Biofilm Prevention and Removal in Non-Target Pseudomonas Strain by Siphovirus-like Coliphage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteriophage
2.2. Bacterial Strains and Growth Conditions
2.3. Antimicrobial Activity
2.4. Anti-Biofilm Assay
2.5. Biofilm-Degrading Assay
2.6. Transmission Electron Microscopy
2.7. Data Analysis
3. Results
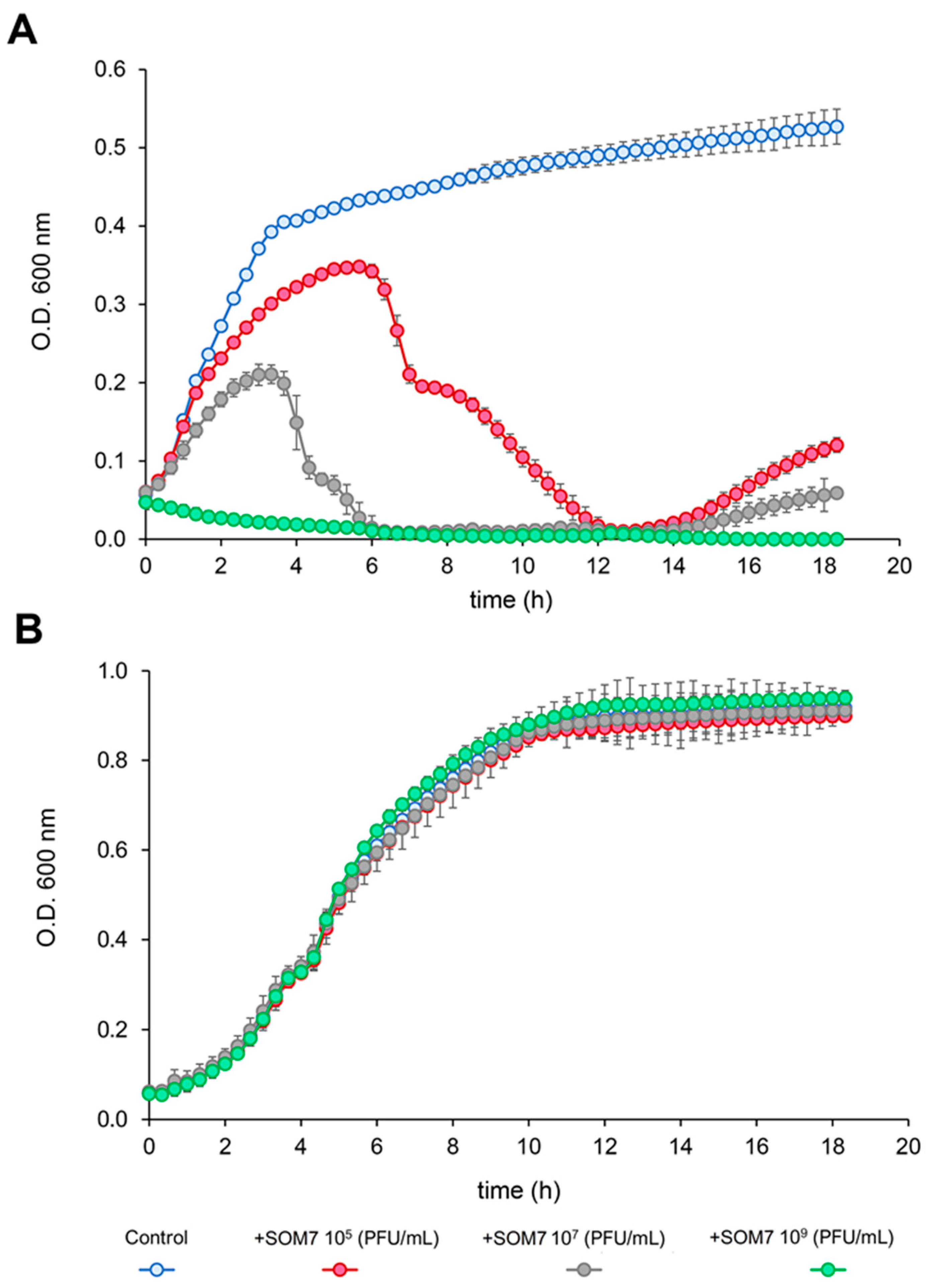
3.1. Antimicrobial Activity of SOM7 Phage
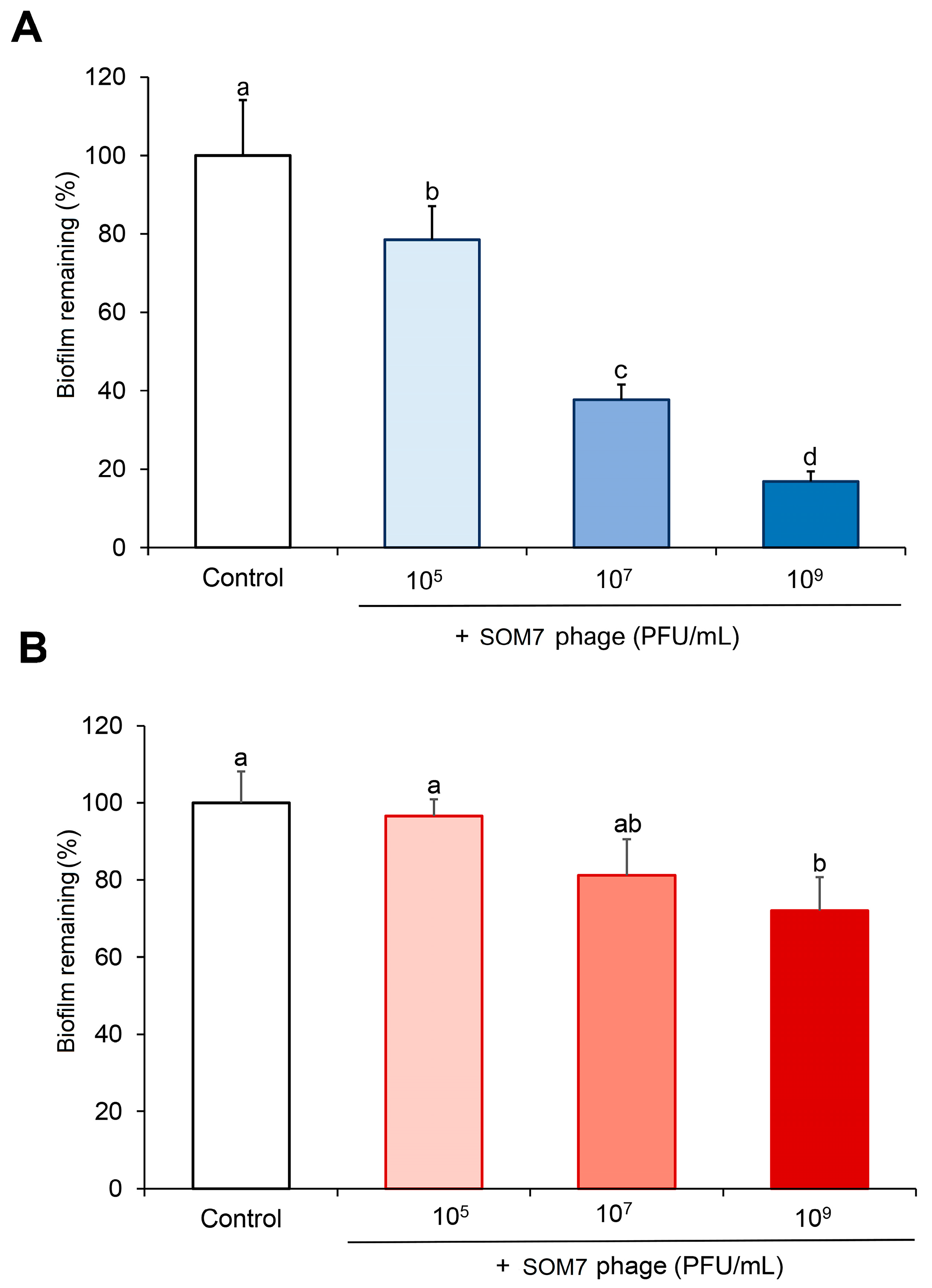
3.2. Anti-Biofilm and Biofilm-Degrading Activity of SOM7 Phage against Non-Target Gram-Negative Bacteria
3.3. TEM Assessment of Phage–Bacteria Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021. 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/341666/9789240027336-eng.pdf (accessed on 7 October 2024).
- Economou, V.; Gousia, P. Agriculture and Food Animals as a Source of Antimicrobial-Resistant Bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Mukane, L.; Racenis, K.; Rezevska, D.; Petersons, A.; Kroica, J. Anti-Biofilm Effect of Bacteriophages and Antibiotics against Uropathogenic Escherichia coli. Antibiotics 2022, 11, 1706. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.A.; Sahukhal, G.S.; Elasri, M.O.; Urban, M.W. Phage-Bacterium War on Polymeric Surfaces: Can Surface-Anchored Bacteriophages Eliminate Microbial Infections? Biomacromolecules 2013, 14, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, E.; Ahmadi, P.; Abedpour-Dehkordi, E.; Arbab-Soleimani, N.; Khamesipour, F. Biofilm Formation, Antimicrobial Susceptibility, Serogroups and Virulence Genes of Uropathogenic E. coli Isolated from Clinical Samples in Iran. Antimicrob. Resist. Infect. Control 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Chen, X.P.; Ali, L.; Wu, L.-Y.; Liu, C.; Gang, C.X.; Huang, Q.F.; Ruan, J.H.; Bao, S.Y.; Rao, Y.P.; Yu, D. Biofilm Formation Plays a Role in the Formation of Multidrug-Resistant Escherichia coli toward Nutrients in Microcosm Experiments. Front. Microbiol. 2018, 9, 367. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 530515. [Google Scholar] [CrossRef]
- Sooriyakumar, P.; Bolan, N.; Kumar, M.; Singh, L.; Yu, Y.; Li, Y.; Weralupitiya, C.; Vithanage, M.; Ramanayaka, S.; Sarkar, B. Biofilm Formation and Its Implications on the Properties and Fate of Microplastics in Aquatic Environments: A Review. J. Hazard. Mater. Adv. 2022, 6, 100077. [Google Scholar] [CrossRef]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef]
- Ruhal, R.; Kataria, R. Biofilm Patterns in Gram-Positive and Gram-Negative Bacteria. Microbiol. Res. 2021, 251, 126829. [Google Scholar] [CrossRef]
- Bikard, D.; Marraffini, L.A. Innate and Adaptive Immunity in Bacteria: Mechanisms of Programmed Genetic Variation to Fight Bacteriophages. Innate Immun. Antigen Process. 2012, 24, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Teklemariam, A.D.; Al-Hindi, R.R.; Qadri, I.; Alharbi, M.G.; Ramadan, W.S.; Ayubu, J.; Al-Hejin, A.M.; Hakim, R.F.; Hakim, F.F.; Hakim, R.F.; et al. The Battle between Bacteria and Bacteriophages: A Conundrum to Their Immune System. Antibiotics 2023, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Watanabe, S.; Miyanaga, K.; Kiga, K.; Sasahara, T.; Aiba, Y.; Tan, X.-E.; Veeranarayanan, S.; Thitiananpakorn, K.; Nguyen, H.M.; et al. A Comprehensive Review on Phage Therapy and Phage-Based Drug Development. Antibiotics 2024, 13, 870. [Google Scholar] [CrossRef] [PubMed]
- Brives, C.; Pourraz, J. Phage Therapy as a Potential Solution in the Fight against AMR: Obstacles and Possible Futures. Palgrave Commun. 2020, 6, 100. [Google Scholar] [CrossRef]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and Efficacy of Phage Therapy in Difficult-to-Treat Infections: A Systematic Review. Lancet Infect. Dis. 2022, 22, e208–e220. [Google Scholar] [CrossRef]
- Jo, S.J.; Kwon, J.; Kim, S.G.; Lee, S.-J. The Biotechnological Application of Bacteriophages: What to Do and Where to Go in the Middle of the Post-Antibiotic Era. Microorganisms 2023, 11, 2311. [Google Scholar] [CrossRef]
- Bisen, M.; Kharga, K.; Mehta, S.; Jabi, N.; Kumar, L. Bacteriophages in Nature: Recent Advances in Research Tools and Diverse Environmental and Biotechnological Applications. Environ. Sci. Pollut. Res. 2024, 31, 22199–22242. [Google Scholar] [CrossRef]
- Muniesa, M.; Mocé-Llivina, L.; Katayama, H.; Jofre, J. Bacterial Host Strains That Support Replication of Somatic Coliphages. Antonie van Leeuwenhoek 2003, 83, 305–315. [Google Scholar] [CrossRef]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus Taxonomy: The Database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef]
- ISO 10705-2; Water Quality. Detection and Enumeration of Bacteriophages—Part 2: Enumeration of Somatic Coliphages. International Organization for Standardization: Geneva, Switzerland, 2000.
- Luong, T.; Salabarria, A.-C.; Edwards, R.A.; Roach, D.R. Standardized Bacteriophage Purification for Personalized Phage Therapy. Nat. Protoc. 2020, 15, 2867–2890. [Google Scholar] [CrossRef]
- Muniesa, M.; Ballesté, E.; Imamovic, L.; Pascual-Benito, M.; Toribio-Avedillo, D.; Lucena, F.; Blanch, A.; Jofre, J. Bluephage: A Rapid Method for the Detection of Somatic Coliphages Used as Indicators of Fecal Pollution in Water. Water Res. 2018, 128, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.K.-H.; Delgado-Charro, B.; Bolhuis, A. A Microtiter Plate-Based Quantitative Method to Monitor the Growth Rate of Dermatophytes and Test Antifungal Activity. J. Microbiol. Methods 2019, 165, 105722. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, e2437. [Google Scholar] [CrossRef]
- Al-kafaween, M.A.; Hilmi, A.B.M.; Jaffar, N.; Al-Jamal, H.A.; Zahri, M.K.; Jibril, F.I. Antibacterial and Antibiofilm Activities of Malaysian Trigona Honey against Pseudomonas aeruginosa ATCC 10145 and Streptococcus Pyogenes ATCC 19615. Jordan J. Biol. Sci. 2020, 13, 69–76. [Google Scholar]
- Mörgelin, M. Negative Staining and Transmission Electron Microscopy of Bacterial Surface Structures. In Bacterial Pathogenesis: Methods and Protocols; Nordenfelt, P., Collin, M., Eds.; Springer: New York, NY, USA, 2017; pp. 211–217. ISBN 978-1-4939-6673-8. [Google Scholar] [CrossRef]
- Tizro, P.; Choi, C.; Khanlou, N. Sample Preparation for Transmission Electron Microscopy. In Biobanking: Methods and Protocols; Yong, W.H., Ed.; Springer: New York, NY, USA, 2019; pp. 417–424. ISBN 978-1-4939-8935-5. [Google Scholar] [CrossRef]
- Sinha, V.; Goyal, A.; Svenningsen, S.L.; Semsey, S.; Krishna, S. In Silico Evolution of Lysis-Lysogeny Strategies Reproduces Observed Lysogeny Propensities in Temperate Bacteriophages. Front. Microbiol. 2017, 8, 1386. [Google Scholar] [CrossRef]
- Xie, Y.; Wahab, L.; Gill, J.J. Development and Validation of a Microtiter Plate-Based Assay for Determination of Bacteriophage Host Range and Virulence. Viruses 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Mitarai, N.; Brown, S.; Sneppen, K. Population Dynamics of Phage and Bacteria in Spatially Structured Habitats Using Phage λ and Escherichia coli. J. Bacteriol. 2016, 198, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Imamovic, L.; Misiakou, M.-A.; van der Helm, E.; Panagiotou, G.; Muniesa, M.; Sommer, M.O.A. Complete Genome Sequence of Escherichia coli Strain WG5. Genome Announc. 2018, 6, 10–1128. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Turner, D.; Shkoporov, A.N.; Lood, C.; Millard, A.D.; Dutilh, B.E.; Alfenas-Zerbini, P.; van Zyl, L.J.; Aziz, R.K.; Oksanen, H.M.; Poranen, M.M. Abolishment of Morphology-Based Taxa and Change to Binomial Species Names: 2022 Taxonomy Update of the ICTV Bacterial Viruses Subcommittee. Arch. Virol. 2023, 168, 74. [Google Scholar] [CrossRef]
- Singh, S.; Pitchers, R.; Hassard, F. Coliphages as Viral Indicators of Sanitary Significance for Drinking Water. Front. Microbiol. 2022, 13, 941532. [Google Scholar] [CrossRef] [PubMed]
- Grose, J.H.; Casjens, S.R. Understanding the Enormous Diversity of Bacteriophages: The Tailed Phages That Infect the Bacterial Family Enterobacteriaceae. Virology 2014, 468, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.R. Isolation and Characterization of Phages for Enterotoxigenic E. coli. Masters’s Thesis, Universidade do Minho, Braga, Guimarães, Portugal, 2023. Available online: https://hdl.handle.net/1822/91556 (accessed on 1 October 2024).
- Henderson, J.C.; Zimmerman, S.M.; Crofts, A.A.; Boll, J.M.; Kuhns, L.G.; Herrera, C.M.; Trent, M.S. The Power of Asymmetry: Architecture and Assembly of the Gram-Negative Outer Membrane Lipid Bilayer. Annu. Rev. Microbiol. 2016, 70, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Maffei, E.; Shaidullina, A.; Burkolter, M.; Heyer, Y.; Estermann, F.; Druelle, V.; Sauer, P.; Willi, L.; Michaelis, S.; Hilbi, H. Systematic Exploration of Escherichia coli Phage–Host Interactions with the BASEL Phage Collection. PLoS Biol. 2021, 19, e3001424. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, F.L.; Vlot, M.; de Jonge, P.A.; Dreesens, L.L.; Beaumont, H.J.; Lavigne, R.; Dutilh, B.E.; Brouns, S.J. Targeting Mechanisms of Tailed Bacteriophages. Nat. Rev. Microbiol. 2018, 16, 760–773. [Google Scholar] [CrossRef]
- Broeker, N.K.; Barbirz, S. Not a Barrier but a Key: How Bacteriophages Exploit Host’s O-antigen as an Essential Receptor to Initiate Infection. Mol. Microbiol. 2017, 105, 353–357. [Google Scholar] [CrossRef]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host Receptors for Bacteriophage Adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef]
- Letarov, A.; Kulikov, E. Adsorption of Bacteriophages on Bacterial Cells. Biochem. Mosc. 2017, 82, 1632–1658. [Google Scholar] [CrossRef]
- Amor, K.; Heinrichs, D.E.; Frirdich, E.; Ziebell, K.; Johnson, R.P. Whitfield Chris Distribution of Core Oligosaccharide Types in Lipopolysaccharides from Escherichia coli. Infect. Immun. 2000, 68, 1116–1124. [Google Scholar] [CrossRef]
- Piya, D.; Lessor, L.; Koehler, B.; Stonecipher, A.; Cahill, J.; Gill, J.J. Genome-Wide Screens Reveal Escherichia coli Genes Required for Growth of T1-like Phage LL5 and V5-like Phage LL12. Sci. Rep. 2020, 10, 8058. [Google Scholar] [CrossRef]
- Korf, I.H.E.; Meier-Kolthoff, J.P.; Adriaenssens, E.M.; Kropinski, A.M.; Nimtz, M.; Rohde, M.; van Raaij, M.J.; Wittmann, J. Still Something to Discover: Novel Insights into Escherichia coli Phage Diversity and Taxonomy. Viruses 2019, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Cinquerrui, S.; Mancuso, F.; Vladisavljević, G.T.; Bakker, S.E.; Malik, D.J. Nanoencapsulation of Bacteriophages in Liposomes Prepared Using Microfluidic Hydrodynamic Flow Focusing. Front. Microbiol. 2018, 9, 2172. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle: Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Markoishvili, K.; Tsitlanadze, G.; Katsarava, R.; Glenn, J.; Sulakvelidze, A. A Novel Sustained-Release Matrix Based on Biodegradable Poly(Ester Amide)s and Impregnated with Bacteriophages and an Antibiotic Shows Promise in Management of Infected Venous Stasis Ulcers and Other Poorly Healing Wounds. Int. J. Dermatol. 2002, 41, 453–458. [Google Scholar] [CrossRef]
- Yang, L.-M.C.; Tam, P.Y.; Murray, B.J.; McIntire, T.M.; Overstreet, C.M.; Weiss, G.A.; Penner, R.M. Virus Electrodes for Universal Biodetection. Anal. Chem. 2006, 78, 3265–3270. [Google Scholar] [CrossRef]
- Jikia, D.; Chkhaidze, N.; Imedashvili, E.; Mgaloblishvili, I.; Tsitlanadze, G.; Katsarava, R.; Glenn Morris, J., Jr.; Sulakvelidze, A. The Use of a Novel Biodegradable Preparation Capable of the Sustained Release of Bacteriophages and Ciprofloxacin, in the Complex Treatment of Multidrug-resistant Staphylococcus aureus-infected Local Radiation Injuries Caused by Exposure to Sr90. Clin. Exp. Dermatol. 2005, 30, 23–26. [Google Scholar] [CrossRef]
- Deng, Z.; Luo, X.M.; Liu, J.; Wang, H. Quorum Sensing, Biofilm, and Intestinal Mucosal Barrier: Involvement the Role of Probiotic. Front. Cell. Infect. Microbiol. 2020, 10, 538077. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Gutiérrez, D.; Donovan, D.M.; Martínez, B.; Rodríguez, A.; García, P. Phage Lytic Proteins: Biotechnological Applications beyond Clinical Antimicrobials. Crit. Rev. Biotechnol. 2016, 36, 542–552. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Górski, A. Bacteriophages and Lysins in Biofilm Control. Virol. Sin. 2020, 35, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Stachler, E.; Kull, A.; Julian, T.R. Bacteriophage Treatment before Chemical Disinfection Can Enhance Removal of Plastic-Surface-Associated Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2021, 87, e00980-21. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F.; Piccardi, P.; Mancini, S.; Gabard, J.; Moreillon, P.; Entenza, J.M.; Resch, G.; Que, Y.-A. Synergistic Interaction Between Phage Therapy and Antibiotics Clears Pseudomonas aeruginosa Infection in Endocarditis and Reduces Virulence. J. Infect. Dis. 2017, 215, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, W.N.; Concepción-Acevedo, J.; Park, T.; Andleeb, S.; Bull, J.J.; Levin, B.R. Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms. PLoS ONE 2017, 12, e0168615. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-Biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Townsend, E.M.; Moat, J.; Jameson, E. CAUTI’s next Top Model—Model Dependent Klebsiella Biofilm Inhibition by Bacteriophages and Antimicrobials. Biofilm 2020, 2, 100038. [Google Scholar] [CrossRef]
- Park, D.-W.; Lee, J.H.; Park, J.-H. Thymol and Eugenol in Essential Oils Enhance Phage Endolysin LysECP26-Mediated Cell Wall Disruption of Escherichia coli O157: H7. Korean J. Food Sci. Technol. 2021, 53, 819–822. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Wang, J.; Ahn, J. Synergistic Antimicrobial Activity of Essential Oils in Combination with Phage Endolysin against Salmonella Typhimurium in Cooked Ground Beef. Food Control 2024, 157, 110187. [Google Scholar] [CrossRef]
- Ferreres, G.; Ivanova, K.; Torrent-Burgués, J.; Tzanov, T. Multimodal Silver-Chitosan-Acylase Nanoparticles Inhibit Bacterial Growth and Biofilm Formation by Gram-Negative Pseudomonas aeruginosa Bacterium. J. Colloid Interface Sci. 2023, 646, 576–586. [Google Scholar] [CrossRef]
- Ferreres, G.; Ivanova, K.; Ivanov, I.; Tzanov, T. Nanomaterials and Coatings for Managing Antibiotic-Resistant Biofilms. Antibiotics 2023, 12, 310. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Fiandra, L.; Mantecca, P.; Catelani, T.; Natan, M.; Banin, E.; Jacobi, G.; Tzanov, T. Antibacterial, Antibiofilm, and Antiviral Farnesol-Containing Nanoparticles Prevent Staphylococcus aureus from Drug Resistance Development. Int. J. Mol. Sci. 2022, 23, 7527. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.; Ivanova, A.; Hoyo, J.; Pérez-Rafael, S.; Tzanov, T. Nano-Formulation Endows Quorum Quenching Enzyme-Antibiotic Hybrids with Improved Antibacterial and Antibiofilm Activities against Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 7632. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lv, P.; Wang, L.-G.; Long, F.; Ma, X.-L.; Liu, C.; Feng, Y.-J.; Yang, M.-F.; Xiao, X. Impact of Nano-TiO2 on Horizontal Transfer of Resistance Genes Mediated by Filamentous Phage Transduction. Environ. Sci. Nano 2020, 7, 1214–1224. [Google Scholar] [CrossRef]
- Domingo-Calap, P.; Delgado-Martínez, J. Bacteriophages: Protagonists of a Post-Antibiotic Era. Antibiotics 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Kifelew, L.G.; Warner, M.S.; Morales, S.; Thomas, N.; Gordon, D.L.; Mitchell, J.G.; Speck, P.G. Efficacy of Lytic Phage Cocktails on Staphylococcus aureus and Pseudomonas aeruginosa in Mixed-Species Planktonic Cultures and Biofilms. Viruses 2020, 12, 559. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Willett, J.L.; Dunny, G.M.; Duerkop, B.A. Phage Infection and Sub-Lethal Antibiotic Exposure Mediate Enterococcus Faecalis Type VII Secretion System Dependent Inhibition of Bystander Bacteria. PLoS Genet. 2021, 17, e1009204. [Google Scholar] [CrossRef]
- De Sordi, L.; Khanna, V.; Debarbieux, L. The Gut Microbiota Facilitates Drifts in the Genetic Diversity and Infectivity of Bacterial Viruses. Cell Host Microbe 2017, 22, 801–808. [Google Scholar] [CrossRef]
- Reyes, A.; Wu, M.; McNulty, N.P.; Rohwer, F.L.; Gordon, J.I. Gnotobiotic Mouse Model of Phage–Bacterial Host Dynamics in the Human Gut. Proc. Natl. Acad. Sci. USA 2013, 110, 20236–20241. [Google Scholar] [CrossRef]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe 2019, 25, 803–814. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, L.M.; Havryliuk, O.; Infante, N.; Muniesa, M.; Morató, J.; Mariychuk, R.; Tzanov, T. Biofilm Prevention and Removal in Non-Target Pseudomonas Strain by Siphovirus-like Coliphage. Biomedicines 2024, 12, 2291. https://doi.org/10.3390/biomedicines12102291
Pérez LM, Havryliuk O, Infante N, Muniesa M, Morató J, Mariychuk R, Tzanov T. Biofilm Prevention and Removal in Non-Target Pseudomonas Strain by Siphovirus-like Coliphage. Biomedicines. 2024; 12(10):2291. https://doi.org/10.3390/biomedicines12102291
Chicago/Turabian StylePérez, Leonardo Martín, Olesia Havryliuk, Nury Infante, Maite Muniesa, Jordi Morató, Ruslan Mariychuk, and Tzanko Tzanov. 2024. "Biofilm Prevention and Removal in Non-Target Pseudomonas Strain by Siphovirus-like Coliphage" Biomedicines 12, no. 10: 2291. https://doi.org/10.3390/biomedicines12102291









