver Amplification Method. Front. Bioeng. Biotechnol. 2021, 9, 778269. [Google Scholar] [CrossRef] [PubMed]
- Poosinuntakul, N.; Chanmee, T.; Porntadavity, S.; Chailapakul, O.; Apilux, A. Silver-enhanced colloidal gold dip strip immunoassay integrated with smartphone-based colorimetry for sensitive detection of cardiac marker troponin I. Sci. Rep. 2022, 12, 19866. [Google Scholar] [CrossRef]
- Liu, R.; Liu, X.; Tang, Y.; Wu, L.; Hou, X.; Lv, Y. Highly sensitive immunoassay based on immunogold-silver amplification and inductively coupled plasma mass spectrometric detection. Anal. Chem. 2011, 83, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Broger, T.; Sossen, B.; du Toit, E.; Kerkhoff, A.D.; Schutz, C.; Ivanova Reipold, E.; Ward, A.; Barr, D.A.; Macé, A.; Trollip, A.; et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: A diagnostic accuracy study. Lancet Infect. Dis. 2019, 19, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Zhou, T.; Zhou, P.; Li, J.; Deng, A. Ultrasensitive and specific detection of anticancer drug 5-fluorouracil in blood samples by a surface-enhanced Raman scattering (SERS)-based lateral flow Immunochromatographic assay. Molecules 2022, 27, 4019. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Dankov, A.; Adanalic, M.; Grzeschik, R.; Tran, V.; Pagel-Wieder, S.; Gessler, F.; Spreitzer, I.; Scholz, T.; Schnierle, B.; et al. Rapid and sensitive SERS-based lateral flow test for SARS-CoV2-Specific IgM/IgG antibodies. Anal. Chem. 2021, 93, 12391–12399. [Google Scholar] [CrossRef]
- Tian, R.; Ren, Y.; Wang, T.; Cao, J.; Li, J.; Deng, A. A SERS-based lateral flow immunochromatographic assay using Raman reporter mediated-gap AuNR@Au nanoparticles as the substrate for the detection of enrofloxacin in food samples. Anal. Chim. Acta 2023, 1257, 341152. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Lu, L.; Rong, Z.; Wang, C.; Peng, Y.; Wang, F.; Wang, J.; Sun, M.; Dong, J.; Wang, D.; et al. Portable and multiplexed lateral flow immunoassay reader based on SERS for highly sensitive point-of-care testing. Biosens. Bioelectron. 2020, 168, 112524. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Walkenfort, B.; König, M.; Salehi, M.; Schlücker, S. Rapid, quantitative, and ultrasensitive point-of-care testing: A portable SERS reader for lateral flow assays in clinical chemistry. Angew. Chem. Int. Ed. Engl. 2019, 58, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Shibata, M.; Kitamura, N.; Nagasawa, F.; Takase, M.; Murakoshi, K.; Nobuhiro, A.; Mizumoto, Y.; Ishihara, H.; Tsuboi, Y. Reversible photoinduced-formation and manipulation of a two-dimensional closely packed assembly of polystyrene nanospheres on a metallic nanostructure. J. Phys. Chem. C 2013, 117, 2500–2506. [Google Scholar] [CrossRef]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shen, Y.R. General properties of local plasmons in metal nanostructures. Phys. Rev. Lett. 2006, 97, 206806. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Wang, K.; Alfranca, G.; de la Fuente, J.M.; Cui, D. A plasmonic thermal sensing based portable device for lateral flow assay detection and quantification. Nanoscale Res. Lett. 2020, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Liu, Y.; Zhan, L.; Liu, Y.; Qin, Z. Signal amplification and quantification on lateral flow assays by laser excitation of plasmonic nanomaterials. Theranostics 2020, 10, 4359–4373. [Google Scholar] [CrossRef] [PubMed]
- Perju, A.; Wongkaew, N. Integrating high-performing electrochemical transducers in lateral flow assay. Anal. Bioanal. Chem. 2021, 413, 5535–5549. [Google Scholar] [CrossRef] [PubMed]
- Gondoh-Noda, Y.; Kometani, M.; Nomura, A.; Aono, D.; Karashima, S.; Ushijima, H.; Tamiya, E.; Murayama, T.; Yoneda, T. Feasibility of a novel mobile C-reactive protein-testing device using gold-linked electrochemical immunoassay: Clinical performance study. JMIR MHealth UHealth 2020, 8, e18782. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. Engl. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wang, K.; Xiao, K.; Qin, W.; Hou, Y.; Xu, H.; Yan, X.; Chen, Y.; Cui, D.; He, J. Dual immunomagnetic nanobeads-based lateral flow test strip for simultaneous quantitative detection of carcinoembryonic antigen and neuron specific enolase. Sci. Rep. 2017, 7, 42414. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Tang, Q.; Li, Q. MnFe2O4 nanoclusters as labels for the quantitative detection of D-dimer in a lateral-flow immunochromatographic assay. J. Chin. Chem. Soc. 2019, 66, 297–302. [Google Scholar] [CrossRef]
- Kotitz, R.; Matz, H.; Trahms, L.; Koch, H.; Weitschies, W.; Rheinlander, T.; Semmler, W.; Bunte, T. SQUID based remanence measurements for immunoassays. IEEE Trans. Appl. Supercond. 1997, 7, 3678–3681. [Google Scholar] [CrossRef]
- Enpuku, K.; Inoue, K.; Soejima, K.; Yoshinaga, K.; Kuma, H.; Hamasaki, N. Magnetic immunoassays utilizing magnetic markers and a high-T/sub c/ SQUID. IEEE Trans. Appl. Supercond. 2005, 15, 660–663. [Google Scholar] [CrossRef]
- Sharma, A.; Tok, A.I.Y.; Lee, C.; Ganapathy, R.; Alagappan, P.; Liedberg, B. Magnetic field assisted preconcentration of biomolecules for lateral flow assaying. Sens. Actuators B 2019, 285, 431–437. [Google Scholar] [CrossRef]
- Guo, L.; Shao, Y.; Duan, H.; Ma, W.; Leng, Y.; Huang, X.; Xiong, Y. Magnetic Quantum Dot Nanobead-Based Fluorescent Immunochromatographic Assay for the Highly Sensitive Detection of Aflatoxin B. Anal. Chem. 2019, 91, 4727–4734. [Google Scholar] [CrossRef]
- Kawasaki, H.; Suzuki, H.; Maekawa, M.; Hariyama, T. Combination of the NanoSuit method and gold/platinum particle-based lateral flow assay for quantitative and highly sensitive diagnosis using a desktop scanning electron microscope. J. Pharm. Biomed. Anal. 2021, 196, 113924. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Suzuki, H.; Furuhashi, K.; Yamashita, K.; Ishikawa, J.; Nagura, O.; Maekawa, M.; Miwa, T.; Tandou, T.; Hariyama, T. Highly sensitive and quantitative diagnosis of SARS-CoV-2 using a gold/platinum particle-based lateral flow assay and a desktop scanning electron microscope. Biomedicines 2022, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Takaku, Y.; Suzuki, H.; Ohta, I.; Ishii, D.; Muranaka, Y.; Shimomura, M.; Hariyama, T. A thin polymer membrane, nano-suit, enhancing survival across the continuum between air and high vacuum. Proc. Natl. Acad. Sci. USA 2013, 110, 7631–7635. [Google Scholar] [CrossRef]
- Danthanarayana, A.N.; Nandy, S.; Kourentzi, K.; Vu, B.; Shelite, T.R.; Travi, B.L.; Brgoch, J.; Willson, R.C. Smartphone-readable RPA-LFA for the high-sensitivity detection of Leishmania kDNA using nanophosphor reporters. PLOS Negl. Trop. Dis. 2023, 17, e0011436. [Google Scholar] [CrossRef] [PubMed]
- Chabi, M.; Vu, B.; Brosamer, K.; Smith, M.; Chavan, D.; Conrad, J.C.; Willson, R.C.; Kourentzi, K. Smartphone-read phage lateral flow assay for point-of-care detection of infection. Analyst 2023, 148, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, X.; Liu, L.; Zhang, X.; Yang, X.; Zheng, S.; Rong, Z.; Wang, S. Ultrasensitive and Simultaneous Detection of Two Specific SARS-CoV-2 Antigens in Human Specimens Using Direct/Enrichment Dual-Mode Fluorescence Lateral Flow Immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 40342–40353. [Google Scholar] [CrossRef]
- Suresh, M.R.; Cuello, A.C.; Milstein, C. Advantages of bispecific hybridomas in one-step immunocytochemistry and immunoassays. Proc. Natl. Acad. Sci. USA 1986, 83, 7989–7993. [Google Scholar] [CrossRef] [PubMed]
- Byrne, H.; Conroy, P.J.; Whisstock, J.C.; O’Kennedy, R.J. A tale of two specificities: Bispecific antibodies for therapeutic and diagnostic applications. Trends Biotechnol. 2013, 31, 621–632. [Google Scholar] [CrossRef] [PubMed]


| Items | Conventional LFA | Disadvantages |
|---|---|---|
| Antibody |
|
|
| Membrane |
|
|
| Labels |
|
|
| Readers |
|
|
| Section | LFA Technology | Disadvantages |
|---|---|---|
| 4.2 | Catalytic Signal Amplification | Operability limitations and longer reaction time |
| 4.3 | Raman Scattering Phenomenon | Complex particle synthesis and conjugate reagent production |
| 4.4 | Heat Detection | Combustible nitrocellulose limits commercial use |
| 4.5 | Electrochemical Detection | Protein adhesion complicates detection |
| 4.6 | Magnetic Particles | High cost of detectors |
| 4.7 | Reflected Electron Detection | Detectors lack portability and full automation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiuchi, K.; Aoki, N.; Ohtake, T.; Iwashita, T.; Kawasaki, H. Transitions in Immunoassay Leading to Next-Generation Lateral Flow Assays and Future Prospects. Biomedicines 2024, 12, 2268. https://doi.org/10.3390/biomedicines12102268
Fujiuchi K, Aoki N, Ohtake T, Iwashita T, Kawasaki H. Transitions in Immunoassay Leading to Next-Generation Lateral Flow Assays and Future Prospects. Biomedicines. 2024; 12(10):2268. https://doi.org/10.3390/biomedicines12102268
Chicago/Turabian StyleFujiuchi, Koyu, Noriko Aoki, Tetsurou Ohtake, Toshihide Iwashita, and Hideya Kawasaki. 2024. "Transitions in Immunoassay Leading to Next-Generation Lateral Flow Assays and Future Prospects" Biomedicines 12, no. 10: 2268. https://doi.org/10.3390/biomedicines12102268






