Modelling a Western Lifestyle in Mice: A Novel Approach to Eradicating Aerobic Spore-Forming Bacteria from the Colonic Microbiome and Assessing Long-Term Clinical Outcomes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Super-Clean Conditions Effectively Removed Colonic Spore-Forming Bacteria
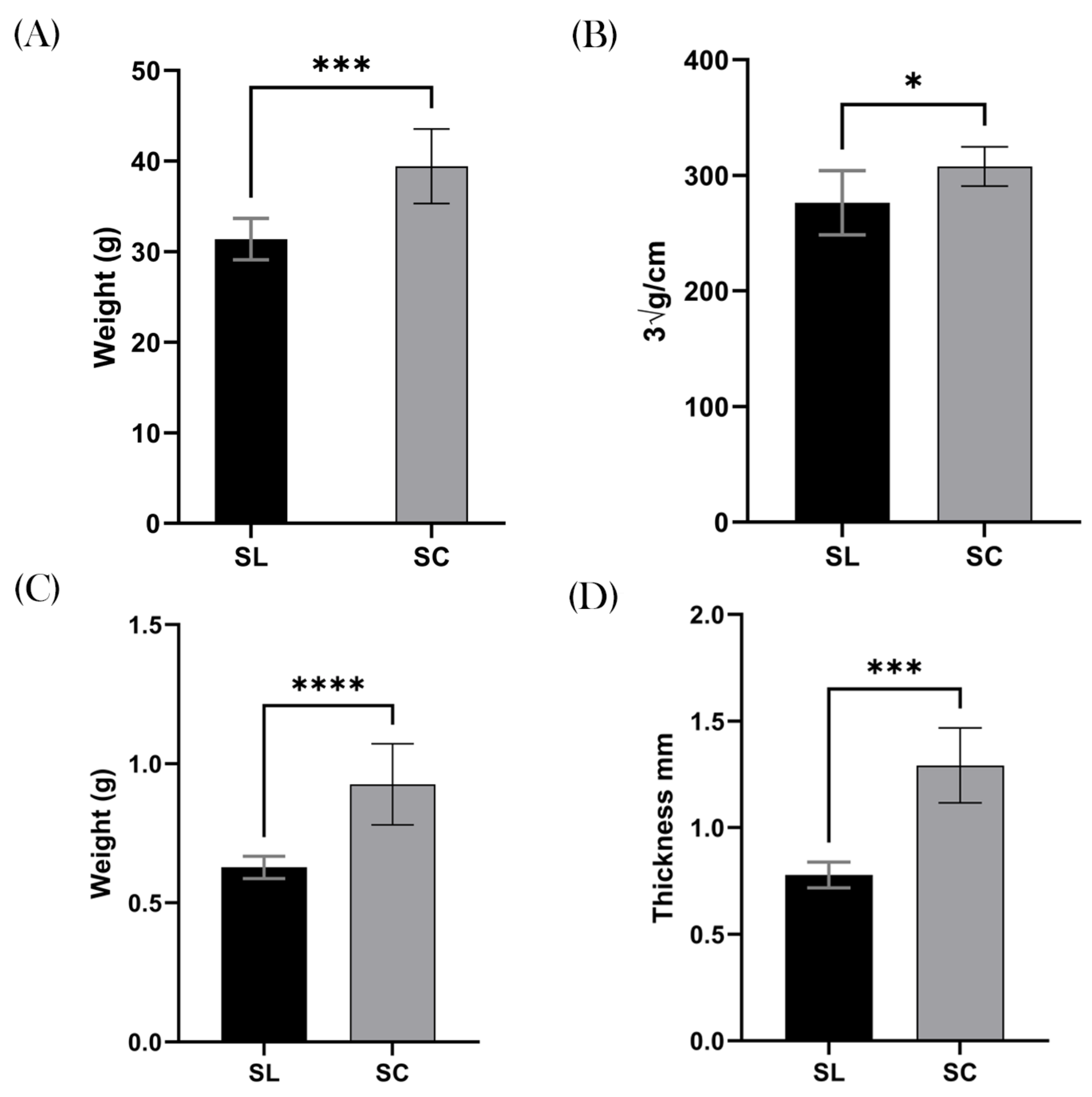
3.2. Weight and Obesity
3.3. Colonic Measurements
3.4. Metabolic Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinross, J.M.; Darzi, A.W.; Nicholson, J.K. Gut microbiome-host interactions in health and disease. Genome Med. 2011, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Blum, W.E.H.; Zechmeister-Boltenstern, S.; Keiblinger, K.M. Does Soil Contribute to the Human Gut Microbiome? Microorganisms 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Anthony, M.A.; Bender, S.F.; van der Heijden, M.G.A. Enumerating soil biodiversity. Proc. Natl. Acad. Sci. USA 2023, 120, e2304663120. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.; Dempsey, E.; Ryan, C.A.; Ross, R.P.; Stanton, C. The Sporobiota of the Human Gut. Gut Microbes 2021, 13, 1863134. [Google Scholar] [CrossRef]
- Saggese, A.; Baccigalupi, L.; Ricca, E. Spore Formers as Beneficial Microbes for Humans and Animals. Appl. Microbiol. 2021, 1, 498–509. [Google Scholar] [CrossRef]
- Nicholson, W.L. Roles of Bacillus endospores in the environment. Cell. Mol. Life Sci. 2002, 59, 410–416. [Google Scholar] [CrossRef]
- Horwell, E.; Vittoria, M.; Hong, H.A.; Bearn, P.; Cutting, S.M. A Family of Cyclic Lipopeptides Found in Human Isolates of Bacillus Ameliorates Acute Colitis via Direct Agonism of Toll-Like Receptor 2 in a Murine Model of Inflammatory Bowel Disease. Dig. Dis. Sci. 2024, 1–13. [Google Scholar] [CrossRef]
- Ferreira, W.T.; Hong, H.A.; Adams, J.R.G.; Hess, M.; Kotowicz, N.K.; Tan, S.; Ferrari, E.; Brisson, A.; Zentek, J.; Soloviev, M.; et al. Environmentally Acquired Bacillus and Their Role in C. difficile Colonization Resistance. Biomedicines 2022, 10, 930. [Google Scholar] [CrossRef]
- Yahya, G.; Ebada, A.; Khalaf, E.M.; Mansour, B.; Nouh, N.A.; Mosbah, R.A.; Saber, S.; Moustafa, M.; Negm, S.; El-Sokkary, M.; et al. Soil-Associated Bacillus Species: A Reservoir of Bioactive Compounds with Potential Therapeutic Activity against Human Pathogens. Microorganisms 2021, 9, 1131. [Google Scholar] [CrossRef]
- Ilinskaya, O.N.; Ulyanova, V.V.; Yarullina, D.R.; Gataullin, I.G. Secretome of Intestinal Bacilli: A Natural Guard against Pathologies. Front. Microbiol. 2017, 8, 1666. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Kaplan, G.G.; Otley, A.R.; Nguyen, G.C.; Underwood, F.E.; Guttmann, A.; Jones, J.; Potter, B.; Catley, C.; Nugent, Z.; et al. Rural and Urban Residence During Early Life is Associated with Risk of Inflammatory Bowel Disease: A Population-Based Inception and Birth Cohort Study. Am. J. Gastroenterol. 2017, 112, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, G.R.; Gerstein, H.C.; Zhang, X.; McQueen, M.; Lear, S.; Lopez-Jaramillo, P.; Mohan, V.; Mony, P.; Gupta, R.; Kutty, V.; et al. Variations in Diabetes Prevalence in Low-, Middle-, and High-Income Countries: Results From the Prospective Urban and Rural Epidemiological Study. Diabetes Care 2016, 39, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.W.K.; Chow, C.M. Childhood asthma epidemiology: Insights from comparative studies of rural and urban populations. Pediatr. Pulmonol. 2008, 43, 107–116. [Google Scholar] [CrossRef]
- Tasnim, N.; Abulizi, N.; Pither, J.; Hart, M.M.; Gibson, D.L. Linking the Gut Microbial Ecosystem with the Environment: Does Gut Health Depend on Where We Live? Front. Microbiol. 2017, 8, 1935. [Google Scholar] [CrossRef]
- Schnorr, S.L. The soil in our microbial DNA informs about environmental interfaces across host and subsistence modalities. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190577. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W. 99th Dahlem Conference on Infection, Inflammation and Chronic Inflammatory Disorders: Darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin. Exp. Immunol. 2010, 160, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.A.; To, E.; Fakhry, S.; Baccigalupi, L.; Ricca, E.; Cutting, S.M. Defining the natural habitat of Bacillus spore-formers. Res. Microbiol. 2009, 160, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Gopal, N.; Hill, C.; Ross, P.R.; Beresford, T.P.; Fenelon, M.A.; Cotter, P.D. The Prevalence and Control of Bacillus and Related Spore-Forming Bacteria in the Dairy Industry. Front. Microbiol. 2015, 6, 1418. [Google Scholar] [CrossRef]
- Gómez-Jódar, I.; Ros-Chumillas, M.; Palop, A. Effect of heating rate on highly heat-resistant spore-forming microorganisms. Food Sci. Technol. Int. 2016, 22, 164–172. [Google Scholar] [CrossRef]
- Park, J.C.; Im, S.-H. Of men in mice: The development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp. Mol. Med. 2020, 52, 1383–1396. [Google Scholar] [CrossRef]
- Broussard, J.L.; Devkota, S. The changing microbial landscape of Western society: Diet, dwellings and discordance. Mol. Metab. 2016, 5, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.M.; Zidar, J.; Ewaldsson, B.; Askevik, K.; Udén, E.; Svensk, E.; Törnqvist, E. Aggression in Group-Housed Male Mice: A Systematic Review. Animals 2022, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Yanai, S.; Endo, S. Functional Aging in Male C57BL/6J Mice Across the Life-Span: A Systematic Behavioral Analysis of Motor, Emotional, and Memory Function to Define an Aging Phenotype. Front. Aging Neurosci. 2021, 13, 697621. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.O. Determination of the surface area of the white rat with its application to the expression of metabolic results. Am. J. Physiol.-Leg. Content 1929, 89, 24–33. [Google Scholar] [CrossRef]
- Spinosa, M.R.; Braccini, T.; Ricca, E.; De Felice, M.; Morelli, L.; Pozzi, G.; Oggioni, M.R. On the fate of ingested spores. Res. Microbiol. 2000, 151, 361–368. [Google Scholar] [CrossRef]
- Leser, T.D.; Knarreborg, A.; Worm, J. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J. Appl. Microbiol. 2008, 104, 1025–1033. [Google Scholar] [CrossRef]
- Tam, N.K.M.; Uyen, N.Q.; Hong, H.A.; Duc, L.H.; Hoa, T.T.; Serra, C.R.; Henriques, A.; Cutting, S.M. The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 2006, 188, 2692–2700. [Google Scholar] [CrossRef]
- Sha, H.; He, X.; Yan, K.; Li, J.; Li, X.; Xie, Y.; Yang, Y.; Deng, Y.; Li, G.; Yang, J. Blocking coprophagy increases the levels of inflammation and depression in healthy mice as well as mice receiving fecal microbiota transplantation from disease model mice donors. APMIS 2023, 131, 351–368. [Google Scholar] [CrossRef]
- Auger, S.; Ramarao, N.; Faille, C.; Fouet, A.; Aymerich, S.; Gohar, M. Biofilm Formation and Cell Surface Properties among Pathogenic and Nonpathogenic Strains of the Bacillus cereus Group. Appl. Environ. Microbiol. 2009, 75, 6616–6618. [Google Scholar] [CrossRef]
- Kennedy, E.A.; King, K.Y.; Baldridge, M.T. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front. Physiol. 2018, 9, 417794. [Google Scholar] [CrossRef]
- Glastras, S.J.; Chen, H.; Teh, R.; McGrath, R.T.; Chen, J.; Pollock, C.A.; Wong, M.; Saad, S. Mouse Models of Diabetes, Obesity and Related Kidney Disease. PLoS ONE 2016, 11, e0162131. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Y.; Yan, H.; Shi, Z.; Evans, M.R.; Yu, X.; Lee, Y.; Chen, S.; Williams, A.; Philippe, J.; Roth, M.; et al. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc. Natl. Acad. Sci. USA 2015, 112, 2503–2508. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kwon, J.; Kim, M.-S.; Park, H.; Ji, Y.; Holzapfel, W.; Hyun, C.K. Protective effects of Bacillus probiotics against high-fat diet-induced metabolic disorders in mice. PLoS ONE 2018, 13, e0210120. [Google Scholar] [CrossRef]
- Wang, P.; Gao, X.; Li, Y.; Wang, S.; Yu, J.; Wei, Y. Bacillus natto regulates gut microbiota and adipose tissue accumulation in a high-fat diet mouse model of obesity. J. Funct. Foods 2020, 68, 103923. [Google Scholar] [CrossRef]
- Cao, G.T.; Dai, B.; Wang, K.L.; Yan, Y.; Xu, Y.L.; Wang, Y.X.; Yang, C.M. Bacillus licheniformis, a potential probiotic, inhibits obesity by modulating colonic microflora in C57BL/6J mice model. J. Appl. Microbiol. 2019, 127, 880–888. [Google Scholar] [CrossRef]
- Wu, D.; Ren, Z.; Pae, M.; Guo, W.; Cui, X.; Merrill, A.H.; Meydani, S. Aging Up-Regulates Expression of Inflammatory Mediators in Mouse Adipose Tissue. J. Immunol. 2007, 179, 4829–4839. [Google Scholar] [CrossRef]
- Christie, G.; Setlow, P. Bacillus spore germination: Knowns, unknowns and what we need to learn. Cell Signal. 2020, 74, 109729. [Google Scholar] [CrossRef]
- Tetz, G.; Tetz, V. Introducing the sporobiota and sporobiome. Gut Pathog. 2017, 9, 38. [Google Scholar] [CrossRef]
- Rook, G.A.W.; Raison, C.L.; Lowry, C.A. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin. Exp. Immunol. 2014, 177, 1–12. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, X.; Yang, T.; Shang, J.; Shang, L.; Mai, H.; Qi, G. Immunomodulation therapy of diabetes by oral administration of a surfactin lipopeptide in NOD mice. Vaccine 2014, 32, 6812–6819. [Google Scholar] [CrossRef]
- Hirt, H. Healthy soils for healthy plants for healthy humans. EMBO Rep. 2020, 21, e51069. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, B.; Müller, H.; Berg, G. An Apple a Day: Which Bacteria Do We Eat With Organic and Conventional Apples? Front. Microbiol. 2019, 10, 475179. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Guo, B.; Guo, Y.; Han, M.; Xu, H.; Luo, R.; Hong, Z.; Zhang, B.; Dong, K.; Wu, J.; et al. Association between Residential Greenness and Gut Microbiota in Chinese Adults. Environ. Int. 2022, 163, 107216. [Google Scholar] [CrossRef] [PubMed]
- Cruells, A.; Cabrera-Rubio, R.; Bustamante, M.; Pelegrí, D.; Cirach, M.; Jimenez-Arenas, P.; Samarra, A.; Martínez-Costa, C.; Collado, M.; Gascon, M. The Influence of Pre- and Postnatal Exposure to Air Pollution and Green Spaces on Infant’s Gut Microbiota: Results from the MAMI Birth Cohort Study. Environ. Res. 2024, 257, 119283. [Google Scholar] [CrossRef]
- Horwell, E.; Bearn, P.; Cutting, S.M. A microbial symphony: A literature review of the factors that orchestrate the colonization dynamics of the human colonic microbiome during infancy and implications for future health. Microbiome Res. Rep. 2024, 3, 50. [Google Scholar] [CrossRef]
- Roslund, M.; Puhakka, R.; Grönroos, M.; Nurminen, N.; Oikarinen, S.; Gazali, A.; Cinek, O.; Kramná, L.; Siter, N.; Vari, H.; et al. Biodiversity Intervention Enhances Immune Regulation and Health-Associated Commensal Microbiota among Daycare Children. Sci. Adv. 2020, 6, eaba2578. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horwell, E.; Ferreira, W.; Hong, H.; Bearn, P.; Cutting, S. Modelling a Western Lifestyle in Mice: A Novel Approach to Eradicating Aerobic Spore-Forming Bacteria from the Colonic Microbiome and Assessing Long-Term Clinical Outcomes. Biomedicines 2024, 12, 2274. https://doi.org/10.3390/biomedicines12102274
Horwell E, Ferreira W, Hong H, Bearn P, Cutting S. Modelling a Western Lifestyle in Mice: A Novel Approach to Eradicating Aerobic Spore-Forming Bacteria from the Colonic Microbiome and Assessing Long-Term Clinical Outcomes. Biomedicines. 2024; 12(10):2274. https://doi.org/10.3390/biomedicines12102274
Chicago/Turabian StyleHorwell, Edward, William Ferreira, Huynh Hong, Philip Bearn, and Simon Cutting. 2024. "Modelling a Western Lifestyle in Mice: A Novel Approach to Eradicating Aerobic Spore-Forming Bacteria from the Colonic Microbiome and Assessing Long-Term Clinical Outcomes" Biomedicines 12, no. 10: 2274. https://doi.org/10.3390/biomedicines12102274





