Effects of Angiotensin-I-Converting Enzyme (ACE) Mutations Associated with Alzheimer’s Disease on Blood ACE Phenotype
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Whole Exome Sequencing
2.3. Chemicals
2.4. Antibodies
2.5. ACE Activity Assay
2.6. Immunological Characterization of the Blood ACE
2.7. Statistical Analysis
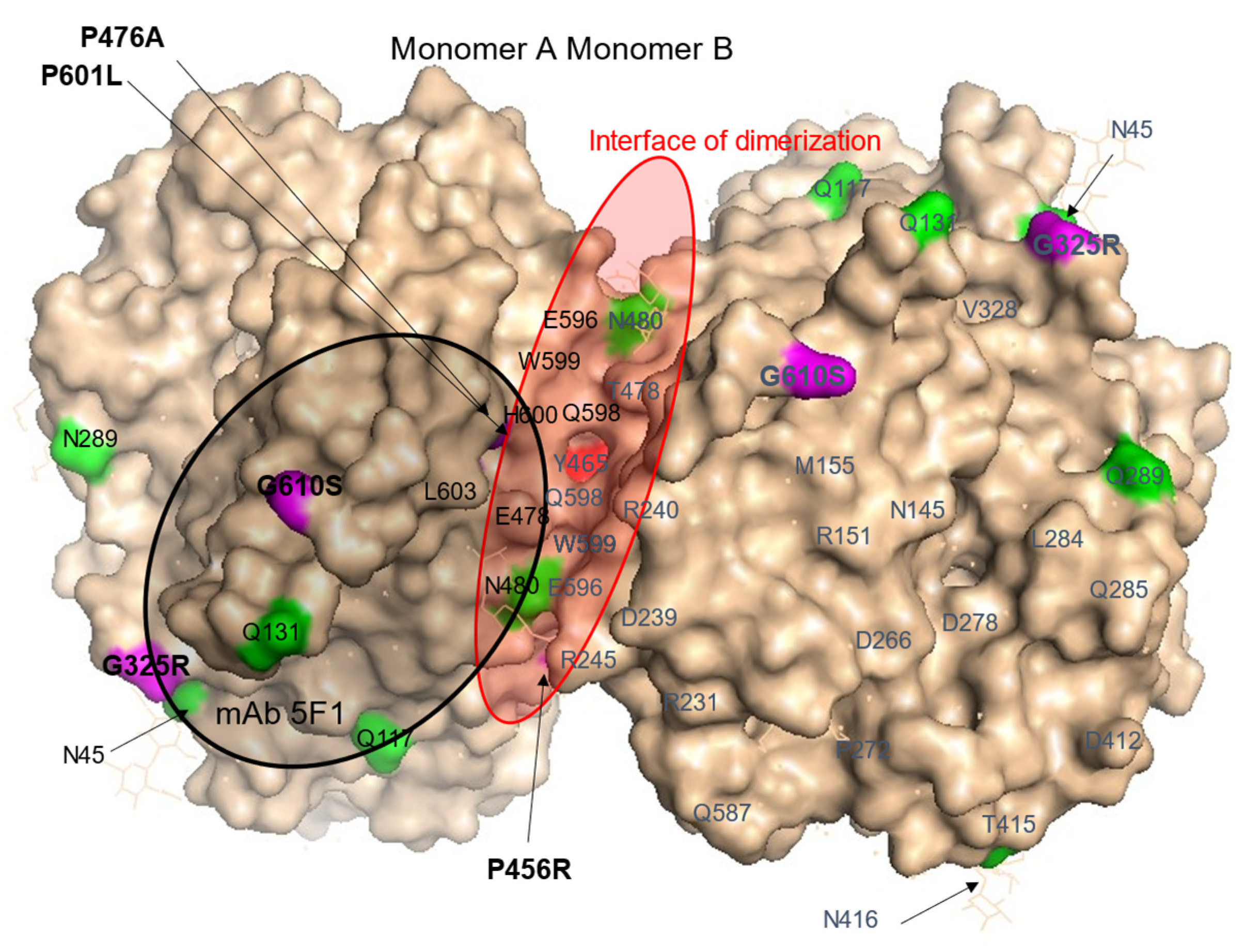
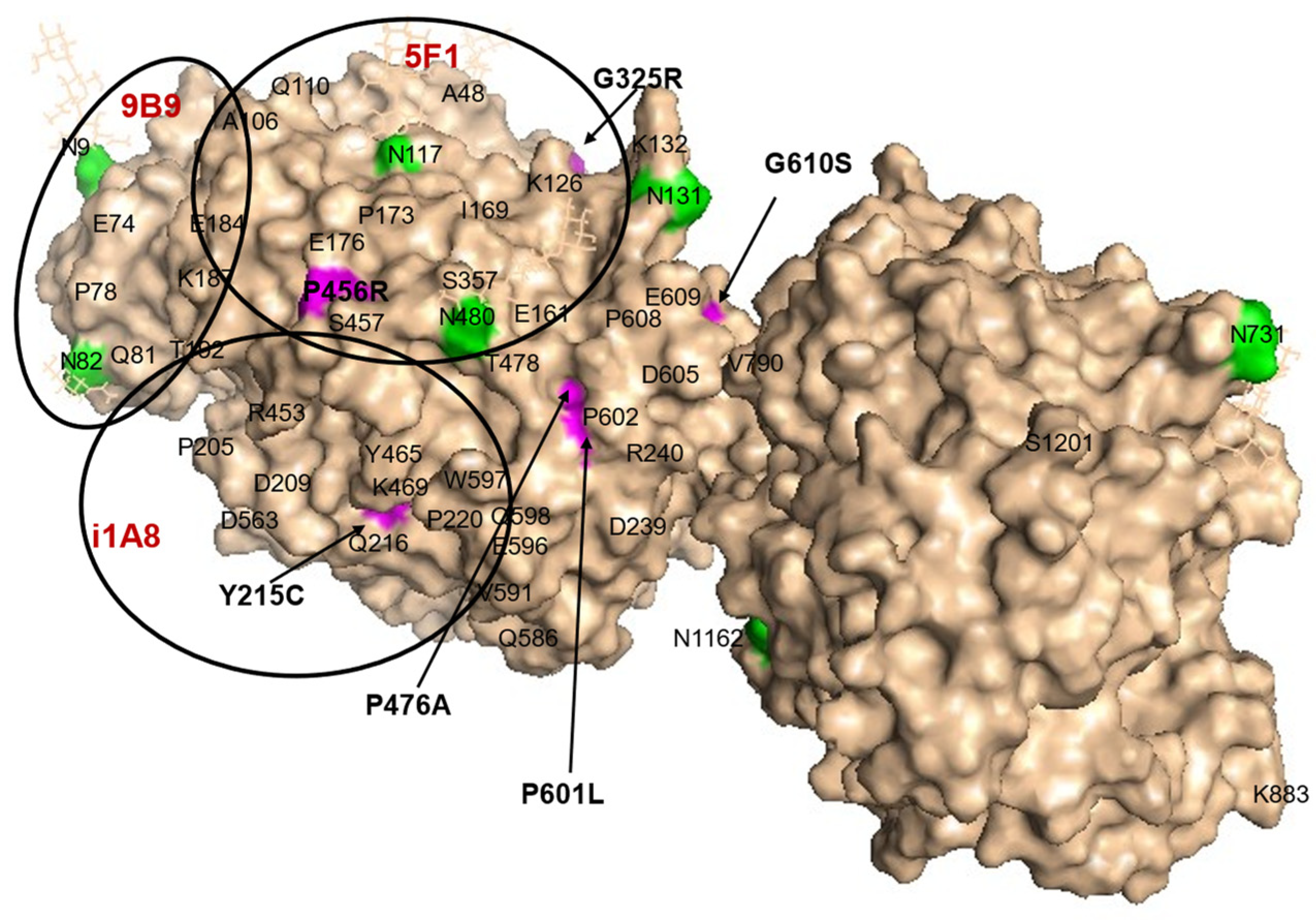
2.8. Localization of AD-Associated ACE Mutations on ACE Globule
3. Results and Discussion
3.1. Quantification of Blood ACE in Carriers of ACE Mutations
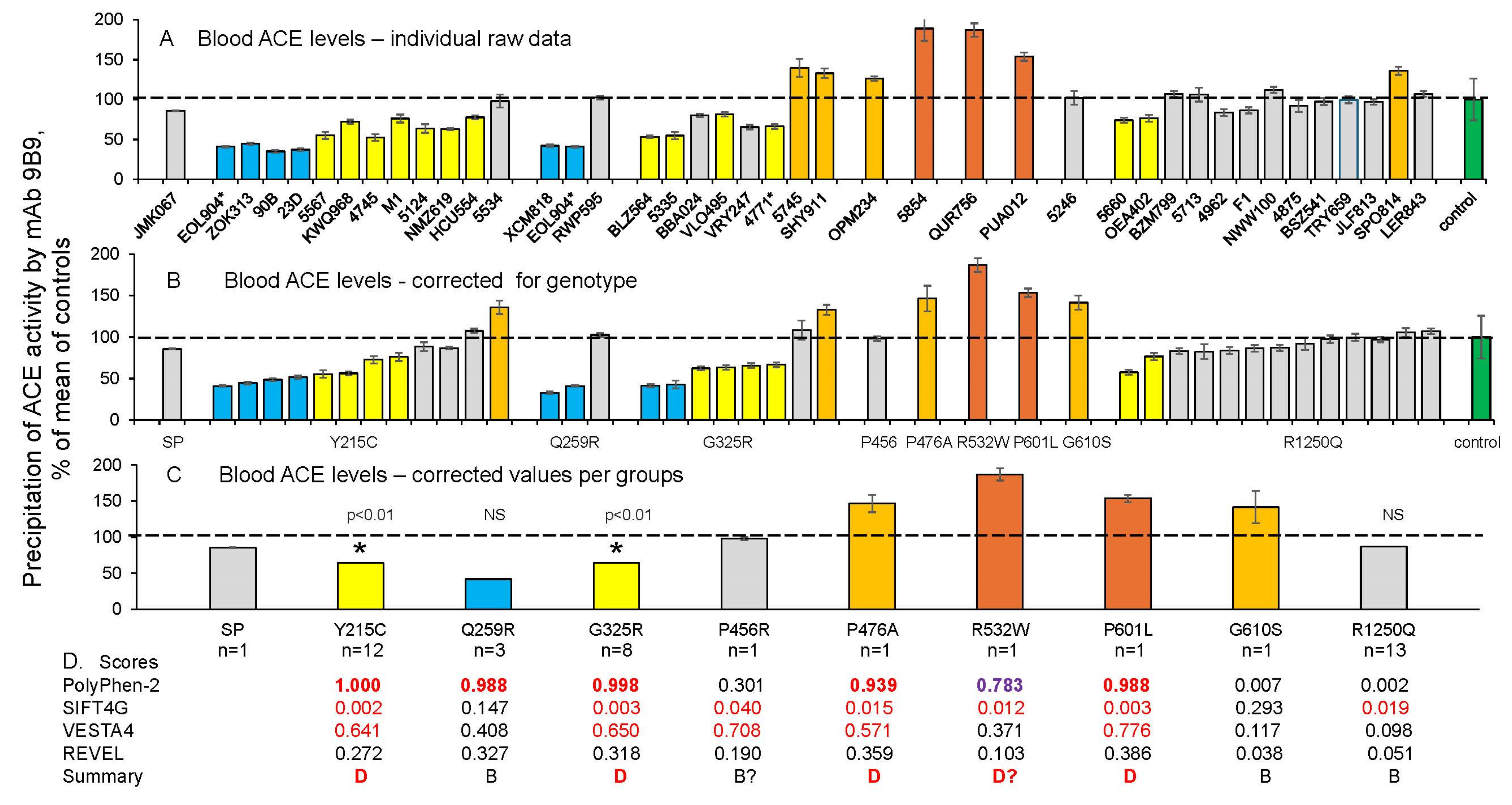
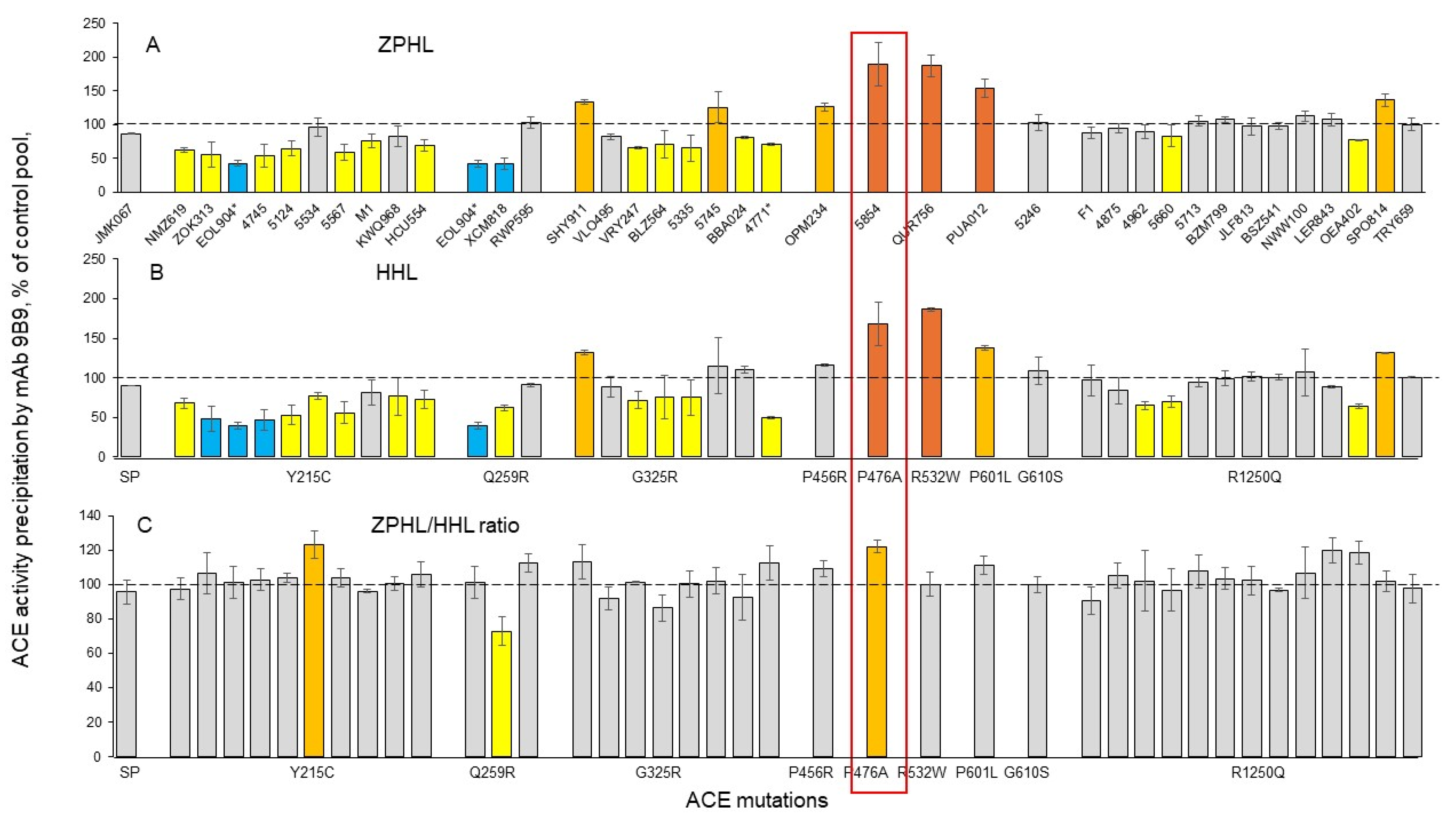
3.2. Detection of Catalytic Abnormalities of Mutant ACEs Using EDTA Plasma Samples


4. Conclusions
- The ACE phenotyping method utilizes the precipitation of ACE by different mAbs in combination with the subsequent measurement of ACE activity [28,31,32,49] to quantitatively estimate the effects of these ACE mutations. It also allows for the identification of patients with transport-deficient ACE mutations that are characterized by a substantial decrease in blood ACE levels (for example, carriers of the common ACE mutations Y215C and G325R).
- Combining the various ACE mAbs at our disposal [29,30] with multiple substrates theoretically allows for the detection of individuals with ACE mutations in the N domain active center that decrease catalytic activity. These mutations may inhibit the ability to hydrolyze N-domain-specific substrates, including Aβ42 [14], which could increase AD risk.
- Using only EDTA-containing plasma samples for ACE phenotyping precludes the direct measurement of blood ACE activity in solution from an individual, but this approach can yield patterns and markers indicative of the effects of ACE mutations. This is achieved through the application of a set of ACE mAbs along with various ACE substrates. This approach is especially useful for transport-deficient ACE mutations. It is conceivable that future clinical trials may target carriers of these transport-deficient ACE mutations to test the effectiveness of a cocktail of chemical and pharmacological chaperones, as well as proteasome inhibitors to rescue the impaired trafficking of mutant ACEs to the cell surface. Such an approach was previously successful as a “proof of concept” in an in vitro model of a rare transport-deficient ACE mutation (Q1069R) [20].
- To estimate the effects of such therapy in future clinical trials, it will be necessary to quantify mutant ACE levels in the blood of patients. We have established such markers for both of the transport-deficient ACE mutations identified in this study: the 2H9/1G12 binding ratio (with ZPHL as a substrate) distinguishes ACE with the Y215C mutation from normal ACE or from ACE with other tested mutations, whereas the 1G12/5F1 binding ratio (also with ZPHL as a substrate) is a marker for the G325R mutation.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Tcw, J.; Goate, A.M. Genetics of β-Amyloid Precursor Protein in Alzheimer’s Disease. Cold Spring Harb. Perspect. Med. 2017, 7, a024539. [Google Scholar] [CrossRef] [PubMed]
- Korovaitseva, G.I.; Bukina, A.; Farrer, L.A.; Rogaev, E.I. Presenilin polymorphism in Alzheimer’s disease. Lancet 1997, 350, 959. [Google Scholar] [CrossRef]
- Sims, R.; Hill, M.; Williams, J. The multiplex model of the genetics of Alzheimer’s disease. Nat. Neurosci. 2020, 23, 311–322. [Google Scholar] [CrossRef]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.D.C.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef]
- Schwartzentruber, J.; Cooper, S.; Liu, J.Z.; Barrio-Hernandez, I.; Bello, E.; Kumasaka, N.; Young, A.M.; Franklin, R.J.; Johnson, T.; Estrada, K.; et al. Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer’s disease risk genes. Nat. Genet. 2021, 53, 392–402. [Google Scholar] [CrossRef]
- Kehoe, P.G.; Russ, C.; McIlroy, S.; Williams, H.; Holmans, P.; Holmes, C.; Liolitsa, D.; Vahidassr, D.; Powell, J.; McGleenon, B.; et al. Variation in DCP1, encoding ACE, is associated with susceptibility to Alzheimer’s disease. Nat. Genet. 1999, 21, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Sassi, C.; Ridge, P.G.; Nalls, M.A.; Gibbs, R.; Ding, J.; Lupton, M.K.; Troakes, C.; Lunnon, K.; Al-Sarraj, S.; Brown, K.S.; et al. Influence of coding variability in APP-Aβ metabolism genes in sporadic Alzheimer’s disease. PLoS ONE 2016, 11, e0150079. [Google Scholar] [CrossRef]
- Sturrock, E.D.; Anthony, C.S.; Danilov, S.M. Peptidyl-dipeptidase a/angiotensin I-converting enzyme. In Handbook of Proteolytic Enzymes; Elsevier: Amsterdam, The Netherlands, 2012; pp. 480–494. [Google Scholar]
- Bernstein, K.E.; Ong, F.S.; Blackwell, W.-L.B.; Shah, K.H.; Giani, J.F.; Gonzalez-Villalobos, R.A.; Shen, X.Z.; Fuchs, S. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol. Rev. 2012, 65, 1–46. [Google Scholar] [CrossRef]
- Hu, J.; Igarashi, A.; Kamata, M.; Nakagawa, H. Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J. Biol. Chem. 2001, 276, 47863–47868. [Google Scholar] [CrossRef]
- Liu, S.; Ando, F.; Fujita, Y.; Liu, J.; Maeda, T.; Shen, X.; Kikuchi, K.; Matsumoto, A.; Yokomori, M.; Tanabe-Fujimura, C.; et al. A clinical dose of angiotensin-converting enzyme (ACE) inhibitor and heterozygous ace deletion exacerbate Alzheimer’s disease pathology in mice. J. Biol. Chem. 2019, 294, 9760–9770. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-Y.; Zhao, Q.-H.; Huang, Q.; Dammer, E.; Chen, S.-D.; Ren, R.-J.; Wang, G. Genetic profiles of familial late-onset Alzheimer’s disease in China: The Shanghai FLOAD Study. Genes. Dis. 2022, 9, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Maeda, T.; Watanabe, A.; Liu, J.; Liu, S.; Oba, R.; Satoh, Y.I.; Komano, H.; Michikawa, M. Aβ42-to-Aβ40- and angiotensin-converting activities in different domains of angiotensin-converting enzyme. J. Biol. Chem. 2009, 284, 31914–31920. [Google Scholar] [CrossRef]
- Danilov, S.M.; Adzhubei, I.A.; Kozuch, A.J.; Petukhov, P.A.; Popova, I.A.; Choudhury, A.; Sengupta, D.; Dudek, S.M. Carriers of heterozygous loss-of-function ACE mutations are at risk for Alzheimer’s disease. Biomedicines 2024, 12, 162. [Google Scholar] [CrossRef]
- Kehoe, P.G.; Katzov, H.; Feuk, L.; Bennet, A.M.; Johansson, B.; Wiman, B.; Faire, U.D.; Cairns, N.J.; Wilcock, G.K.; Brookes, A.J.; et al. Haplotypes extending across ACE are associated with Alzheimer’s disease. Hum. Mol. Genet. 2003, 12, 859–867. [Google Scholar] [CrossRef]
- Corvol, P.; Michaud, A.; Gribouval, O.; Gasc, J.M.; Gubler, M.C. Can we live without a functional renin-angiotensin system? Clin. Exp. Pharmacol. Physiol. 2008, 35, 431–433. [Google Scholar] [PubMed]
- Gribouval, O.; Morinière, V.; Pawtowski, A.; Arrondel, C.; Sallinen, S.-L.; Saloranta, C.; Clericuzio, C.; Viot, G.; Tantau, J.; Blesson, S.; et al. Spectrum of mutations in the renin-angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum. Mut. 2011, 33, 316–326. [Google Scholar] [CrossRef]
- Cuddy, L.K.; Prokopenko, D.; Cunningham, E.P.; Brimberry, R.; Song, P.; Kirchner, R.; Chapman, B.A.; Hofmann, O.; Hide, W.; Procissi, D.; et al. Aβ-accelerated neurodegeneration caused by Alzheimer’s-associated ACE variant R1279Q is rescued by angiotensin system inhibition in mice. Sci. Transl. Med. 2020, 12, eaaz2541. [Google Scholar] [CrossRef]
- Danilov, S.M.; Kalinin, S.; Chen, Z.; Vinokour, E.I.; Nesterovitch, A.B.; Schwartz, D.E.; Gribouval, O.; Gubler, M.-C.; Minshall, R.D. Angiotensin I-converting enzyme Gln1069Arg mutation impairs trafficking to the cell surface resulting in selective denaturation of the C-domain. PLoS ONE 2010, 5, e10438. [Google Scholar] [CrossRef]
- Belova, V.; Pavlova, A.; Afasizhev, R.; Moskalenko, V.; Korzhanova, M.; Krivoy, A.; Cheranev, V.; Nikashin, B.; Bulusheva, I.; Rebrikov, D.; et al. System analysis of the sequencing quality of human whole exome samples on BGI NGS platform. Sci. Rep. 2022, 12, 609. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 138. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Poplin, R.; Chang, P.-C.; Alexander, D.; Schwartz, S.; Colthurst, T.; Ku, A.; Newburger, D.; Dijamco, J.; Nguyen, N.; Afshar, P.T.; et al. A universal SNP and small-indel variant caller using deep neural networks. Nat. Biotech. 2018, 6, 983–987. [Google Scholar] [CrossRef]
- Tan, A.; Abecasis, G.R.; Kang, H.M. Unified Representation of Genetic Variants. Bioinformatics 2015, 31, 2202–2204. [Google Scholar] [CrossRef]
- Li, Q.; Wang, K. InterVar: Clinical interpretation of genetic variants by ACMG-AMP 2015 guideline. Am. J. Hum. Genet 2017, 100, 1–14. [Google Scholar] [CrossRef]
- Danilov, S.M.; Balyasnikova, I.V.; Danilova, A.S.; Naperova, I.A.; Arablinskaya, N.E.; Borisov, S.E.; Metzger, R.; Franke, F.E.; Schwartz, D.E.; Gachok, I.V.; et al. Conformational fingerprinting of the angiotensin I-converting enzyme (ACE). 1. application in sarcoidosis. J. Proteome Res. 2010, 9, 5782–5793. [Google Scholar] [CrossRef]
- Danilov, S.M. Conformational fingerprinting using monoclonal antibodies (on the example of angiotensin I-converting enzyme-ACE). Mol. Biol. 2017, 51, 906–920. [Google Scholar] [CrossRef]
- Popova, I.A.; Lubbe, L.; Petukhov, P.A.; Kalantarov, G.F.; Trakht, I.N.; Chernykh, E.R.; Leplina, O.Y.; Lyubimov, A.V.; Garcia, J.G.; Dudek, S.M.; et al. Epitope mapping of novel monoclonal antibodies to human angiotensin I-converting enzyme. Protein Sci. 2021, 30, 1577–1593. [Google Scholar] [CrossRef]
- Danilov, S.; Savoie, F.; Lenoir, B.; Jeunemaitre, X.; Azizi, M.; Tarnow, L.; Alhenc-Gelas, F. Development of enzyme-linked immunoassays for human angiotensin I converting enzyme suitable for large-scale studies. J. Hypertens. 1996, 14, 719–727. [Google Scholar] [CrossRef]
- Samokhodskaya, L.M.; Jain, M.S.; Kurilova, O.V.; Bobkov, A.P.; Kamalov, A.A.; Dudek, S.M.; Danilov, S.M. Phenotyping angiotensin-converting enzyme in blood: A necessary approach for precision medicine. J. Appl. Lab. Med. 2021, 6, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Danilov, S.M.; Balyasnikova, I.V.; Albrecht, R.F.; Kost, O.A. Simultaneous determination of ACE activity with 2 substrates provides information on the status of somatic ace and allows detection of inhibitors in human blood. J. Cardiovasc. Pharmacol. 2008, 52, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; E Ramensky, V.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Lubbe, L.; Sewell, B.T.; Woodward, J.D.; Sturrock, E.D. Cryo-EM reveals mechanisms of angiotensin I-converting enzyme allostery and dimerization. EMBO J. 2022, 41, e110550. [Google Scholar] [CrossRef]
- Anthony, C.S.; Corradi, H.R.; Schwager, S.L.; Redelinghuys, P.; Georgiadis, D.; Dive, V.; Acharya, K.R.; Sturrock, E.D. The N domain of human angiotensin-I- converting enzyme: The role of N glycosylation and crystal structure in complex with N domain specific phosphinic inhibitor, RXP407. J. Biol. Chem. 2010, 285, 35685–35693. [Google Scholar] [CrossRef] [PubMed]
- Biller, H.; Zissel, G.; Ruprecht, B.; Nauck, M.; Grawitz, A.B.; Muller-Quernheim, J. Genotype-corrected reference values for serum angiotensin-converting enzyme. Eur. Resp. J. 2006, 28, 1085–1090. [Google Scholar] [CrossRef]
- Kruit, A.; Grutters, J.C.; Gerritsen, W.B.; Kos, S.; Wodzig, W.K.; van den Bosch, J.M.; Ruven, H.J. ACE I/D-corrected Z-scores to identify normal and elevated ACE activity in sarcoidosis. Respir. Med. 2007, 101, 510–515. [Google Scholar] [CrossRef]
- Danilov, S.M.; Lünsdorf, H.; Akinbi, H.T.; Nesterovitch, A.B.; Epshtein, Y.; Letsiou, E.; Kryukova, O.V.; Piegeler, T.; Golukhova, E.Z.; Schwartz, D.E.; et al. Lysozyme and bilirubin bind to ACE and regulate its conformation and shedding. Sci. Rep. 2016, 6, 34913. [Google Scholar] [CrossRef]
- Rigat, B.; Hubert, C.; Alhenc-Gelas, F.; Cambien, F.; Corvol, P.; Soubrier, F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Invest. 1990, 86, 1343–1346. [Google Scholar] [CrossRef]
- Rice, G.I.; Jones, A.L.; Grant, P.J.; Carter, A.M.; Turner, A.J.; Hooper, N.M. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension 2006, 48, 914–920. [Google Scholar] [CrossRef]
- Costerousse, O.; Allegrini, J.; Lopez, M.; Alhenc-Gelas, F. Angiotensin I-converting enzyme in human circulating mononuclear cells: Genetic polymorphism of expression in T-lymphocytes. Biochem. J. 1993, 290 Pt 1, 33–40. [Google Scholar] [CrossRef]
- Danser, A.J.; Schalekamp, M.A.; Bax, W.A.; Brink, A.M.v.D.; Saxena, P.R.; Riegger, G.A.; Schunkert, H. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation 1995, 92, 1387–1388. [Google Scholar] [CrossRef] [PubMed]
- Tiret, L.; Rigat, B.; Visvikis, S.; Breda, C.; Corvol, P.; Cambien, F.; Soubrier, F. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am. J. Hum. Genet. 1992, 51, 197–205. [Google Scholar] [PubMed]
- Lopera, F.; Marino, C.; Chandrahas, A.S.; O’Hare, M.; Villalba-Moreno, N.D.; Aguillon, D.; Baena, A.; Sanchez, J.S.; Vila-Castelar, C.; Ramirez Gomez, L.; et al. Resilience to autosomal dominant Alzheimer’s disease in a Reelin-COLBOS heterozygous man. Nat. Med. 2023, 29, 1243–1252. [Google Scholar] [CrossRef]
- Quiroz, Y.T.; Aguillon, D.; Aguirre-Acevedo, D.C.; Vasquez, D.; Zuluaga, Y.; Baena, A.Y.; Madrigal, L.; Hincapié, L.; Sanchez, J.S.; Langella, S.; et al. APOE3 Christchurch Heterozygosity and Autosomal Dominant Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 2156–2164. [Google Scholar]
- Danilov, S.M.; Gordon, K.; Nesterovitch, A.B.; Lünsdorf, H.; Chen, Z.; Castellon, M.; Popova, I.A.; Kalinin, S.; Mendonca, E.; Petukhov, P.A.; et al. Angiotensin I-converting enzyme mutation (Y465D) cause dramatic increase in blood ACE via accelerated ACE shedding due to changes of ACE dimerization. PLoS ONE 2011, 6, e25952. [Google Scholar] [CrossRef]
- Danilov, S.M.; Jain, M.S.; Petukhov, P.A.; Goldman, C.; DiSanto-Rose, M.; Vancavage, R.; Francuzevitch, L.Y.; Samokhodskaya, L.M.; Kamalov, A.A.; Arbieva, Z.H.; et al. Novel ACE mutations mimicking sarcoidosis by increasing blood ACE levels. Transl. Res. 2021, 230, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Danilov, S.M.; Tikhomirova, V.E.; Metzger, R.; Naperova, I.A.; Bukina, T.M.; Goker-Alpan, O.; Tayebi, N.; Gayfullin, N.M.; Schwartz, D.E.; Samokhodskaya, L.M.; et al. ACE phenotyping in Gaucher disease. Mol. Genet. Metab. 2018, 123, 501–510. [Google Scholar] [CrossRef]
- Danilov, S.M.; Watermeyer, J.M.; Balyasnikova, I.V.; Gordon, K.; Kugaevskaya, E.V.; Elisseeva, Y.E.; Albrecht, R.F.; Sturrock, E.D. Fine epitope mapping of mAb 5F1 reveals anticatalytic activity. Biochemistry 2007, 46, 9019–9031. [Google Scholar] [CrossRef]
- Naqvi, N.; Liu, K.; Graham, R.M.; Husain, A. Molecular basis of exopeptidase activity in the C-terminal domain of human angiotensin I-converting enzyme: Insights into the origins of its exopeptidase activity. J. Biol. Chem. 2005, 280, 6669–6675. [Google Scholar] [CrossRef]
- Cozier, G.E.; Lubbe, L.; Sturrock, E.D.; Acharya, K.R. Angiotensin-converting enzyme open for business: Structural insights into the subdomain dynamics. FEBS J. 2020, 288, 2238–2256. [Google Scholar] [CrossRef] [PubMed]
- Kohlstedt, K.; Shoghi, F.; Müller-Esterl, W.; Busse, R.; Fleming, I. CK2 phosphorylates the angiotensin-converting enzyme and regulates its retention in the endothelial cell plasma membrane. Circ. Res. 2002, 91, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Kozuch, A.J.; Petukhov, P.A.; Fagyas, M.; Popova, I.A.; Lindeblad, M.O.; Bobkov, A.P.; Kamalov, A.A.; Toth, A.; Dudek, S.M.; Danilov, S.M. Urinary ACE phenotyping as a research and diagnostic tool: Identification of sex-dependent ACE immunoreactivity. Biomedicines 2023, 11, 953. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryukova, O.V.; Islanov, I.O.; Zaklyazminskaya, E.V.; Korostin, D.O.; Belova, V.A.; Cheranev, V.V.; Repinskaia, Z.A.; Tonevitskaya, S.A.; Petukhov, P.A.; Dudek, S.M.; et al. Effects of Angiotensin-I-Converting Enzyme (ACE) Mutations Associated with Alzheimer’s Disease on Blood ACE Phenotype. Biomedicines 2024, 12, 2410. https://doi.org/10.3390/biomedicines12102410
Kryukova OV, Islanov IO, Zaklyazminskaya EV, Korostin DO, Belova VA, Cheranev VV, Repinskaia ZA, Tonevitskaya SA, Petukhov PA, Dudek SM, et al. Effects of Angiotensin-I-Converting Enzyme (ACE) Mutations Associated with Alzheimer’s Disease on Blood ACE Phenotype. Biomedicines. 2024; 12(10):2410. https://doi.org/10.3390/biomedicines12102410
Chicago/Turabian StyleKryukova, Olga V., Igor O. Islanov, Elena V. Zaklyazminskaya, Dmitry O. Korostin, Vera A. Belova, Valery V. Cheranev, Zhanna A. Repinskaia, Svetlana A. Tonevitskaya, Pavel A. Petukhov, Steven M. Dudek, and et al. 2024. "Effects of Angiotensin-I-Converting Enzyme (ACE) Mutations Associated with Alzheimer’s Disease on Blood ACE Phenotype" Biomedicines 12, no. 10: 2410. https://doi.org/10.3390/biomedicines12102410
APA StyleKryukova, O. V., Islanov, I. O., Zaklyazminskaya, E. V., Korostin, D. O., Belova, V. A., Cheranev, V. V., Repinskaia, Z. A., Tonevitskaya, S. A., Petukhov, P. A., Dudek, S. M., Kost, O. A., Rebrikov, D. V., & Danilov, S. M. (2024). Effects of Angiotensin-I-Converting Enzyme (ACE) Mutations Associated with Alzheimer’s Disease on Blood ACE Phenotype. Biomedicines, 12(10), 2410. https://doi.org/10.3390/biomedicines12102410







