Ketamine, Esketamine, and Arketamine: Their Mechanisms of Action and Applications in the Treatment of Depression and Alleviation of Depressive Symptoms
Abstract
:1. Introduction
2. Ketamine
3. Esketamine
4. Arketamine
| Condition | References |
|---|---|
| Cognitive impairments | [16,34,75,76,77,78,79,80,81,82,83,84,85,86] |
| COVID-19 | [87,88,89,90,91,92,93] |
| Inflammatory disease | [36,37,78,91,94,95,96,97,98] |
| Ischemic stroke | [99,100,101,102,103] |
| Multiple sclerosis | [48,92] |
| Organophosphate poisoning | [104,105,106,107,108] |
| Osteoporosis | [109,110,111,112,113] |
| Parkinson’s disease | [114,115,116,117,118] |
| Perioperative anesthesia | [13,96,119,120,121,122,123,124,125,126] |
| Substance use disorder | [127,128,129] |
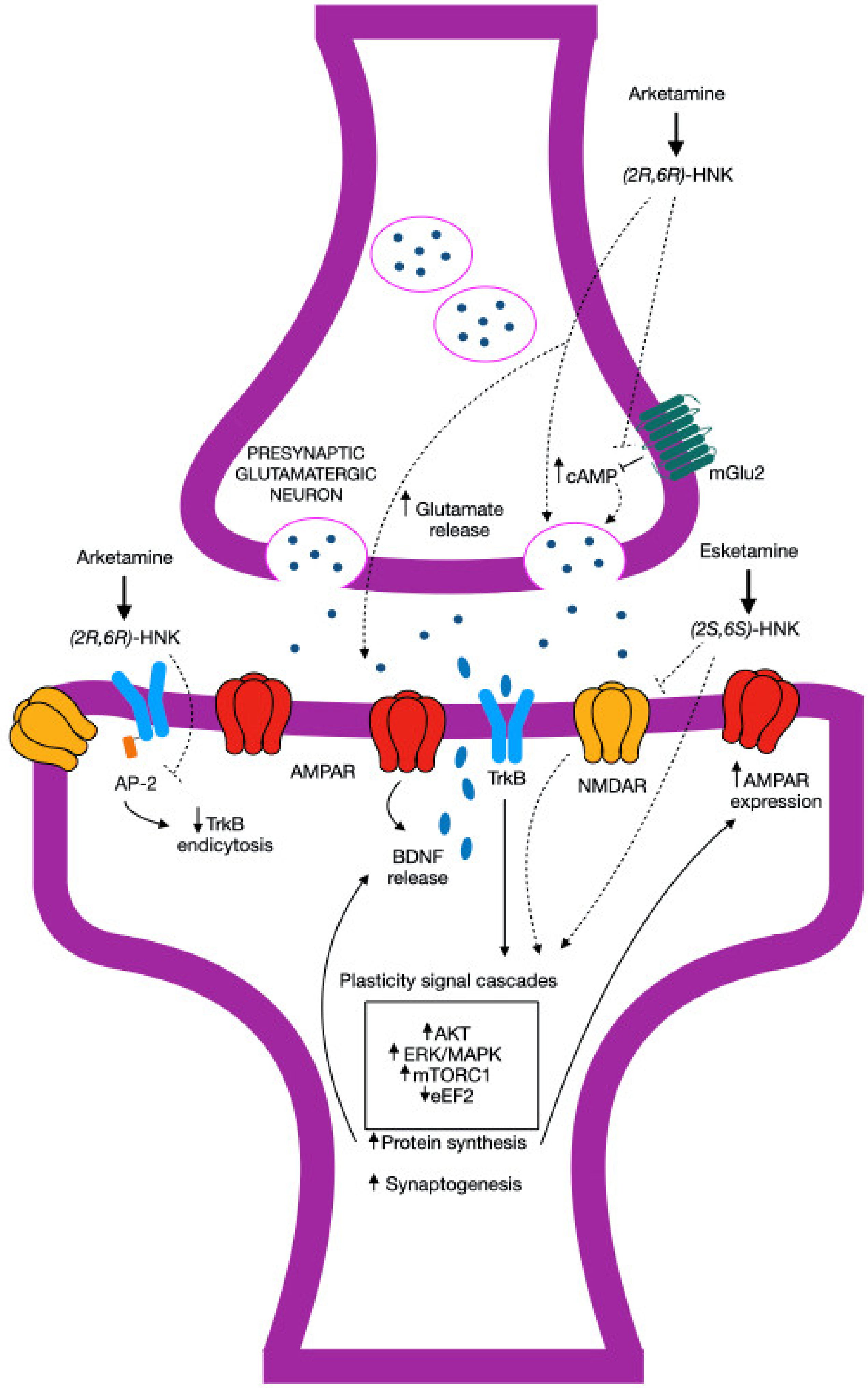
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ball, C.; Westhorpe, R. Intravenous Induction Agents: Ketamine. Anaesth. Intensive Care 2002, 30, 115. [Google Scholar] [CrossRef]
- Mion, G. History of anaesthesia The ketamine story—Past, present and future. Eur. J. Anaesthesiol. 2017, 34, 571–575. [Google Scholar] [CrossRef]
- Denomme, N.B.S. The Domino Effect: Ed Domino’s early studies of Psychoactive Drugs. J. Psychoact. Drugs 2018, 50, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Das, J. Repurposing of Drugs-The Ketamine Story. J. Med. Chem. 2020, 63, 13514–13525. [Google Scholar] [CrossRef]
- Bloomfield, A.; Chan, N.; Fryml, L.; Horace, R.; Pyati, S. Ketamine for Chronic Pain and Mental Health: Regulations, Legalities, and the Growth of Infusion Clinics. Curr. Pain Headache Rep. 2023, 27, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, J.; Jiang, H.; Xie, K. Ketamine in the Management of Acute Pain: A Comprehensive Meta-Analysis. J. Coll. Physicians Surg. Pak. 2024, 34, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Merelman, A.H.; Perlmutter, M.C.; Strayer, R.J. Alternatives to Rapid Sequence Intubation: Contemporary Airway Management with Ketamine. W. J. Emerg. Med. 2019, 20, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.A.; Paciullo, C.A. Subdissociative Ketamine Use in the Emergency Department. Adv. Emerg. Nurs. J. 2019, 41, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mashour, G.A. Ketamine and the paradox of anaesthetic state transitions. Br. J. Anaesth. 2024, 132, 224–226. [Google Scholar] [CrossRef]
- Tian, F.; Lewis, L.D.; Zhou, D.W.; Balanza, G.A.; Paulk, A.C.; Zelmann, R.; Peled, N.; Soper, D.; Santa Cruz Mercado, L.A.; Peterfreund, R.A.; et al. Characterizing brain dynamics during ketamine-induced dissociation and subsequent interactions with propofol using human intracranial neurophysiology. Nat. Commun. 2023, 14, 1748. [Google Scholar] [CrossRef]
- McMurray, M.; Orthober, R.; Huecker, M. Ketamine’s love story with the heart: A Takotsubo twist. Am. J. Emerg. Med. 2024, 77, 232.e5–232.e7. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.L.; Silvay, G. Ketamine: An update on the first twenty-five years of clinical experience. Can. J. Anaesth. 1989, 36, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Barrett, W.; Buxhoeveden, M.; Dhillon, S. Ketamine: A versatile tool for anesthesia and analgesia. Curr. Opin. Anaesthesiol. 2020, 33, 633–638. [Google Scholar] [CrossRef]
- Nowacka, A.; Borczyk, M. Ketamine applications beyond anesthesia—A literature review. Eur. J. Pharmacol. 2019, 860, 172547. [Google Scholar] [CrossRef] [PubMed]
- Wilkowska, A.; Wiglusz, M.S.; Jakuszkowiak-Wojten, K.; Cubała, W.J. Ketamine and Lamotrigine Combination in Psychopharmacology: Systematic Review. Cells 2022, 11, 645. [Google Scholar] [CrossRef]
- Passie, T.; Adams, H.A.; Logemann, F.; Brandt, S.D.; Wiese, B.; Karst, M. Comparative effects of (S)-ketamine and racemic (R/S)-ketamine on psychopathology, state of consciousness and neurocognitive performance in healthy volunteers. Eur. Neuropsychopharmacol. 2021, 44, 92–104. [Google Scholar] [CrossRef]
- Feder, A.; Rutter, S.B.; Schiller, D.; Charney, D.S. The emergence of ketamine as a novel treatment for posttraumatic stress disorder. Adv. Pharmacol. 2020, 89, 261–286. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; Pereira, E.F.R.; Albuquerque, E.X.; Thomas, C.J.; Zarate, C.A., Jr.; et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef]
- Fukumoto, K.; Duman, R.S. (2R,6R)-Hydroxynorketamine, a Metabolite of Ketamine: The Antidepressant Actions and the Mechanisms. In New Rapid-Acting Antidepressants; Hashimoto, K., Manto, M., Eds.; Contemporary Clinical Neuroscience; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Schwenk, E.S.; Pradhan, B.; Nalamasu, R.; Stolle, L.; Wainer, I.W.; Cirullo, M.; Olson, A.; Pergolizzi, J.V.; Torjman, M.C.; Viscusi, E.R. Ketamine in the Past, Present, and Future: Mechanisms, Metabolites, and Toxicity. Curr. Pain Headache Rep. 2021, 25, 57. [Google Scholar] [CrossRef]
- Kadriu, B.; Ballard, E.D.; Henter, I.D.; Murata, S.; Gerlus, N.; Zarate, C.A., Jr. Neurobiological biomarkers of response to ketamine. Adv. Pharmacol. 2020, 89, 195–235. [Google Scholar] [CrossRef]
- Marguilho, M.; Figueiredo, I.; Castro-Rodrigues, P. A unified model of ketamine’s dissociative and psychedelic properties. J. Psychopharmacol. 2023, 37, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.A.; Werner, C. From the racemate to the eutomer: (S)-ketamine. Renaissance of a substance? Anaesthesist 1997, 46, 1026–1042. [Google Scholar] [CrossRef] [PubMed]
- Lii, T.R.; Singh, V. Ketamine for Complex Regional Pain Syndrome: A Narrative Review Highlighting Dosing Practices and Treatment Response. Anesthesiol. Clin. 2023, 41, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Kohtala, S. Ketamine-50 years in use: From anesthesia to rapid antidepressant effects and neurobiological mechanisms. Pharmacol. Rep. 2021, 73, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chang, L.; Hashimoto, K. A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacol. Biochem. Behav. 2020, 190, 172870. [Google Scholar] [CrossRef]
- Himmelseher, S.; Kochs, E.F. Ready for a “breakthrough” with ketamine? A look at recent pharmacological insights! Curr. Opin. Anaesthesiol. 2021, 34, 393–401. [Google Scholar] [CrossRef]
- Schatzberg, A.F. Mechanisms of Action of Ketamine and Esketamine. Am. J. Psychiatry 2021, 178, 1130. [Google Scholar] [CrossRef]
- Kheirkhah, M.; Nugent, A.C.; Livinski, A.A.; Neely, L.; Johnson, S.C.; Henter, I.D.; Varnosfaderani, S.D.; Price, R.B.; Hejazi, N.; Yavi, M.; et al. Exploring the impact of music on response to ketamine/esketamine: A scoping review. Neurosci. Biobehav. Rev. 2024, 162, 105693. [Google Scholar] [CrossRef]
- Kalkman, H.O. Activation of σ1-Receptors by R-Ketamine May Enhance the Antidepressant Effect of S-Ketamine. Biomedicines 2023, 11, 2664. [Google Scholar] [CrossRef]
- Feeney, A.; Papakostas, G.I. Pharmacotherapy: Ketamine and Esketamine. Psychiatr. Clin. N. Am. 2023, 46, 277–290. [Google Scholar] [CrossRef]
- Zhang, K.; Hashimoto, K. An update on ketamine and its two enantiomers as rapid-acting antidepressant. Expert Rev. Neurother. 2019, 19, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Trimmel, H.; Helbok, R.; Staudinger, T.; Jaksch, W.; Messerer, B.; Schöchl, H.; Likar, R. S(+)-ketamine: Current trends in emergency and intensive care medicine. Wien. Klin. Wochenschr. 2018, 130, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; Ago, Y.; Igarashi, H.; Higuchi, M.; Tanuma, M.; Shimazaki, Y.; Kawai, T.; Seiriki, K.; Hayashida, M.; Yamaguchi, S.; et al. (R)-ketamine restores anterior insular cortex activity and cognitive deficits in social isolation-reared mice. Mol. Psychiatry 2024, 29, 1406–1416. [Google Scholar] [CrossRef]
- Brown, G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflamm. 2019, 16, 180. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, J.; Fujita, Y.; Qu, Y.; Shan, J.; Wan, X.; Wang, X.; Ishima, T.; Kobayashi, K.; Wang, L.; et al. Nuclear factor of activated T cells 4 in the prefrontal cortex is required for prophylactic actions of (R)-ketamine. Transl. Psychiatry 2022, 12, 27. [Google Scholar] [CrossRef]
- Fujita, Y.; Hashimoto, Y.; Hashimoto, H.; Chang, L.; Hashimoto, K. Dextran sulfate sodium-induced inflammation and colitis in mice are ameliorated by (R)-ketamine but not (S)-ketamine: A role of TrkB signaling. Eur. J. Pharmacol. 2021, 897, 173954. [Google Scholar] [CrossRef]
- Zhang, K.; Sakamoto, A.; Chang, L.; Qu, Y.; Wang, S.; Pu, Y.; Tan, Y.; Wang, X.; Fujita, Y.; Ishima, T.; et al. Splenic NKG2D confers resilience versus susceptibility in mice after chronic social defeat stress: Beneficial effects of (R)-ketamine. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 447–456. [Google Scholar] [CrossRef]
- Hashimoto, K. Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine. Biochem. Pharmacol. 2020, 177, 113935. [Google Scholar] [CrossRef]
- Hashimoto, K. Are NMDA and opioid receptors involved in the antidepressant actions of ketamine? Proc. Natl. Acad. Sci. USA 2020, 117, 11200–11201. [Google Scholar] [CrossRef]
- Jelen, L.A.; Young, A.H.; Stone, J.M. Ketamine: A tale of two enantiomers. J. Psychopharmacol. 2021, 35, 109–123. [Google Scholar] [CrossRef]
- Ma, L.; Hashimoto, K. The role of hippocampal KCNQ2 channel in antidepressant actions of ketamine. Neuron 2022, 110, 2201–2203. [Google Scholar] [CrossRef]
- Scotton, E.; Antqueviezc, B.; Vasconcelos, M.F.; Dalpiaz, G.; Géa, L.P.; Ferraz Goularte, J.; Colombo, R.; Ribeiro Rosa, A. Is (R)-ketamine a potential therapeutic agent for treatment-resistant depression with less detrimental side effects? A review of molecular mechanisms underlying ketamine and its enantiomers. Biochem. Pharmacol. 2022, 198, 114963. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chang, L.; Hashimoto, K. Molecular mechanisms underlying the antidepressant actions of arketamine: Beyond the NMDA receptor. Mol. Psychiatry 2022, 27, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Rickards, H. Depression in neurological disorders: Parkinson’s disease, multiple sclerosis, and stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. S1), i48–i52. [Google Scholar] [CrossRef] [PubMed]
- Hesdorffer, D.C. Comorbidity between neurological illness and psychiatric disorders. CNS Spectr. 2016, 21, 230–238. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Hashimoto, K. (R)-ketamine as prophylactic and therapeutic drug for neurological disorders: Beyond depression. Neurosci. Biobehav. Rev. 2022, 139, 104762. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Wan, X.; Tan, Y.; Qu, Y.; Shan, J.; Yang, Y.; Ma, L.; Hashimoto, K. (R)-ketamine ameliorates demyelination and facilitates remyelination in cuprizone-treated mice: A role of gut-microbiota-brain axis. Neurobiol. Dis. 2022, 165, 105635. [Google Scholar] [CrossRef]
- Nikolin, S.; Rodgers, A.; Schwaab, A.; Bahji, A.; Zarate, C.A., Jr.; Vazquez, G.; Loo, C. Ketamine for the treatment of major depression: A systematic review and meta-analysis. eClinicalMedicine 2023, 62, 102127. [Google Scholar] [CrossRef]
- Meshkat, S.; Haikazian, S.; Di Vincenzo, J.D.; Fancy, F.; Johnson, D.; Chen-Li, D.; McIntyre, R.S.; Mansur, R.; Rosenblat, J.D. Oral ketamine for depression: An updated systematic review. World J. Biol. Psychiatry 2023, 24, 545–557. [Google Scholar] [CrossRef]
- Mandal, S.; Sinha, V.K.; Goyal, N. Efficacy of ketamine therapy in the treatment of depression. Indian J. Psychiatry 2019, 61, 480–485. [Google Scholar] [CrossRef]
- Aleksandrova, L.R.; Phillips, A.G.; Yu Wang, Y.T. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J. Psychiatry Neurosci. 2017, 42, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.K.; Kavalali, E.T.; Monteggia, L.M. Ketamine and rapid antidepressant action: New treatments and novel synaptic signaling mechanisms. Neuropsychopharmacology 2024, 49, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Schatzberg, A.F. Ketamine: Promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology 2015, 40, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, O. Esketamine for treatment-resistant depression: A review of clinical evidence (Review). Exp. Ther. Med. 2023, 25, 111. [Google Scholar] [CrossRef]
- Yang, C.; Kobayashi, S.; Nakao, K.; Dong, C.; Han, M.; Qu, Y.; Ren, Q.; Zhang, J.-C.; Ma, M.; Toki, H.; et al. AMPA receptor activation-independent antidepressant actions of ketamine metabolite (S)-norketamine. Biol. Psychiatry 2018, 84, 591–600. [Google Scholar] [CrossRef]
- Farmer, C.A.; Gilbert, J.R.; Moaddel, R.; George, J.; Adeojo, L.; Lovett, J.; Nugent, A.C.; Kadriu, B.; Yuan, P.; Gould, T.D.; et al. Ketamine metabolites, clinical response, and gamma power in a randomized, placebo-controlled, crossover trial for treatment-resistant major depression. Neuropsychopharmacology 2020, 45, 1398–1404. [Google Scholar] [CrossRef]
- Canuso, C.M.; Singh, J.B.; Fedgchin, M.; Alphs, L.; Lane, R.; Lim, P.; Pinter, C.; Hough, D.; Sanacora, G.; Manji, H.; et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: Results of a double-blind, randomized, placebo-controlled study. Am. J. Psychiatry 2018, 175, 620–630. [Google Scholar] [CrossRef]
- Daly, E.J.; Singh, J.B.; Fedgchin, M.; Cooper, K.; Lim, P.; Shelton, R.C.; Thase, M.E.; Winokur, A.; Van Nueten, L.; Manji, H.; et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 2018, 75, 139–148. [Google Scholar] [CrossRef]
- Daly, E.J.; Trivedi, M.H.; Janik, A.; Li, H.; Zhang, Y.; Li, X.; Lane, R.; Lim, P.; Duca, A.R.; Hough, D.; et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 2019, 76, 893–903. [Google Scholar] [CrossRef]
- Popova, V.; Daly, E.J.; Trivedi, M.; Cooper, K.; Lane, R.; Lim, P.; Mazzucco, C.; Hough, D.; Thase, M.E.; Shelton, R.C.; et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: A randomizeddouble-blind active-controlled study. Am. J. Psychiatry 2019, 176, 428–438. [Google Scholar] [CrossRef]
- Fedgchin, M.; Trivedi, M.; Daly, E.J.; Melkote, R.; Lane, R.; Lim, P.; Vitagliano, D.; Blier, P.; Fava, M.; Liebowitz, M.; et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: Results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int. J. Neuropsychopharmacol. 2019, 22, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Ochs-Ross, R.; Daly, E.J.; Zhang, Y.; Lane, R.; Lim, P.; Morrison, R.L.; Hough, D.; Manji, H.; Drevets, W.C.; Sanacora, G.; et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression—TRANSFORM-3. Am. J. Geriatr. Psychiatry 2020, 28, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Annetta, M.G.; Iemma, D.; Garisto, C.; Tafani, C.; Proietti, R. Ketamine: New indications for an old drug. Curr. Drug Targets 2005, 6, 789–794. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, D.; Wu, Z.; Huang, C.; Xu, X.; Xu, X.; Liu, C.; Hashimoto, K.; Yang, C. A bibliometric analysis of research on (R)-ketamine from 2002 to 2021. Neuropharmacology 2022, 218, 109207. [Google Scholar] [CrossRef]
- Zanos, P.; Highland, J.N.; Liu, X.; Troppoli, T.A.; Georgiou, P.; Lovett, J.; Morris, P.J.; Stewart, B.W.; Thomas, C.J.; Thompson, S.M.; et al. (R)-Ketamine exerts antidepressant actions partly via conversion to (2R,6R)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses. Br. J. Pharmacol. 2019, 176, 2573–2592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pu, Y.; Liu, J.; Li, L.; An, C.; Wu, Y.; Zhang, W.; Zhang, W.; Qu, S.; Yan, W. Exploring the multifaceted potential of (R)-ketamine beyond antidepressant applications. Front. Pharmacol. 2024, 15, 1337749. [Google Scholar] [CrossRef]
- Leal, G.; Bandeira, I.; Correia-Melo, F.; Telles, M.; Mello, R.; Vieira, F.; Lima, C.S.; Jesus-Nunes, A.P.; Guerreiro-Costa, L.N.F.; Marback, R.F.; et al. Intravenous arketamine for treatment-resistant depression: Open-label pilot study. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 577–582. [Google Scholar] [CrossRef]
- Leal, G.C.; Souza-Marques, B.; Mello, R.P.; Bandeira, I.D.; Caliman-Fontes, A.T.; Carneiro, B.A.; Faria-Guimarães, D.; Guerreiro-Costa, L.N.F.; Jesus-Nunes, A.P.; Silva, S.S.; et al. Arketamine as adjunctive therapy for treatment-resistant depression: A placebo-controlled pilot study. J. Affect. Disord. 2023, 330, 7–15. [Google Scholar] [CrossRef]
- Bandeira, I.D.; Leal, G.C.; Correia-Melo, F.S.; Souza-Marques, B.; Silva, S.S.; Lins-Silva, D.H.; Mello, R.P.; Vieira, F.; Dorea-Bandeira, I.; Faria-Guimarães, D.; et al. Arketamine for bipolar depression: Open-label, dose-escalation, pilot study. J. Psychiatr. Res. 2023, 164, 229–234. [Google Scholar] [CrossRef]
- Chinese Clinical Trial Registry. Efficacy and Safety of Ketamine, S-Ketamine and R-Ketamine in Treatment Resistant Depression: A Randomized Controlled Trial. Available online: https://www.chictr.org.cn/showproj.html?proj=26844 (accessed on 12 September 2024).
- PR Newswire. Perception Neuroscience’s PCN-101 (R-Ketamine) Demonstrates Tolerability in Phase 1 Single Ascending Dose Study. Available online: https://www.prnewswire.com/news-releases/perception-neurosciences-pcn-101-r-ketamine-demonstrates-tolerability-in-phase-1-single-ascending-dose-study-301231491.html (accessed on 12 September 2024).
- Grunebaum, M.F.; Galfalvy, H.C.; Choo, T.H.; Parris, M.S.; Burke, A.K.; Suckow, R.F.; Cooper, T.B.; Mann, J.J. Ketamine metabolite pilot study in a suicidal depression trial. J. Psychiatr. Res. 2019, 117, 129–134. [Google Scholar] [CrossRef]
- ClinicalTrials. Phase 1 Evaluation of (2R,6R)-Hydroxynorketamine. Available online: https://clinicaltrials.gov/study/NCT04711005 (accessed on 12 September 2024).
- Tan, Y.; Fujita, Y.; Pu, Y.; Chang, L.; Qu, Y.; Wang, X.; Hashimoto, K. Repeated intermittent administration of (R)-ketamine during juvenile and adolescent stages prevents schizophrenia-relevant phenotypes in adult offspring after maternal immune activation: A role of TrkB signaling. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Ide, S.; Ikekubo, Y.; Mishina, M.; Hashimoto, K.; Ikeda, K. Cognitive impairment that is induced by (R)-ketamine is abolished in NMDA GluN2D receptor subunit knockout mice. Int. J. Neuropsychopharmacol. 2019, 22, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Fujita, Y.; Qu, Y.; Chang, L.; Pu, Y.; Wang, S.; Wang, X.; Hashimoto, K. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent repeated intermittent administration of (R)-ketamine, but not (S)-ketamine: Role of BDNF-TrkB signaling. Pharmacol. Biochem. Behav. 2020, 188, 172839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, L.; Wan, X.; Shan, J.; Qu, Y.; Hashimoto, K. (R)-ketamine attenuates LPS-induced endotoxin-derived deliriumthrough inhibition of neuroinflammation. Psychopharmacology 2021, 238, 2743–2753. [Google Scholar] [CrossRef]
- Li, S.H.; Abd-Elrahman, K.S.; Ferguson, S.S.G. Targeting mGluR2/3 for treatment of neurodegenerative and neuropsychiatric diseases. Pharmacol. Ther. 2022, 239, 108275. [Google Scholar] [CrossRef]
- Pałucha-Poniewiera, A.; Bobula, B.; Rafało-Ulińska, A. The antidepressant-like activity and cognitive enhancing effects of the combined administration of (R)-Ketamine and LY341495 in the CUMS model of depression in mice are related to themodulation of excitatory synaptic transmission and LTP in the PFC. Pharmaceuticals 2023, 16, 288. [Google Scholar] [CrossRef]
- Pothorszki, D.; Koncz, S.; Török, D.; Papp, N.; Bagdy, G. Unique effects of (R)-ketamine compared to (S)-ketamine on EEG theta power in rats. Pharmaceuticals 2024, 17, 194. [Google Scholar] [CrossRef]
- Popik, P.; Hogendorf, A.; Bugno, R.; Khoo, S.; Zajdel, P.; Malikowska-Racia, N.; Nikiforuk, A.; Golebiowska, J. Effects of ketamine optical isomers, psilocybin, psilocin and norpsilocin on time estimation and cognition in rats. Psychopharmacology 2022, 239, 1689–1703. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiang, H.; Cai, Y.; Meng, S.S.; Zhang, Y.; aQiu, P. Systematic evaluation of the associations between mental disorders and dementia: An umbrella review of systematic reviews and meta-analyses. J. Affect. Disord. 2022, 307, 301–309. [Google Scholar] [CrossRef]
- Sabates, J.; Chiu, W.H.; Loi, S.; Lampit, A.; Gavelin, H.M.; Chong, T.; Launder, N.; Goh, A.M.Y.; Brodtmann, A.; Lautenschlager, N.; et al. The associations between neuropsychiatric symptoms and cognition in people with dementia: A systematic review and meta-analysis. Neuropsychol. Rev. 2024, 34, 581–597. [Google Scholar] [CrossRef]
- Hashimoto, K. Arketamine for cognitive impairment in psychiatric disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1513–1525. [Google Scholar] [CrossRef] [PubMed]
- Shafique, H.; Demers, J.C.; Biesiada, J.; Golani, L.K.; Cerne, R.; Smith, J.L.; Szostak, M.; Witkin, J.M. (R)-(-)-Ketamine: The Promise of a Novel Treatment for Psychiatric and Neurological Disorders. Int. J. Mol. Sci. 2024, 25, 6804. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.J.; Elliott, M.; Seminerio, M.J.; Matsumoto, R.R. Evaluation of sigma (σ) receptors in the antidepressant-like effects of ketamine in vitro and in vivo. Eur. Neuropsychopharmacol. 2012, 22, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Ortoleva, J. Consider Adjunctive Ketamine in mechanically ventilated coronavirus disease-2019 Patients. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2580. [Google Scholar] [CrossRef] [PubMed]
- Akinosoglou, K.; Gogos, A.; Papageorgiou, C.; Angelopoulos, E.; Gogos, C. Ketamine in COVID-19 patients: Thinking out of the box. J. Med. Virol. 2021, 93, 4069–4070. [Google Scholar] [CrossRef]
- Hashimoto, K. Repurposing of CNS drugs to treat COVID-19 infection: Targeting the sigma-1 receptor. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 249–258. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, L.; Hashimoto, Y.; Wan, X.; Shan, J.; Qu, Y.; Hashimoto, K. (R)-ketamine ameliorates lethal inflammatory responses and multi-organ injury in mice induced by cecum ligation and puncture. Life Sci. 2021, 284, 119882. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Tan, Y.; Qu, Y.; Shan, J.; Hashimoto, K. (R)-ketamine ameliorates the progression of experimental autoimmune encephalomyelitis in mice. Brain Res. Bull. 2021, 177, 316–323. [Google Scholar] [CrossRef]
- Vollenweider, F.; Leenders, K.; Oye, I.; Hell, D.; Angst, J. Differential psychopathology and patterns of cerebral glucose utilization produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur. Neuropsychopharmacol. 1997, 7, 25–38. [Google Scholar] [CrossRef]
- Lu, Y.; Ding, X.; Wu, X.; Huang, S. Ketamine inhibits LPS-mediated BV2 microglial inflammation via NMDA receptor blockage. Fundam. Clin. Pharmacol. 2020, 34, 229–237. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Zhou, C.; Wu, H. Ketamine inhibits LPS-induced HGMB1 release in vitro and in vivo. Int. Immunopharmacol. 2014, 23, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Cao, Q.; Luo, S.; He, L.; Yang, C.; Chen, J.; Qi, Q.; Hashimoto, K.; Zhang, J.-C. Microglial ERKNRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol. Psychiatry 2022, 27, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D.; Vallerand, I.A.; Shaheen, A.A.; Lowerison, M.W.; Swain, M.G.; Barnabe, C.; Patten, S.B.; Kaplan, G.G. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut 2019, 68, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, T.; Wu, Z.; Wang, Y.; Liu, H.; Zhang, B.; Yang, S.; Wang, D.; Huang, C.; Duan, J.; et al. The role of CD38 in inflammation-induced depression-like behavior and the antidepressant effect of (R)-ketamine. Brain Behav. Immun. 2024, 115, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Abdoulaye, I.A.; Wu, S.S.; Chibaatar, E.; Yu, D.F.; Le, K.; Cao, X.J.; Guo, Y.-J. Ketamine induces lasting antidepressant effects by modulating the NMDAR/CaMKIImediated synaptic plasticity of the hippocampal dentate gyrus in depressive stroke model. Neural Plast. 2021, 2021, 6635084. [Google Scholar] [CrossRef]
- Shu, L.; Li, T.; Han, S.; Ji, F.; Pan, C.; Zhang, B.; Li, J. Inhibition of neuronspecific CREB dephosphorylation is involved in propofol and ketamine-induced neuroprotection against cerebral ischemic injuries of mice. Neurochem. Res. 2012, 37, 49–58. [Google Scholar] [CrossRef]
- Zhang, L.M.; Wu, Z.Y.; Liu, J.Z.; Li, Y.; Lv, J.M.; Wang, L.Y.; Shan, Y.D.; Song, R.-X.; Miao, H.-T.; Zhang, W.; et al. Subanesthetic dose of S-ketamine improved cognitive dysfunction via the inhibition of hippocampal astrocytosis in a mouse model of post-stroke chronic stress. J. Psychiatr. Res. 2023, 158, 1–14. [Google Scholar] [CrossRef]
- Johnston, J.N.; Henter, I.D.; Zarate, C.A., Jr. The antidepressant actions of ketamine and its enantiomers. Pharmacol. Ther. 2023, 246, 108431. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Chang, L.; Qu, Y.; Pu, Y.; Wang, S.; Fujita, Y.; Ishima, T.; Chen, J.; Hashimoto, K. Neuronal brain injury after cerebral ischemic stroke is ameliorated after subsequent administration of (R)-ketamine, but not (S)-ketamine. Pharmacol. Biochem. Behav. 2020, 191, 172904. [Google Scholar] [CrossRef]
- Ribeiro, A.; Zhu, J.; Kronfol, M.; Jahr, F.; Younis, R.; Hawkins, E.; McClay, J.L.; Deshpande, L.S. Molecular mechanisms for the antidepressant-like effects of a low-dose ketamine treatment in a DFP-based rat model for Gulf War Illness. Neurotoxicology 2020, 80, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hawkins, E.; Phillips, K.; Deshpande, L.S. Assessment of ketamine and its enantiomers in an organophosphate-based rat model for features of Gulf War Illness. Int. J. Environ. Res. Public Health 2020, 17, 4710. [Google Scholar] [CrossRef]
- Zanos, P.; Gould, T. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Ide, S.; Ikeda, K. Mechanisms of the antidepressant effects of ketamine enantiomers and their metabolites. Biol. Psychiatry 2018, 84, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Ide, S.; Ikekubo, Y.; Mishina, M.; Hashimoto, K.; Ikeda, K. Role of NMDA receptor GluN2D subunit in the antidepressant effects of enantiomers of ketamine. J. Pharmacol. Sci. 2017, 135, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Kadriu, B.; Gold, P.; Luckenbaugh, D.; Lener, M.; Ballard, E.; Niciu, M.J.; Henter, I.D.; Park, L.T.; De Sousa, R.T.; Yuan, P.; et al. Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol. Psychiatry 2018, 23, 1626–1631. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, M.; Dong, C.; Hashimoto, K. Role of inflammatory bone markers in the antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Int. J. Neuropsychopharmacol. 2018, 21, 1025–1030. [Google Scholar] [CrossRef]
- Xiong, Z.; Fujita, Y.; Zhang, K.; Pu, Y.; Chang, L.; Ma, M.; Chen, J.; Hashimoto, K. Beneficial effects of (R)-ketamine, but not its metabolite (2R,6R)-hydroxynorketamine, in the depression-like phenotype, inflammatory bone markers, and bone mineral density in a chronic social defeat stress model. Behav. Brain Res. 2019, 368, 111904. [Google Scholar] [CrossRef]
- Wan, X.; Eguchi, A.; Fujita, Y.; Ma, L.; Wang, X.; Yang, Y.; Qu, Y.; Chang, L.; Zhang, J.; Mori, C.; et al. Effects of (R)-ketamine on reduced bone mineral density in ovariectomized mice: A role of gut microbiota. Neuropharmacology 2022, 213, 109139. [Google Scholar] [CrossRef]
- Wan, X.; Eguchi, A.; Chang, L.; Mori, C.; Hashimoto, K. Beneficial effects of arketamine on the reduced bone mineral density in susceptible mice after chronic social defeat stress: Role of the gut-microbiota-bone-brain axis. Neuropharmacology 2023, 228, 109466. [Google Scholar] [CrossRef]
- Vecchia, D.D.; Kanazawa, L.K.S.; Wendler, E.; de Almeida Soares Hocayen, P.; Bruginski, E.; Campos, F.R.; Stern, C.A.J.; Vital, M.A.B.F.; Miyoshi, E.; Wöhr, M.; et al. Effects of ketamine on vocal impairment, gait changes, and anhedonia induced by bilateral 6-OHDA infusion into the substantia nigra pars compacta in rats: Therapeutic implications for Parkinson’s disease. Behav. Brain Res. 2018, 342, 1–10. [Google Scholar] [CrossRef]
- Vecchia, D.D.; Kanazawa, L.K.S.; Wendler, E.; Hocayen, P.A.S.; Vital, M.; Takahashi, R.N.; Da Cunha, C.; Miyoshi, E.; Andreatini, R. Ketamine reversed short-term memory impairment and depressive-like behavior in animal model of Parkinson’s disease. Brain Res. Bull. 2021, 168, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.C.; Song, J.J.; Wang, Y.; Chen, Y.; Hong, D.X. Neuron-protective effect of subanesthestic-dosage ketamine on mice of Parkinson’s disease. Asian Pac. J. Trop. Med. 2017, 10, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Fujita, A.; Fujita, Y.; Pu, Y.; Chang, L.; Hashimoto, K. MPTP-induced dopaminergic neurotoxicity in mouse brain is attenuated after subsequent intranasal administration of (R)-ketamine: A role of TrkB signaling. Psychopharmacology 2020, 237, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shirayama, Y.; Zhang, J.; Ren, Q.; Yao, W.; Ma, M.; Dong, C.; Hashimoto, K. R-ketamine: A rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl. Psychiatry 2015, 5, e632. [Google Scholar] [CrossRef] [PubMed]
- White, P.; Ham, J.; Way, W.; Trevor, A. Pharmacology of ketamine isomers in surgical patients. Anesthesiology 1980, 52, 231–239. [Google Scholar] [CrossRef]
- White, P.; Schüttler, J.; Shafer, A.; Stanski, D.; Horai, Y.; Trevor, A. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br. J. Anaesth. 1985, 57, 197–203. [Google Scholar] [CrossRef]
- Olofsen, E.; Kamp, J.; Henthorn, T.; van Velzen, M.; Niesters, M.; Sarton, E.; Dahan, A. Ketamine psychedelic and antinociceptive effects are connected. Anesthesiology 2022, 136, 792–801. [Google Scholar] [CrossRef]
- Geisslinger, G.; Hering, W.; Thomann, P.; Knoll, R.; Kamp, H.; Brune, K. Pharmacokinetics and pharmacodynamics of ketamine enantiomers in surgical patients using a stereoselective analytical method. Br. J. Anaesth. 1993, 70, 666–671. [Google Scholar] [CrossRef]
- Kamp, J.; van Velzen, M.; Aarts, L.; Niesters, M.; Dahan, A.; Olofsen, E. Stereoselective ketamine effect on cardiac output: A population pharmacokinetic/pharmacodynamic modelling study in healthy volunteers. Br. J. Anaesth. 2021, 127, 23–31. [Google Scholar] [CrossRef]
- Jonkman, K.; van der Schrier, R.; van Velzen, M.; Aarts, L.; Olofsen, E.; Sarton, E.; Niesters, M.; Dahan, A. Differential role of nitric oxide in the psychedelic symptoms induced by racemic ketamine and esketamine in human volunteers. Br. J. Anaesth. 2018, 120, 1009–1018. [Google Scholar] [CrossRef]
- de Carvalho, C.; Lopes, M.; Constantino, L.; Hoeller, A.; de Melo, H.; Guarnieri, R.; Linhares, M.N.; Bortolotto, Z.A.; Prediger, R.D.; Latini, A.; et al. The ERK phosphorylation levels in the amygdala predict anxiety symptoms in humans and MEK/ERK inhibition dissociates innate and learned defensive behaviors in rats. Mol. Psychiatry 2021, 26, 7257–7269. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ren, Q.; Qu, Y.; Zhang, J.; Ma, M.; Dong, C.; Hashimoto, K. Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol. Psychiatry 2018, 83, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Mateus, C.F.; Malcolm, R.J.; Brady, K.T.; Back, S.E. Efficacy of ketamine in the treatment of substance use disorders: A systematic review. Front. Psychiatry 2018, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.; Kranzler, J.; Kaniecki, K.; Popik, P.; Smith, J.; Hashimoto, K.; Sporn, J. R-(-)-ketamine modifies behavioral effects of morphine predicting efficacy as a novel therapy for opioid use disorder. Pharmacol. Biochem. Behav. 2020, 194, 172927. [Google Scholar] [CrossRef] [PubMed]
- Shafique, H.; Witkin, J.; Smith, J.; Kaniecki, K.; Sporn, J.; Holuj, M.; Krawczyk, M.; Kuziak, A.; Popik, P. Rapid tolerance to behavioral effects of ethanol in rats: Prevention by R-(-)-ketamine. Pharmacol. Biochem. Behav. 2021, 203, 173152. [Google Scholar] [CrossRef]
- Highland, J.N.; Zanos, P.; Riggs, L.M.; Georgiou, P.; Clark, S.M.; Morris, P.J.; Moaddel, R.; Thomas, C.J.; Zarate, C.A., Jr.; Pereira, E.F.R.; et al. Hydroxynorketamines: Pharmacology and Potential Therapeutic Applications. Pharmacol. Rev. 2021, 73, 763–791. [Google Scholar] [CrossRef]


| Antidepressant effects—animal studies |
| Antidepressant effects: arketamine > racemic ketamine and esketamine |
| Racemic ketamine, esketamine, and arketamine |
| Decrease in immobility time in the forced swim test (FST)/or tail suspension test TST |
| Increase in sucrose preference in the sucrose preference test (SPT) |
| Side effects—animal studies |
| Side effects: arketamine < racemic ketamine and esketamine |
| Racemic ketamine and esketamine |
| Hyperlocomotion |
| Psychomimetic effects |
| Rewarding effects |
| Abuse liability |
| Arketamine |
| Mild effects on locomotion |
| Cognitive process profile (CPP) scores, motor coordinator deficits, and prepulse inhibition (PPI) |
| No serious adverse events were reported |
| Antidepressant effects—humans |
| Racemic ketamine, esketamine, and arketamine |
| Reduced score on the Montgomery–Åsberg Depression Rating Scale (MADRS)/Hamilton Depression Rating Scale (HDRS) |
| Ketamine therapy includes, among others, depression (even treatment-resistant), anxiety, suicidal ideation, post-traumatic stress disorder (PTSD), obsessive–compulsive disorder (OCD), neuropathic pain, chronic pain, substance abuse and eating disorders; esketamine: treatment-resistant depression and major depressive disorder with acute suicidal ideation or behavior |
| Side effects—humans |
| Racemic ketamine and esketamine |
| Headache |
| Dizziness |
| Dissociation |
| Rewarding effects |
| Abuse liability |
| Cognitive dysfunction |
| Arketamine |
| No serious adverse events were reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawczak, P.; Feszak, I.; Bączek, T. Ketamine, Esketamine, and Arketamine: Their Mechanisms of Action and Applications in the Treatment of Depression and Alleviation of Depressive Symptoms. Biomedicines 2024, 12, 2283. https://doi.org/10.3390/biomedicines12102283
Kawczak P, Feszak I, Bączek T. Ketamine, Esketamine, and Arketamine: Their Mechanisms of Action and Applications in the Treatment of Depression and Alleviation of Depressive Symptoms. Biomedicines. 2024; 12(10):2283. https://doi.org/10.3390/biomedicines12102283
Chicago/Turabian StyleKawczak, Piotr, Igor Feszak, and Tomasz Bączek. 2024. "Ketamine, Esketamine, and Arketamine: Their Mechanisms of Action and Applications in the Treatment of Depression and Alleviation of Depressive Symptoms" Biomedicines 12, no. 10: 2283. https://doi.org/10.3390/biomedicines12102283






