Machine Learning Reveals Microbial Taxa Associated with a Swim across the Pacific Ocean
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Sample Processing and Sequencing
2.3. Statistical Analysis
3. Results
3.1. Excessive Exercise Significantly Alters Microbial Community Structure
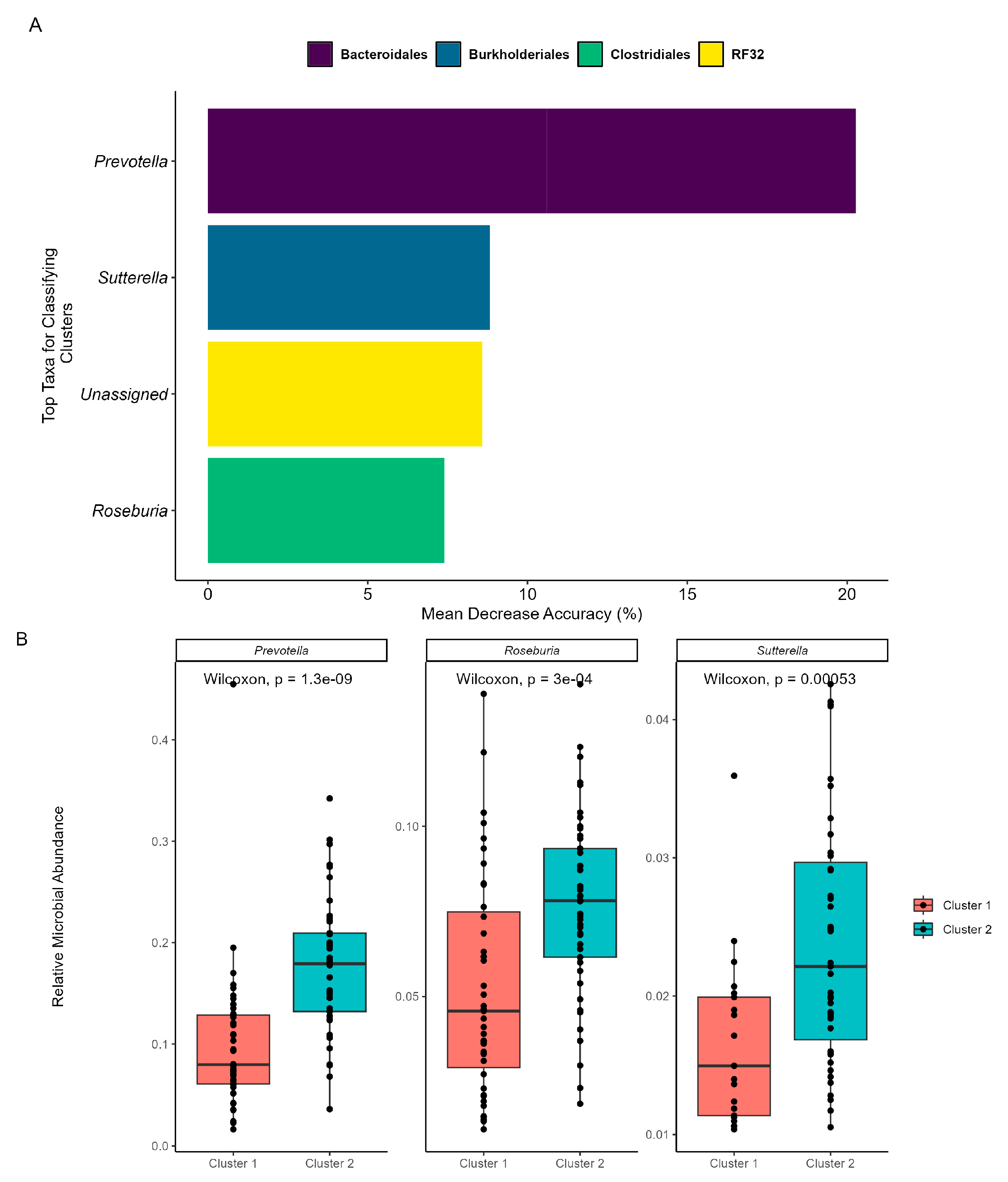
3.2. Machine Learning Reveals Microbial Taxa That Shape Swim Time Course
4. Discussion
4.1. Physiological Measures Indicate Maladaptive Responses to EE
4.2. Microbial Diversity and EE
4.3. GM Alterations: Microbial Adaptations and Maladaptations in Response to EE
4.4. Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bellicha, A.; Van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of Exercise Training on Weight Loss, Body Composition Changes, and Weight Maintenance in Adults with Overweight or Obesity: An Overview of 12 Systematic Reviews and 149 Studies. Obes. Rev. 2021, 22 (Suppl. S4), e13256. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Franklin, B.A.; Fagard, R.; Farquhar, W.B.; Kelley, G.A.; Ray, C.A. Exercise and Hypertension. Med. Sci. Sports Exerc. 2004, 36, 533–553. [Google Scholar] [CrossRef]
- Kramer, A. An Overview of the Beneficial Effects of Exercise on Health and Performance. In Physical Exercise for Human Health [Internet]; Xiao, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1228, pp. 3–22. Available online: http://link.springer.com/10.1007/978-981-15-1792-1_1 (accessed on 4 January 2024).
- Flores-Opazo, M.; McGee, S.L.; Hargreaves, M. Exercise and GLUT4. Exerc. Sport Sci. Rev. 2020, 48, 110–118. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Patil, H.R.; Lavie, C.J.; Magalski, A.; Vogel, R.A.; McCullough, P.A. Potential Adverse Cardiovascular Effects From Excessive Endurance Exercise. Mayo Clin. Proc. 2012, 87, 587–595. [Google Scholar] [CrossRef]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A. Prevention, Diagnosis, and Treatment of the Overtraining Syndrome: Joint Consensus Statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013, 45, 186–205. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kratz, M.; Damman, C.J.; Hullarg, M. Mechanisms Linking the Gut Microbiome and Glucose Metabolism. J. Clin. Endocrinol. Metab. 2016, 101, 1445–1454. [Google Scholar] [CrossRef]
- Moghetti, P.; Bacchi, E.; Brangani, C.; Donà, S.; Negri, C. Metabolic Effects of Exercise. In Frontiers of Hormone Research [Internet]; Lanfranco, F., Strasburger, C.J., Eds.; S. Karger AG: Basel, Switzerland, 2016; pp. 44–57. Available online: https://www.karger.com/Article/FullText/445156 (accessed on 4 January 2024).
- Durk, R.P.; Castillo, E.; Márquez-Magaña, L.; Grosicki, G.J.; Bolter, N.D.; Lee, C.M.; Bagley, J.R. Gut Microbiota Composition Is Related to Cardiorespiratory Fitness in Healthy Young Adults. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 249–253. [Google Scholar] [CrossRef]
- John, G.K.; Mullin, G.E. The Gut Microbiome and Obesity. Curr. Oncol. Rep. 2016, 18, 45. [Google Scholar] [CrossRef]
- Cataldi, S.; Poli, L.; Şahin, F.N.; Patti, A.; Santacroce, L.; Bianco, A.; Greco, G.; Ghinassi, B.; Di Baldassarre, A.; Fischetti, F. The Effects of Physical Activity on the Gut Microbiota and the Gut–Brain Axis in Preclinical and Human Models: A Narrative Review. Nutrients 2022, 14, 3293. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Puddu, A.; Sanguineti, R.; Montecucco, F.; Viviani, G.L. Evidence for the Gut Microbiota Short-Chain Fatty Acids as Key Pathophysiological Molecules Improving Diabetes. Mediat. Inflamm. 2014, 2014, 162021. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome Potentiates Endurance Exercise through Intestinal Acetate Production. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rué, O.; Mariadassou, M.; et al. Gut Bacteria Are Critical for Optimal Muscle Function: A Potential Link with Glucose Homeostasis. Am. J. Physiol. -Endocrinol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory Fitness as a Predictor of Intestinal Microbial Diversity and Distinct Metagenomic Functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; O’Keefe, E.L.; Lavie, C.J. The Goldilocks Zone for Exercise: Not Too Little, Not Too Much. Mo. Med. 2018, 115, 98–105. [Google Scholar]
- Etxebarria, N.; Mujika, I.; Pyne, D. Training and Competition Readiness in Triathlon. Sports 2019, 7, 101. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, S.; Huang, H.; Liang, J.; Wu, Y.; Li, C.; Yuan, H.; Zhao, X.; Lai, X.; Hou, S. Influence of Excessive Exercise on Immunity, Metabolism, and Gut Microbial Diversity in an Overtraining Mice Model. Scand. J. Med. Sci. Sports 2018, 28, 1541–1551. [Google Scholar] [CrossRef]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef]
- Olshansky, B.; Ricci, F.; Fedorowski, A. Importance of resting heart rate. Trends Cardiovasc. Med. 2022, 33, 502–515. [Google Scholar] [CrossRef]
- Kyle, U.G.; Schutz, Y.; Dupertuis, Y.M.; Pichard, C. Body composition interpretation. Nutrition 2003, 19, 597–604. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar]
- Lee, M.G.; Park, K.S.; Kim, D.U.; Choi, S.M.; Kim, H.J. Effects of high-intensity exercise training on body composition, abdominal fat loss, and cardiorespiratory fitness in middle-aged Korean females. Appl. Physiol. Nutr. Metab. 2012, 37, 1019–1027. [Google Scholar] [CrossRef]
- Langleite, T.M.; Jensen, J.; Norheim, F.; Gulseth, H.L.; Tangen, D.S.; Kolnes, K.J.; Heck, A.; Storås, T.; Grøthe, G.; Dahl, M.A.; et al. Insulin Sensitivity, Body Composition and Adipose Depots Following 12 w Combined Endurance and Strength Training in Dysglycemic and Normoglycemic Sedentary Men. Arch. Physiol. Biochem. 2016, 122, 167–179. [Google Scholar] [CrossRef]
- Strasser, B.; Spreitzer, A.; Haber, P. Fat Loss Depends on Energy Deficit Only, Independently of the Method for Weight Loss. Ann. Nutr. Metab. 2007, 51, 428–432. [Google Scholar] [CrossRef]
- Osilla, E.V.; Safadi, A.O.; Sharma, S. Calories. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK499909/ (accessed on 4 January 2024).
- Shepherd, J.T. Circulatory response to exercise in health. Circulation 1987, 76 Pt 2, VI3–VI10. [Google Scholar]
- Reimers, A.; Knapp, G.; Reimers, C.D. Effects of Exercise on the Resting Heart Rate: A Systematic Review and Meta-Analysis of Interventional Studies. J. Clin. Med. 2018, 7, 503. [Google Scholar] [CrossRef] [PubMed]
- Hafen, B.; Sharma, S. Oxygen Saturation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK525974/ (accessed on 4 February 2024).
- Sharman, J.E.; LaGerche, A. Exercise blood pressure: Clinical relevance and correct measurement. J. Hum. Hypertens. 2015, 29, 351–358. [Google Scholar] [CrossRef]
- Purdom, T.; Cook, M.; Colleran, H.; Stewart, P.; San Diego, L. Low Energy Availability (LEA) and Hypertension in Black Division I Collegiate Athletes: A Novel Pilot Study. Sports 2023, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of Physical Activities: An Update of Activity Codes and MET Intensities. Med. Sci. Sports Exerc. 2000, 32 (Suppl. S9), S498–S516. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine; Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. (Eds.) ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018; 472p. [Google Scholar]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The Athletic Gut Microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and Disease Markers Correlate with Gut Microbiome Composition across Thousands of People. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Cullen, J.M.A.; Shahzad, S.; Dhillon, J. A systematic review on the effects of exercise on gut microbial diversity, taxonomic composition, and microbial metabolites: Identifying research gaps and future directions. Front. Physiol. 2023, 14, 1292673. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Pugh, J.; Wosinska, L.; Quilter, K.; Mattimoe, D.; Allen, J.; Joyce, S.A.; O’Sullivan, O.; Close, G.L. Ultra-Endurance Triathlon Competition Shifts Fecal Metabolome Independent of Changes to Microbiome Composition. J. Appl. Physiol. 2023, 135, 549–558. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Sun, S.; Lulla, A.; Sioda, M.; Winglee, K.; Wu, M.C.; Jacobs, D.R., Jr.; Shikany, J.M.; Lloyd-Jones, D.M.; Launer, L.J.; Fodor, A.A.; et al. Gut Microbiota Composition and Blood Pressure: The CARDIA Study. Hypertension 2019, 73, 998–1006. [Google Scholar] [CrossRef]
- Šoltys, K.; Lendvorský, L.; Hric, I.; Baranovičová, E.; Penesová, A.; Mikula, I.; Bohmer, M.; Budiš, J.; Vávrová, S.; Grones, J.; et al. Strenuous Physical Training, Physical Fitness, Body Composition and Bacteroides to Prevotella Ratio in the Gut of Elderly Athletes. Front. Physiol. 2021, 12, 670989. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.; Blond, M.B.; Hansen, T.H.; Rosenkilde, M.; Quist, J.S.; Gram, A.S.; Ekstrøm, C.T.; Hansen, T.; Stallknecht, B. Structured Exercise Alters the Gut Microbiota in Humans with Overweight and Obesity—A Randomized Controlled Trial. Int. J. Obes. 2020, 44, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Blekhman, R. Population dynamics of the human gut microbiome: Change is the only constant. Genome Biol. 2019, 20, 150. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The Gut Microbiome in Atherosclerotic Cardiovascular Disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. In Bugs as Drugs: Therapeutic Microbes for the Prevention and Treatment of Disease; Britton, R.A., Cani, P.D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Microbiol. Spectr.; Volume 5, pp. 73–98. [Google Scholar]
- Petriz, B.A.; Castro, A.P.; Almeida, J.A.; Gomes, C.P.; Fernandes, G.R.; Kruger, R.H.; Pereira, R.W.; Franco, O.L. Exercise Induction of Gut Microbiota Modifications in Obese, Non-Obese and Hypertensive Rats. BMC Genom. 2014, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. eBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community Characteristics of the Gut Microbiomes of Competitive Cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut Microbiota Dysbiosis Contributes to the Development of Hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia Intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Mondot, S.; Lachkar, L.; Doré, J.; Blottière, H.M.; Hanachi, M. Roseburia, a decreased bacterial taxon in the gut microbiota of patients suffering from anorexia nervosa. Eur. J. Clin. Nutr. 2022, 76, 1486–1489. [Google Scholar] [CrossRef]
- Smiljanec, K.; Lennon, S.L. Sodium, hypertension, and the gut: Does the gut microbiota go salty? Am. J. Physiol.-Heart Circ. Physiol. 2019, 317, H1173–H1182. [Google Scholar] [CrossRef]
- Hiippala, K.; Kainulainen, V.; Kalliomäki, M.; Arkkila, P.; Satokari, R. Mucosal Prevalence and Interactions with the Epithelium Indicate Commensalism of Sutterella spp. Front. Microbiol. 2016, 7, 1706. Available online: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01706/full (accessed on 19 January 2024). [CrossRef]
- Mukhopadhya, I.; Hansen, R.; Nicholl, C.E.; Alhaidan, Y.A.; Thomson, J.M.; Berry, S.H.; Pattinson, C.; Stead, D.A.; Russell, R.K.; El-Omar, E.M.; et al. A Comprehensive Evaluation of Colonic Mucosal Isolates of Sutterella Wadsworthensis from Inflammatory Bowel Disease. PLoS ONE 2011, 6, e27076. [Google Scholar] [CrossRef]
- Hansen, R.; Berry, S.H.; Mukhopadhya, I.; Thomson, J.M.; Saunders, K.A.; Nicholl, C.E.; Bisset, W.M.; Loganathan, S.; Mahdi, G.; Kastner-Cole, D.; et al. The Microaerophilic Microbiota of De-Novo Paediatric Inflammatory Bowel Disease: The BISCUIT Study. PLoS ONE 2013, 8, e58825. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Lennon, G.; O’Sullivan, O.; Docherty, N.; Balfe, A.; Maguire, A.; Mulcahy, H.E.; Doherty, G.; O’Donoghue, D.; Hyland, J.; et al. Spatial Variation of the Colonic Microbiota in Patients with Ulcerative Colitis and Control Volunteers. Gut 2015, 64, 1553–1561. [Google Scholar] [CrossRef]
- Vanhaecke, T.; Bretin, O.; Poirel, M.; Tap, J. Drinking Water Source and Intake Are Associated with Distinct Gut Microbiota Signatures in US and UK Populations. J. Nutr. 2022, 152, 171–182. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed]
- Thurber, C.; Dugas, L.R.; Ocobock, C.; Carlson, B.; Speakman, J.R.; Pontzer, H. Extreme events reveal an alimentary limit on sustained maximal human energy expenditure. Sci. Adv. 2019, 5, eaaw0341. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H.; Durazo-Arvizu, R.; Dugas, L.R.; Plange-Rhule, J.; Bovet, P.; Forrester, T.E.; Lambert, E.V.; Cooper, R.S.; Schoeller, D.A.; Luke, A. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Curr. Biol. 2016, 26, 410–417. [Google Scholar] [CrossRef] [PubMed]
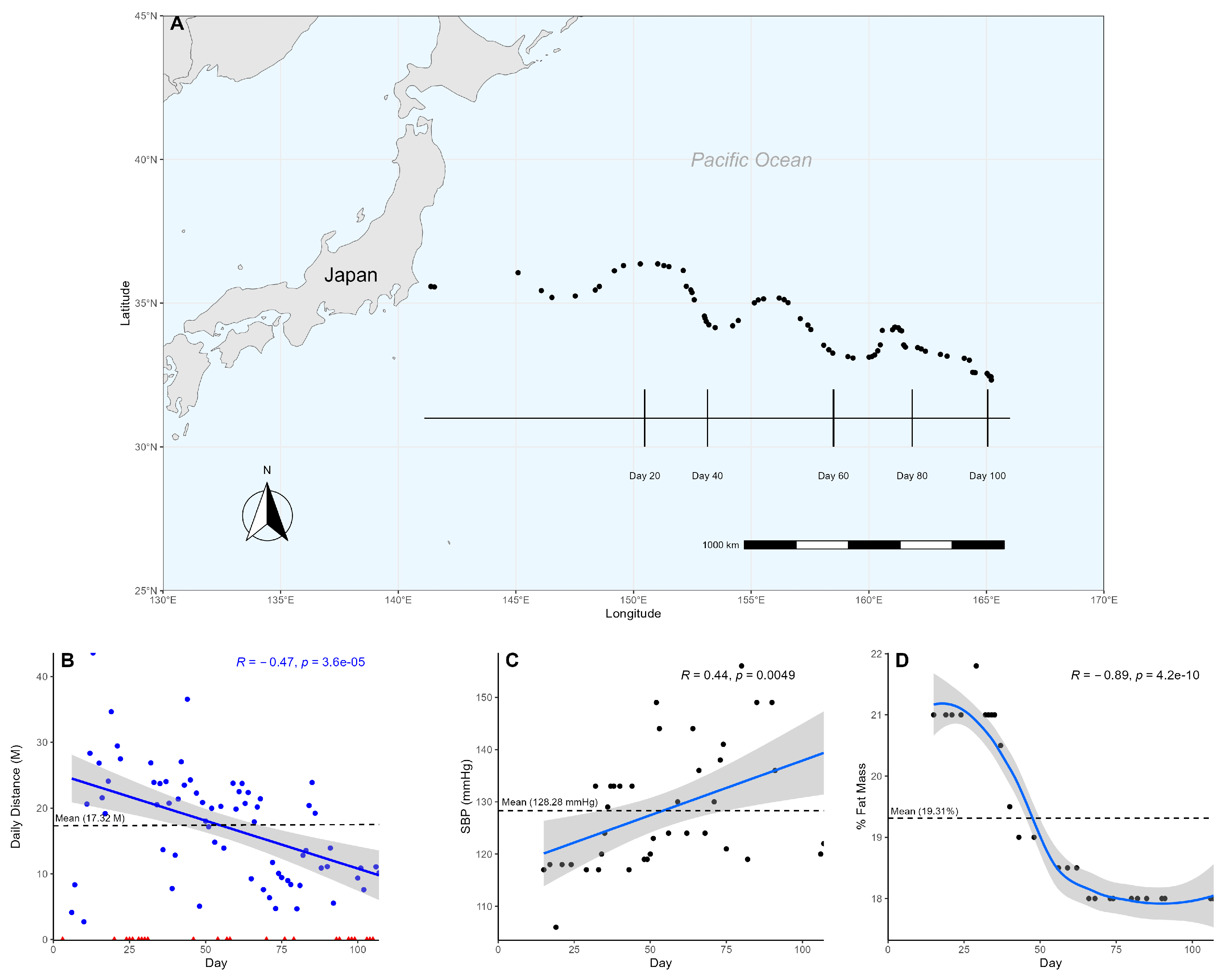


| Variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Counts | M ± SD | p Value |
|---|---|---|---|---|---|---|---|
| Daily swim distance | 22.39 ± 11.78 | 20.24 ± 6.92 | 14.66 ± 6.86 | 12.58 ± 5.04 | 69 | 17.32 ± 8.44 | ** |
| Heart rate | 64.4 ± 9.07 | 67.35 ± 5.43 | 64.09 ± 6.63 | 64.83 ± 8.33 | 39 | 65.67 ± 6.64 | 0.59 |
| Blood pressure | 115.4 ± 5.27 | 127.24 ± 9.71 | 133.45 ± 10.73 | 132.5 ± 14.18 | 39 | 128.28 ± 11.50 | * |
| Body fat composition | 21.00 ± 0.00 | 20.42 ± 1.01 | 18.19 ± 0.26 | 18.00 ± 0.00 | 27 | 19.31 ± 1.41 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, G.; Reczek, S.; Omozusi, O.; Hogue, T.; Cook, M.D.; Hampton-Marcell, J. Machine Learning Reveals Microbial Taxa Associated with a Swim across the Pacific Ocean. Biomedicines 2024, 12, 2309. https://doi.org/10.3390/biomedicines12102309
Lewis G, Reczek S, Omozusi O, Hogue T, Cook MD, Hampton-Marcell J. Machine Learning Reveals Microbial Taxa Associated with a Swim across the Pacific Ocean. Biomedicines. 2024; 12(10):2309. https://doi.org/10.3390/biomedicines12102309
Chicago/Turabian StyleLewis, Garry, Sebastian Reczek, Osayenmwen Omozusi, Taylor Hogue, Marc D. Cook, and Jarrad Hampton-Marcell. 2024. "Machine Learning Reveals Microbial Taxa Associated with a Swim across the Pacific Ocean" Biomedicines 12, no. 10: 2309. https://doi.org/10.3390/biomedicines12102309
APA StyleLewis, G., Reczek, S., Omozusi, O., Hogue, T., Cook, M. D., & Hampton-Marcell, J. (2024). Machine Learning Reveals Microbial Taxa Associated with a Swim across the Pacific Ocean. Biomedicines, 12(10), 2309. https://doi.org/10.3390/biomedicines12102309






