Diabetes Control Status and Severity of Depression: Insights from NHANES 2005–2020
Abstract
1. Introduction
2. Materials and Methods
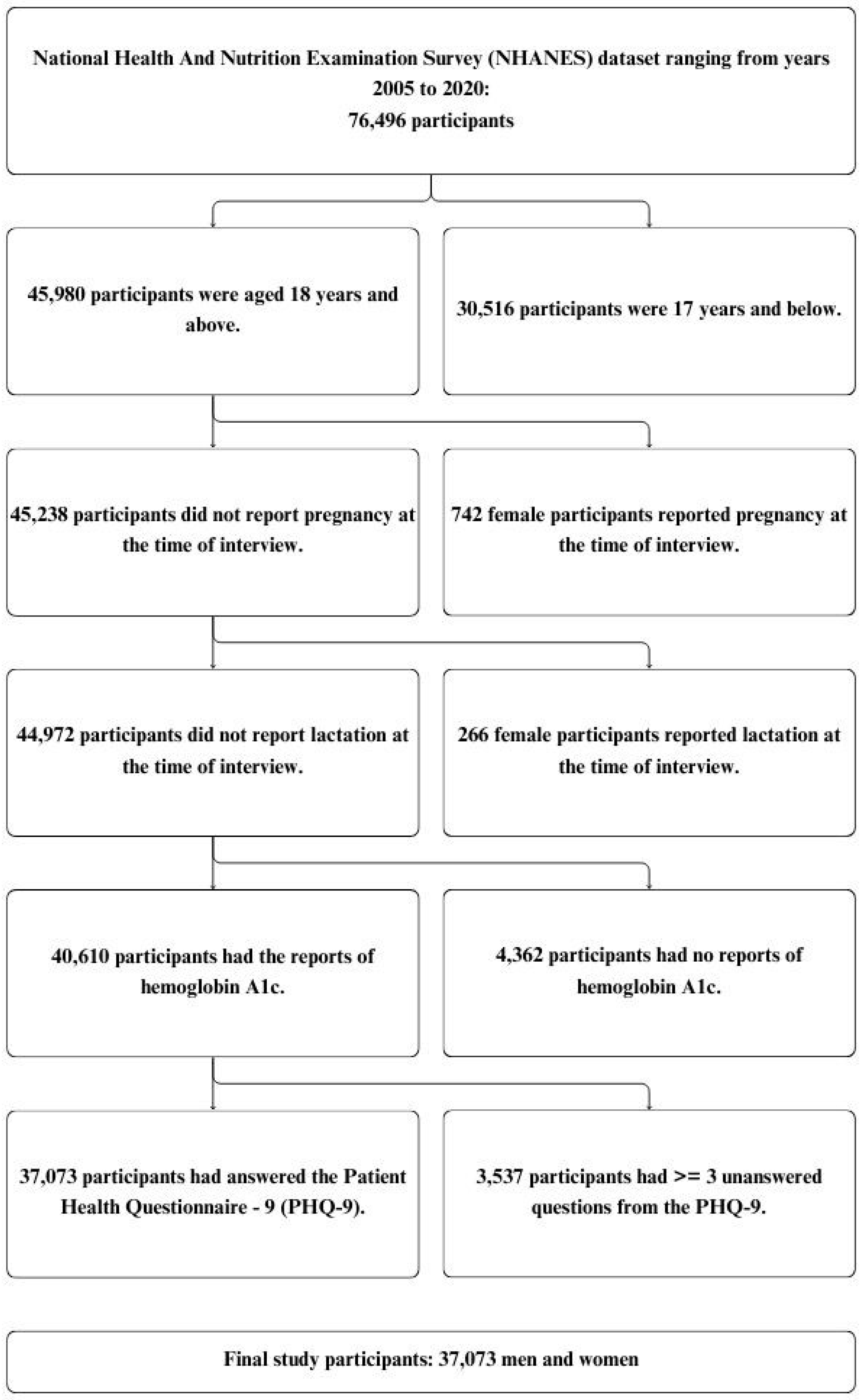
2.1. Source of Data
2.2. Statistical Analysis
2.2.1. Examining the Association between Glycemic Control Status and Various Severities of Depression
2.2.2. Examining the Associations between Diabetes Control Status and Various Severities of Depression
2.2.3. Examining the Association between Diabetes Control Status and Various Severities of Depression among Participants with Diabetes, Stratified by BMI and Race/Ethnicity
3. Results
3.1. Glycemic Control Status and Various Severities of Depression
3.2. Diabetes Control Status and Various Severities of Depression
3.3. Diabetes Control Status and Various Severities of Depression among Participants with Diabetes, Stratified by BMI and Race/Ethnicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lloyd, C.E.; Roy, T.; Nouwen, A.; Chauhan, A.M. Epidemiology of Depression in Diabetes: International and Cross-Cultural Issues. J. Affect. Disord. 2012, 142, S22–S29. [Google Scholar] [CrossRef]
- Roy, T.; Lloyd, C.E. Epidemiology of Depression and Diabetes: A Systematic Review. J. Affect. Disord. 2012, 142, S8–S21. [Google Scholar] [CrossRef]
- Collins, M.M.; Corcoran, P.; Perry, I.J. Anxiety and Depression Symptoms in Patients with Diabetes. Diabet. Med. 2009, 26, 153–161. [Google Scholar] [CrossRef]
- Campayo, A.; de Jonge, P.; Roy, J.F.; Saz, P.; de la Cámara, C.; Quintanilla, M.A.; Marcos, G.; Santabárbara, J.; Lobo, A. Depressive Disorder and Incident Diabetes Mellitus: The Effect of Characteristics of Depression. Am. J. Psychiatry 2010, 167, 580–588. [Google Scholar] [CrossRef]
- Lindekilde, N.; Nefs, G.; Henriksen, J.E.; Lasgaard, M.; Schram, M.; Rubin, K.; Rutters, F.; Kivimaki, M.; Pouwer, F. Psychiatric Disorders as Risk Factors for the Development of Type 2 Diabetes Mellitus: An Umbrella Review Protocol. BMJ Open 2019, 9, e024981. [Google Scholar] [CrossRef]
- Coleman, J. Anxiety and Diabetes. Available online: https://www.thediabetescouncil.com/anxiety-and-diabetes/ (accessed on 12 March 2024).
- Egede, L.E.; Dismuke, C.E. Serious Psychological Distress and Diabetes: A Review of the Literature. Curr. Psychiatry Rep. 2012, 14, 15–22. [Google Scholar] [CrossRef]
- Markowitz, S.; Gonzalez, J.S.; Wilkinson, J.L.; Safren, S.A. Treating Depression in Diabetes: Emerging Findings. Psychosomatics 2011, 52, 1–18. [Google Scholar] [CrossRef]
- Rubin, R.R.; Peyrot, M. Psychological Issues and Treatments for People with Diabetes. J. Clin. Psychol. 2001, 57, 457–478. [Google Scholar] [CrossRef]
- Pouwer, F. Should We Screen for Emotional Distress in Type 2 Diabetes Mellitus? Nat. Rev. Endocrinol. 2009, 5, 665–671. [Google Scholar] [CrossRef]
- Tabák, A.G.; Akbaraly, T.N.; Batty, G.D.; Kivimäki, M. Depression and Type 2 Diabetes: A Causal Association? Lancet Diabetes Endocrinol. 2014, 2, 236–245. [Google Scholar] [CrossRef]
- Fisher, L.; Hessler, D.M.; Polonsky, W.H.; Mullan, J. When Is Diabetes Distress Clinically Meaningful?: Establishing Cut Points for the Diabetes Distress Scale. Diabetes Care 2012, 35, 259–264. [Google Scholar] [CrossRef]
- Meurs, M.; Roest, A.M.; Wolffenbuttel, B.H.R.; Stolk, R.P.; de Jonge, P.; Rosmalen, J.G.M. Association of Depressive and Anxiety Disorders with Diagnosed versus Undiagnosed Diabetes: An Epidemiological Study of 90,686 Participants. Psychosom. Med. 2016, 78, 233–241. [Google Scholar] [CrossRef]
- Mosili, P.; Mkhize, B.C.; Sibiya, N.H.; Ngubane, P.S.; Khathi, A. Review of the Direct and Indirect Effects of Hyperglycemia on the HPA Axis in T2DM and the Co-Occurrence of Depression. BMJ Open Diabetes Res. Care 2024, 12, e003218. [Google Scholar] [CrossRef]
- Barnard, K.; Peveler, R.C.; Holt, R.I.G. Antidepressant Medication as a Risk Factor for Type 2 Diabetes and Impaired Glucose Regulation: Systematic review. Diabetes Care 2013, 36, 3337–3345. [Google Scholar] [CrossRef]
- Darwish, L.; Beroncal, E.; Sison, M.V.; Swardfager, W. Depression in People with Type 2 Diabetes: Current Perspectives. Diabetes Metab. Syndr. Obesity Targets Ther. 2018, 11, 333–343. [Google Scholar] [CrossRef]
- Basiri, R.; Seidu, B.; Rudich, M. Exploring the Interrelationships between Diabetes, Nutrition, Anxiety, and Depression: Implications for Treatment and Prevention Strategies. Nutrients 2023, 15, 4226. [Google Scholar] [CrossRef]
- Hunter, J.C.; DeVellis, B.M.; Jordan, J.M.; Kirkman, M.S.; Linnan, L.A.; Rini, C.; Fisher, E.B. The Association of Depression and Diabetes across Methods, Measures, and Study Contexts. Clin. Diabetes Endocrinol. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Svenningsson, I.; Björkelund, C.; Marklund, B.; Gedda, B. Anxiety and Depression in Obese and Normal-Weight Individuals with Diabetes Type 2: A Gender Perspective. Scand. J. Caring Sci. 2012, 26, 349–354. [Google Scholar] [CrossRef][Green Version]
- Golden, S.H.; Lazo, M.; Carnethon, M.; Bertoni, A.G.; Schreiner, P.J.; Roux, A.V.D.; Lee, H.B.; Lyketsos, C. Examining a Bidirectional Association between Depressive Symptoms and Diabetes. JAMA 2008, 299, 2751–2759. [Google Scholar] [CrossRef]
- Katon, W.J.; Russo, J.E.; Heckbert, S.R.; Lin, E.H.B.; Ciechanowski, P.; Ludman, E.; Young, B.; Von Korff, M. The Relationship between Changes in Depression Symptoms and Changes in Health Risk Behaviors in Patients with Diabetes. Int. J. Geriatr. Psychiatry 2010, 25, 466–475. [Google Scholar] [CrossRef]
- Bădescu, S.V.; Tătaru, C.; Kobylinska, L.; Georgescu, E.L.; Zahiu, D.M.; Zăgrean, A.M.; Zăgrean, L. The Association between Diabetes Mellitus and Depression. J. Med. Life 2016, 9, 120–125. [Google Scholar]
- NHANES. Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=1999 (accessed on 12 March 2024).
- National Health and Nutrition Examination Survey (NHANES)|CMS. Available online: https://www.cms.gov/About-CMS/Agency-Information/OMH/resource-center/hcps-and-researchers/data-tools/sgm-clearinghouse/nhanes (accessed on 2 August 2024).
- NHANES. About the National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 2 August 2024).
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- CDC Diabetes and Mental Health. Available online: https://www.cdc.gov/diabetes/living-with/mental-health.html (accessed on 12 March 2024).
- Li, C.; Shang, S.; Liang, W. Physical Activity Types, Physical Activity Levels and Risk of Diabetes in General Adults: The NHANES 2007–2018. Int. J. Environ. Res. Public Health 2023, 20, 1398. [Google Scholar] [CrossRef]
- Lim, E.; Davis, J.; Chen, J.J. The Association of Race/Ethnicity, Dietary Intake, and Physical Activity with Depression. J. Racial Ethn. Health Disparities 2021, 8, 315–331. [Google Scholar] [CrossRef]
- Egede, L.E.; Poston, M.E. Racial/Ethnic Differences in Leisure-Time Physical Activity Levels among Individuals with Diabetes. Diabetes Care 2004, 27, 2493–2494. [Google Scholar] [CrossRef]
- Sartorius, N. Depression and Diabetes. Dialogues Clin. Neurosci. 2018, 20, 47–52. [Google Scholar] [CrossRef]
- Engum, A. The Role of Depression and Anxiety in Onset of Diabetes in a Large Population-Based Study. J. Psychosom. Res. 2007, 62, 31–38. [Google Scholar] [CrossRef]
- Wiltink, J.; Beutel, M.E.; Till, Y.; Ojeda, F.M.; Wild, P.S.; Münzel, T.; Blankenberg, S.; Michal, M. Prevalence of Distress, Comorbid Conditions and Well Being in the General Population. J. Affect. Disord. 2011, 130, 429–437. [Google Scholar] [CrossRef]
- Gary-Webb, T.L.; Baptiste-Roberts, K.; Pham, L.; Wesche-Thobaben, J.; Patricio, J.; Pi-Sunyer, F.X.; Brown, A.F.; Jones-Corneille, L.; Brancati, F.L.; Look AHEAD Research Group. Neighborhood Socioeconomic Status, Depression, and Health Status in the Look AHEAD (Action for Health in Diabetes) Study. BMC Public Health 2011, 11, 349. [Google Scholar] [CrossRef]
- Ludman, E.J.; Katon, W.; Russo, J.; Von Korff, M.; Simon, G.; Ciechanowski, P.; Lin, E.; Bush, T.; Walker, E.; Young, B. Depression and Diabetes Symptom Burden. Gen. Hosp. Psychiatry 2004, 26, 430–436. [Google Scholar] [CrossRef]
- Renn, B.N.; Feliciano, L.; Segal, D.L. The Bidirectional Relationship of Depression and Diabetes: A Systematic Review. Clin. Psychol. Rev. 2011, 31, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Wium-Andersen, I.K.; Hengeveld, E.M.; Rungby, J.; Jørgensen, M.B.; Osler, M.; Wium-Andersen, M.K. Hemoglobin A1c-Levels and Subsequent Risk of Depression in Individuals with and without Diabetes. J. Diabetes Its Complicat. 2021, 35, 107946. [Google Scholar] [CrossRef] [PubMed]
- Penckofer, S.; Quinn, L.; Byrn, M.; Ferrans, C.; Miller, M.; Strange, P. Does Glycemic Variability Impact Mood and Quality of Life? Diabetes Technol. Ther. 2012, 14, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.H.B.; Rutter, C.M.; Katon, W.; Heckbert, S.R.; Ciechanowski, P.; Oliver, M.M.; Ludman, E.J.; Young, B.A.; Williams, L.H.; McCulloch, D.K.; et al. Depression and Advanced Complications of Diabetes: A Prospective Cohort Study. Diabetes Care 2009, 33, 264–269. [Google Scholar] [CrossRef]

| Variable (Number of Participants 1) | Total Participants (n = 37,073) | Normoglycemia (n = 22,393) | Prediabetes (n = 8815) | Diabetes (n = 5865) |
|---|---|---|---|---|
| Depression Categories | ||||
| No Depression | 76.63% | 77.80% | 76.91% | 69.47% |
| Mild Depression | 15.53% | 15.12% | 15.01% | 18.72% |
| Moderate to Severe Depression | 7.84% | 7.07% | 8.08% | 11.82% |
| Age Categories | ||||
| 18–34 Years | 28.38% | 38.16% | 9.86% | 4.28% |
| 35–44 Years | 17.18% | 19.55% | 13.56% | 9.82% |
| 45–54 Years | 19.02% | 18.23% | 20.88% | 20.35% |
| 55–64 Years | 16.72% | 12.72% | 24.23% | 26.70% |
| 65+ Years | 18.70% | 11.34% | 31.47% | 38.86% |
| Sex | ||||
| Male | 49.73% | 49.90% | 47.70% | 52.19% |
| Female | 50.27% | 50.10% | 52.30% | 47.81% |
| Race/Ethnic Groups | ||||
| Non-Hispanic White | 67.87% | 70.73% | 62.69% | 60.39% |
| Non-Hispanic Black | 10.68% | 8.41% | 15.59% | 15.20% |
| Mexican American | 8.45% | 8.26% | 8.29% | 9.82% |
| Non-Mexican Hispanic | 5.71% | 5.70% | 5.55% | 6.08% |
| Other Races—Including Multi-Racial | 7.29% | 6.91% | 7.88% | 8.51% |
| Body Mass Index (BMI) Categories | ||||
| Normal Weight | 1.60% | 2.01% | 0.99% | 0.31% |
| Underweight | 28.01% | 33.96% | 18.59% | 10.14% |
| Overweight | 32.50% | 33.65% | 33.08% | 24.97% |
| Class I Obese | 20.50% | 18.14% | 23.51% | 28.83% |
| Class II Obese | 9.54% | 7.04% | 13.17% | 17.56% |
| Class III Obese | 7.11% | 4.57% | 9.97% | 16.75% |
| Missing | 0.73% | 0.62% | 0.70% | 1.44% |
| Physical Activity Categories | ||||
| No Activity | 18.83% | 14.65% | 24.00% | 33.80% |
| Quartile 1 | 19.25% | 18.22% | 21.56% | 21.16% |
| Quartile 2 | 24.46% | 25.85% | 21.28% | 21.93% |
| Quartile 3 | 18.60% | 20.53% | 15.95% | 12.16% |
| Quartile 4 | 18.84% | 20.72% | 17.18% | 10.95% |
| Missing | 0.02% | 0.03% | 0.02% | 0.00% |
| Glycemic Control Status | Number | Depression Categories | |||
|---|---|---|---|---|---|
| Mild Depression | Moderate to Severe Depression | ||||
| Odds Ratio | 95%CI | Odds Ratio | 95%CI | ||
| Model 1 * | |||||
| Normoglycemia | 22,393 | 1 (reference) | 1 (reference) | ||
| Prediabetes | 8815 | 1.07 | 0.96–1.19 | 1.25 | 1.09–1.45 |
| Diabetes (HbA1C < 5.7%) | 367 | 2.11 | 1.42–3.15 | 2.00 | 1.40–2.88 |
| Diabetes (5.7% ≤ HbA1c < 10.0%) | 4915 | 1.48 | 1.32–1.66 | 2.14 | 1.81–2.54 |
| Diabetes (HbA1c ≥ 10.0%) | 583 | 1.71 | 1.22–2.40 | 2.86 | 2.07–3.94 |
| Model 2 ** | |||||
| Normoglycemia | 22,393 | 1 (reference) | 1 (reference) | ||
| Prediabetes | 8815 | 0.97 | 0.87–1.09 | 1.10 | 0.95–1.28 |
| Diabetes (HbA1C < 5.7%) | 367 | 1.90 | 1.26–2.85 | 1.70 | 1.19–2.42 |
| Diabetes (5.7% ≤ HbA1c < 10.0%) | 4915 | 1.24 | 1.10–1.40 | 1.63 | 1.38–1.92 |
| Diabetes (HbA1c ≥ 10.0%) | 583 | 1.49 | 1.04–2.12 | 2.31 | 1.68–3.17 |
| Diabetes Control Status | Number | Depression Categories | |||
|---|---|---|---|---|---|
| Mild Depression | Moderate to Severe Depression | ||||
| Odds Ratio | 95%CI | Odds Ratio | 95%CI | ||
| Model 1 * | |||||
| Diabetes (HbA1C < 5.7%) | 367 | 1.46 | 0.99–2.16 | 0.94 | 0.65–1.36 |
| Diabetes (5.7% ≤ HbA1c < 10.0%) | 4915 | 1 (reference) | 1 (reference) | ||
| Diabetes (HbA1c ≥ 10.0%) | 583 | 1.24 | 0.87–1.76 | 1.42 | 1.01–2.01 |
| Model 2 ** | |||||
| Diabetes (HbA1C < 5.7%) | 367 | 1.54 | 1.02–2.34 | 1.07 | 0.75–1.54 |
| Diabetes (5.7% ≤ HbA1c < 10.0%) | 4915 | 1 (reference) | 1 (reference) | ||
| Diabetes (HbA1c ≥ 10.0%) | 583 | 1.26 | 0.89–1.79 | 1.53 | 1.07–2.19 |
| Diabetes Control Status | Depression Categories | |||||||
| Mild Depression | Moderate to Severe Depression | |||||||
| Non-Hispanic Whites | Other Races/Ethnicities | Non-Hispanic Whites | Other Races/Ethnicities | |||||
| Odds Ratio | 95%CI | Odds Ratio | 95%CI | Odds Ratio | 95%CI | Odds Ratio | 95%CI | |
| HbA1C < 5.7% | 1.53 | 0.81–2.88 | 1.66 | 1.08–2.54 | 0.74 | 0.39–1.42 | 1.81 | 1.24–2.64 |
| 5.7% ≤ HbA1c < 10.0% | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| HbA1c ≥ 10.0% | 1.44 | 0.72–2.85 | 1.13 | 0.79–1.63 | 1.93 | 0.99–3.74 | 1.42 | 0.99–2.04 |
| Diabetes Control Status | Depression Categories | |||||||
| Mild Depression | Moderate to Severe Depression | |||||||
| BMI < 25 kg/m2 | BMI ≥ 25 kg/m2 | BMI < 25 kg/m2 | BMI ≥ 25 kg/m2 | |||||
| Odds Ratio | 95%CI | Odds Ratio | 95%CI | Odds Ratio | 95%CI | Odds Ratio | 95%CI | |
| HbA1C < 5.7% | 0.50 | 0.20–1.29 | 1.62 | 1.03–2.55 | 4.29 | 1.81–10.17 | 0.74 | 0.50–1.10 |
| 5.7% ≤ HbA1c < 10.0% | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| HbA1c ≥ 10.0% | 1.51 | 0.53–4.28 | 1.13 | 0.79–1.62 | 2.25 | 0.84–5.99 | 1.44 | 0.98–2.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basiri, R.; Rajanala, Y.; Kassem, M.; Cheskin, L.J.; Frankenfeld, C.L.; Farvid, M.S. Diabetes Control Status and Severity of Depression: Insights from NHANES 2005–2020. Biomedicines 2024, 12, 2276. https://doi.org/10.3390/biomedicines12102276
Basiri R, Rajanala Y, Kassem M, Cheskin LJ, Frankenfeld CL, Farvid MS. Diabetes Control Status and Severity of Depression: Insights from NHANES 2005–2020. Biomedicines. 2024; 12(10):2276. https://doi.org/10.3390/biomedicines12102276
Chicago/Turabian StyleBasiri, Raedeh, Yatisha Rajanala, Megan Kassem, Lawrence J. Cheskin, Cara L. Frankenfeld, and Maryam S. Farvid. 2024. "Diabetes Control Status and Severity of Depression: Insights from NHANES 2005–2020" Biomedicines 12, no. 10: 2276. https://doi.org/10.3390/biomedicines12102276
APA StyleBasiri, R., Rajanala, Y., Kassem, M., Cheskin, L. J., Frankenfeld, C. L., & Farvid, M. S. (2024). Diabetes Control Status and Severity of Depression: Insights from NHANES 2005–2020. Biomedicines, 12(10), 2276. https://doi.org/10.3390/biomedicines12102276






