Comparison of Malondialdehyde, Acetylcholinesterase, and Apoptosis-Related Markers in the Cortex and Hippocampus of Cognitively Dysfunctional Mice Induced by Scopolamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Experimental Protocol
2.2.1. Evaluation of Scopolamine Duration
2.2.2. Design of Scopolamine Dose Experiment
2.3. Behavior Test
2.3.1. Morris Water Maze
2.3.2. Novel Object Recognition Test
2.4. Lipid Peroxidation
2.5. AChE Activity
2.6. Western Blotting
2.7. Immunohistochemical Analyses of BDNF, Nerve Growth Factor (NGF), and MAP2 Marker Expression
2.8. Statistical Analyses
3. Results
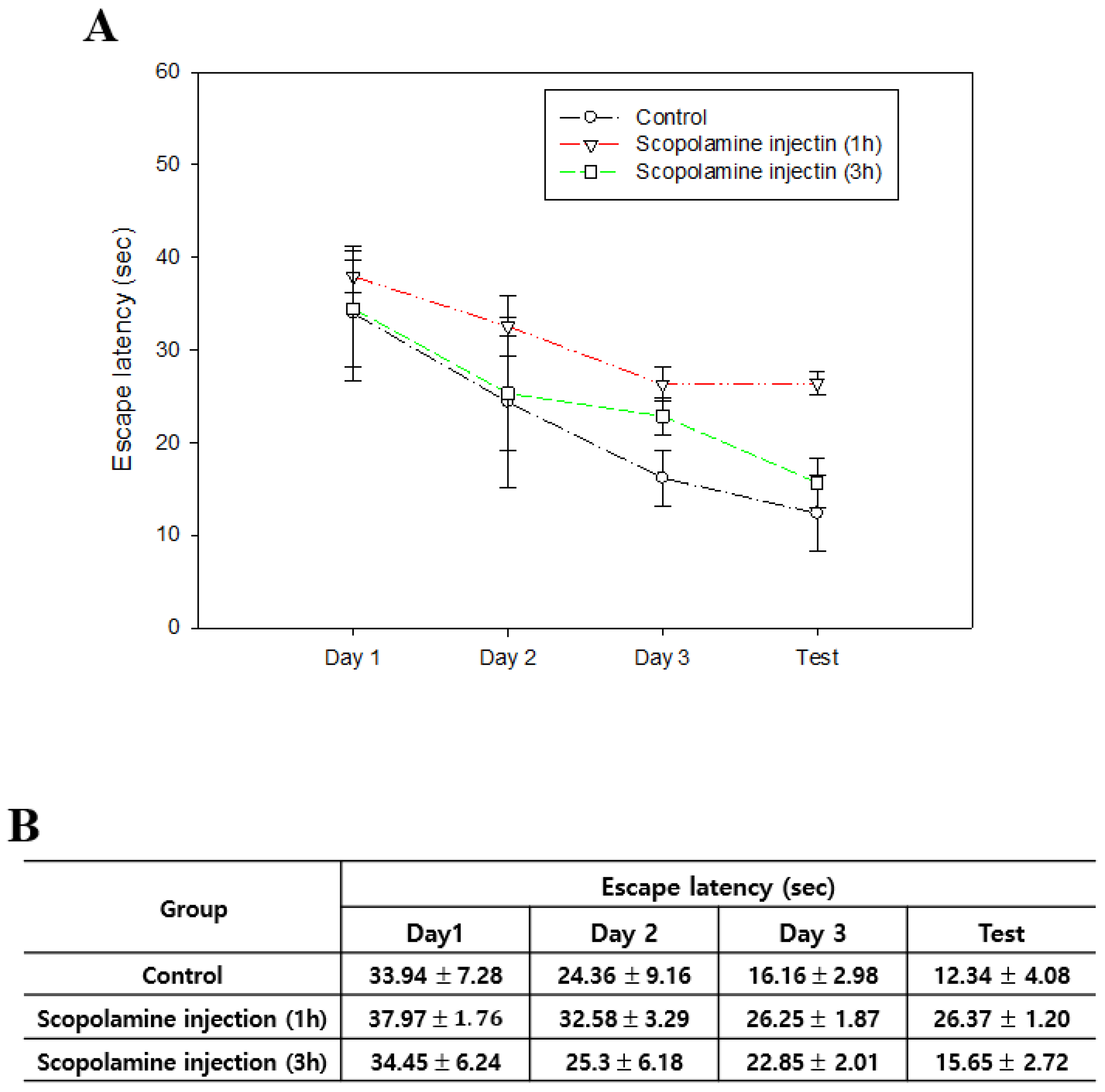
3.1. Cognitive Impairment Caused by Scopolamine Does Not Persist Long-Term
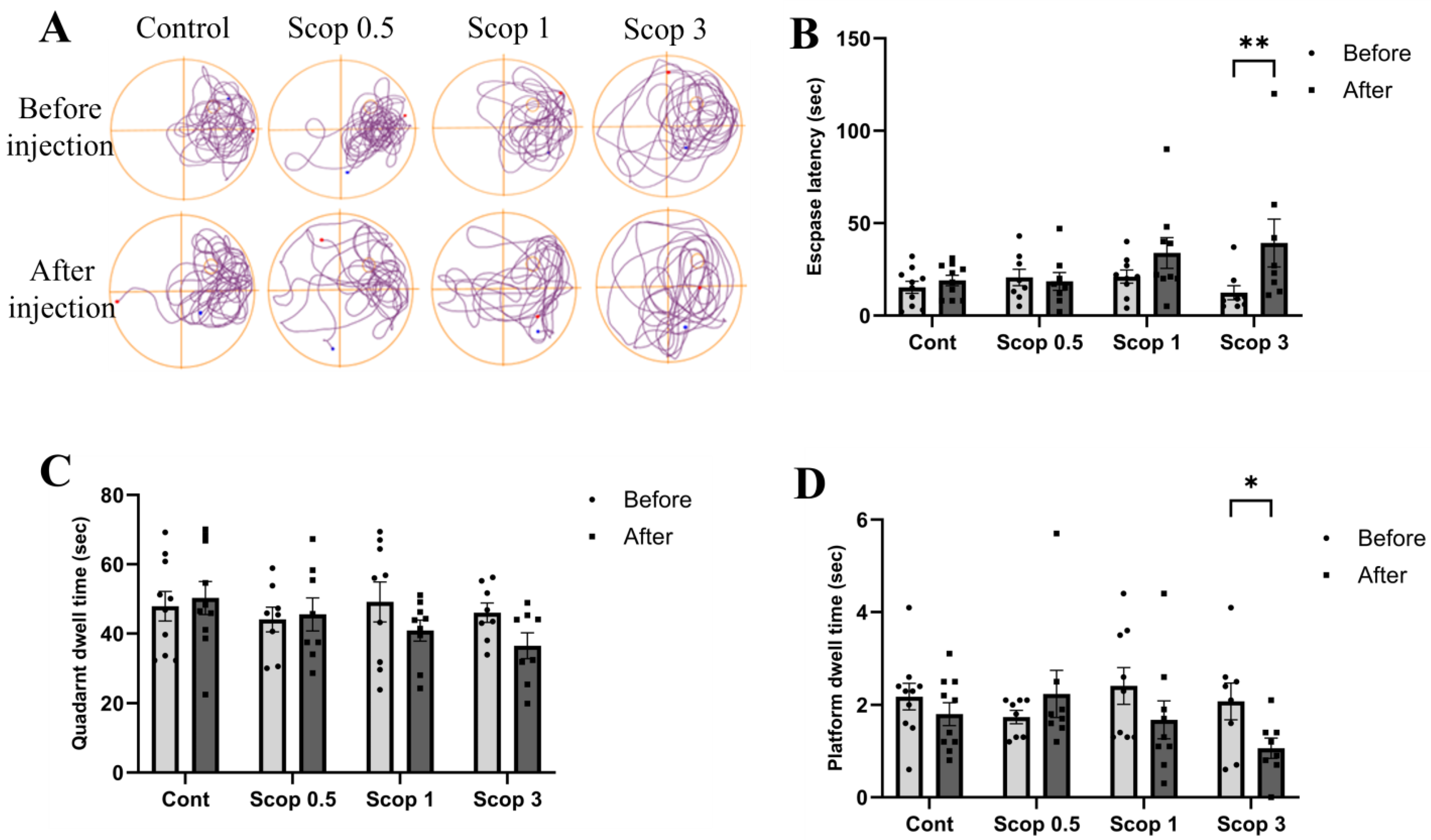
3.2. Spatial Learning and Memory Impairment Due to Scopolamine Depends on the Dose
3.3. Scopolamine-Induced Recognition Memory Impairment Is Reduced at Certain Doses
3.4. Immunohistochemical Analysis of BDNF, NGF, and MAP2 Marker Expression
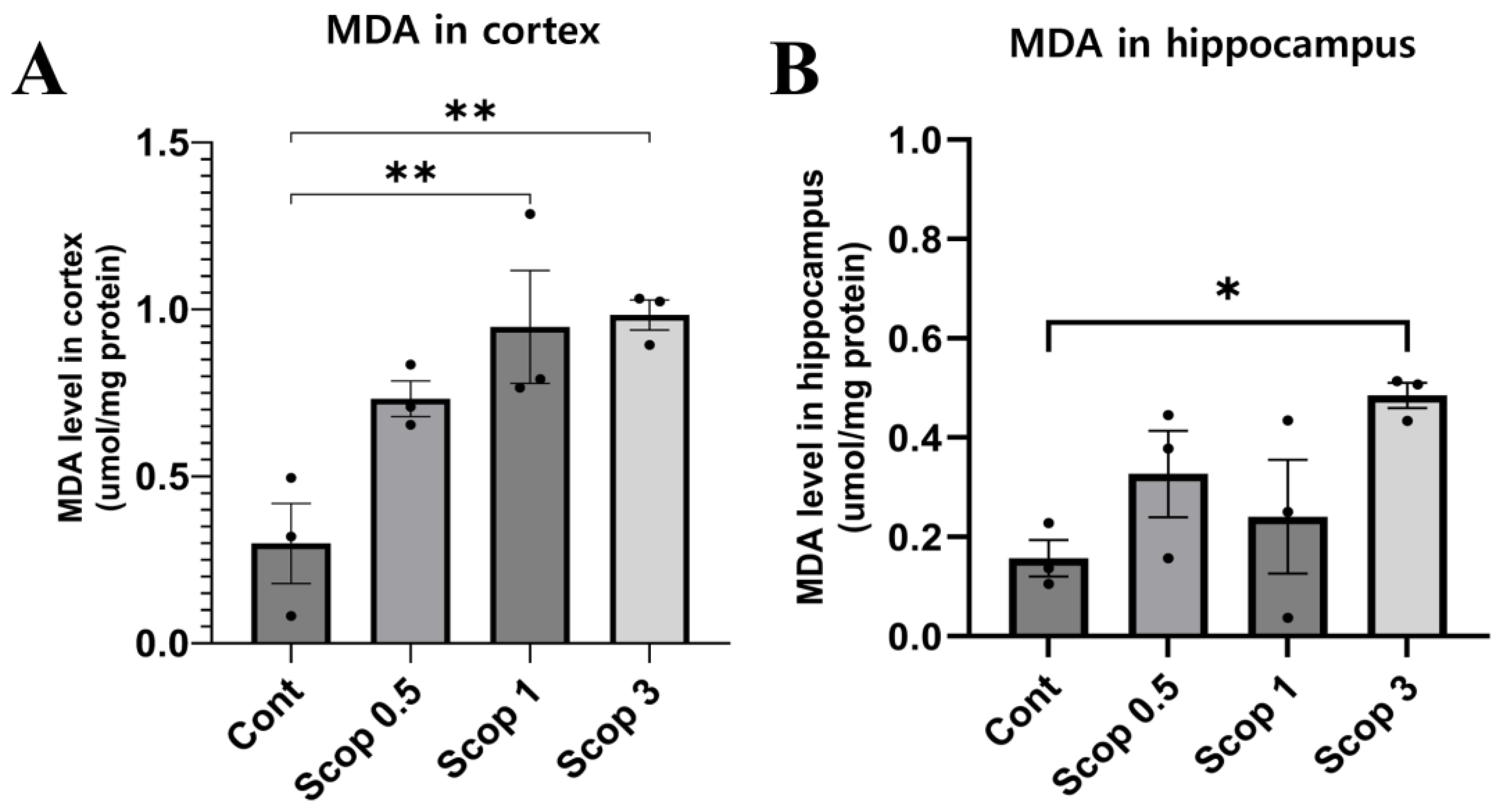
3.5. Changes in Malondialdehyde Level in the Brain Following Scopolamine Administration
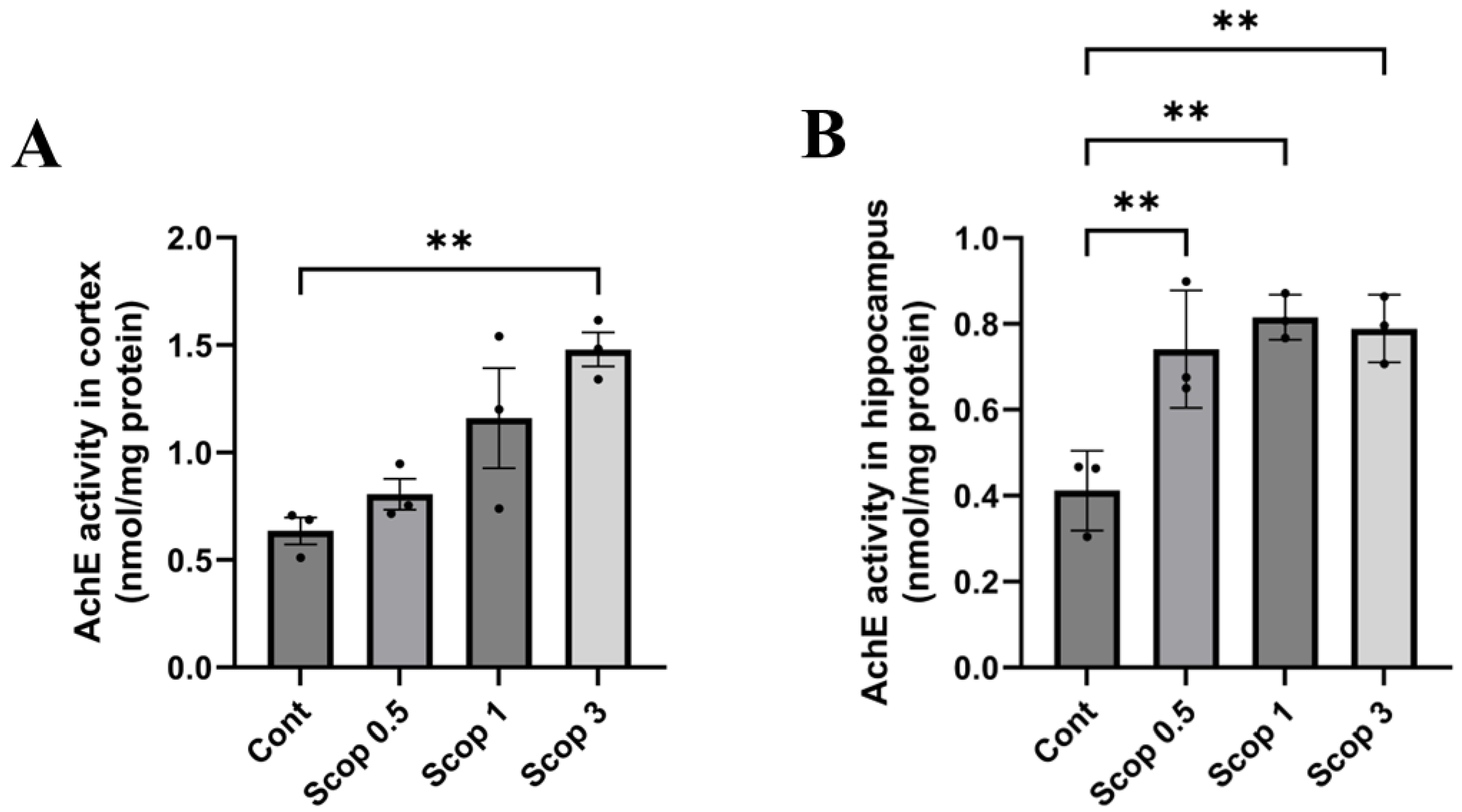
3.6. Changes in Acetylcholinesterase (AChE) Activity in the Brain Following Scopolamine Administration
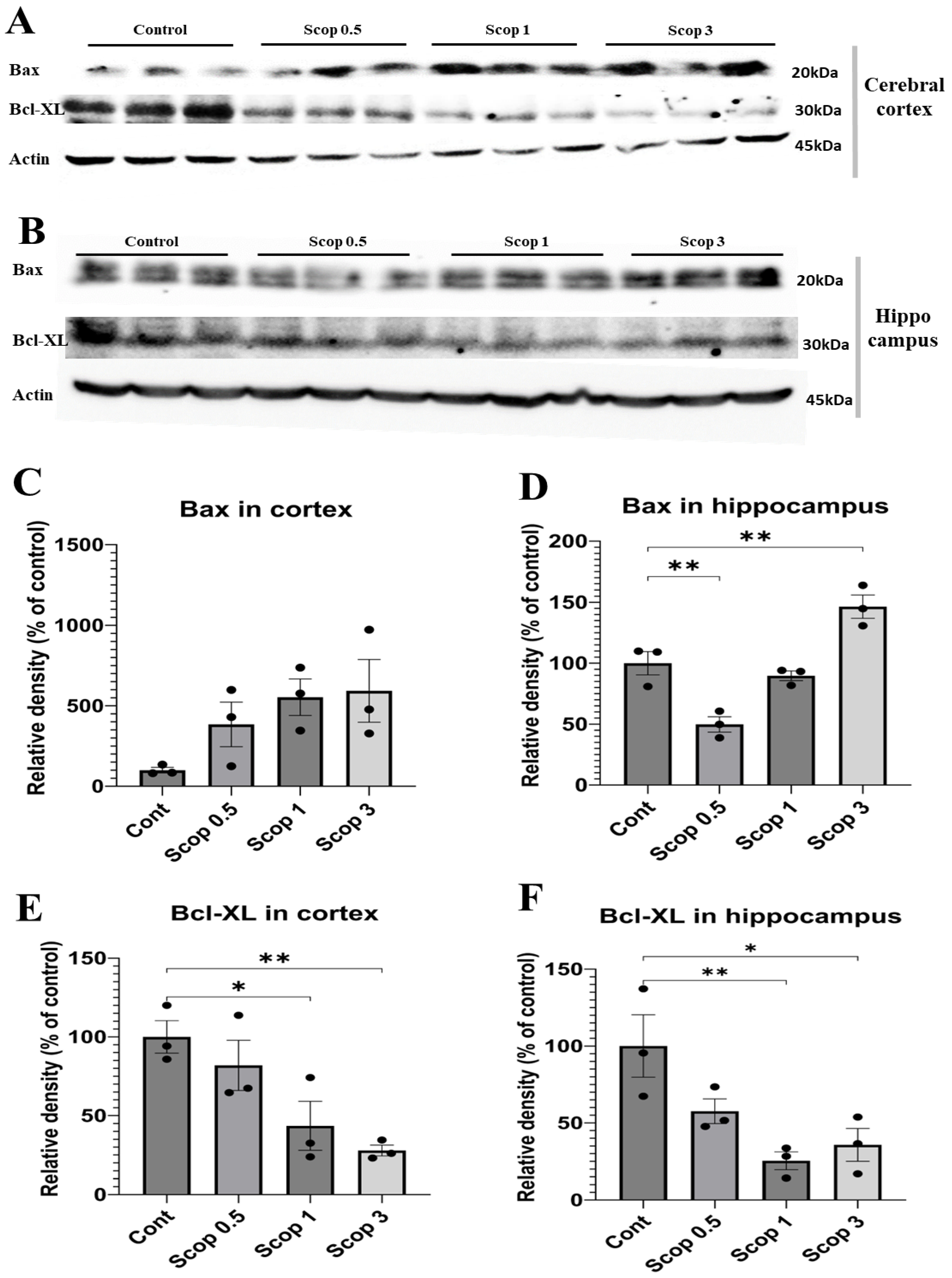
3.7. Neuronal Cell Death Level Effect Depending on Scopolamine Dose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Camargos, E.R.; de Souza, L.C.; Teixeira, A.L. Animal models of neurodegenerative diseases. Braz J Psychiatry. 2013, 35, S82–S91. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Res 2018, 7, 1161. [Google Scholar] [CrossRef]
- Lanctôt, K.L.; Amatniek, J.; Ancoli-Israel, S.; Arnold, S.E.; Ballard, C.; Cohen-Mansfield, J.; Ismail, Z.; Lyketsos, C.; Miller, D.S.; Musiek, E.; et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimer’s Dement. 2017, 3, 440–449. [Google Scholar] [CrossRef]
- Amor, S.; Peferoen, L.A.; Vogel, D.Y.; Breur, M.; van der Valk, P.; Baker, D.; van Noort, J.M. Inflammation in neurodegenerative diseases—An update. Immunology 2014, 142, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.R.; Awan, N.R.; Yar, M. Neurodegenerative diseases and effective drug delivery: A review of challenges and novel therapeutics. J. Control. Release 2021, 330, 1152–1167. [Google Scholar] [CrossRef]
- Martin, J.B. Molecular basis of the neurodegenerative disorders. N. Engl. J. Med. 1999, 340, 1970–1980. [Google Scholar] [CrossRef]
- Aigner, L.; Arber, S.; Kapfhammer, J.P.; Laux, T.; Schneider, C.; Botteri, F.; Brenner, H.R.; Caroni, P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell 1995, 83, 269–278. [Google Scholar] [CrossRef]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin Rescue Oxidative Stress-Mediated Neuroinflammation/ Neurodegeneration and Memory Impairment in Scopolamine-Induced Amnesia Mice Model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Ibach, B.; Haen, E. Acetylcholinesterase inhibition in Alzheimer’s Disease. Curr. Pharm. Des. 2004, 10, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Rountree, S.D.; Chan, W.; Pavlik, V.N.; Darby, E.J.; Siddiqui, S.; Doody, R.S. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer disease. Alzheimer’s Res. Ther. 2009, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Balez, R.; Ooi, L. Getting to NO Alzheimer’s Disease: Neuroprotection versus Neurotoxicity Mediated by Nitric Oxide. Oxid. Med. Cell Longev. 2016, 2016, 3806157. [Google Scholar] [CrossRef] [PubMed]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell Adh. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.C. Small nanobody drugs win big backing from pharma. Nat. Med. 2013, 19, 1355–1356. [Google Scholar] [CrossRef]
- Arrowsmith, J. Phase II failures: 2008–2010. Nat. Rev. Drug. Discov. 2011, 10, 328–329. [Google Scholar] [CrossRef]
- Denayer, T.; Stöhr, T.; Van Roy, M. Animal models in translational medicine: Validation and prediction. New Horiz. Transl. Med. 2014, 2, 5–11. [Google Scholar] [CrossRef]
- Capurro, V.; Busquet, P.; Lopes, J.P.; Bertorelli, R.; Tarozzo, G.; Bolognesi, M.L.; Piomelli, D.; Reggiani, A.; Cavalli, A. Pharmacological characterization of memoquin, a multi-target compound for the treatment of Alzheimer’s disease. PLoS ONE 2013, 8, e56870. [Google Scholar] [CrossRef]
- Zhang, G.R.; Cheng, X.R.; Zhou, W.X.; Zhang, Y.X. Age-related expression of calcium/calmodulin-dependent protein kinase II A in the hippocampus and cerebral cortex of senescence accelerated mouse prone/8 mice is modulated by anti-Alzheimer’s disease drugs. Neuroscience 2009, 159, 308–315. [Google Scholar] [CrossRef]
- Vanguilder, H.D.; Freeman, W.M. The hippocampal neuroproteome with aging and cognitive decline: Past progress and future directions. Front. Aging. Neurosci. 2011, 3, 8. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Heo, S.; McLaren, M.; Pence, B.D.; Martin, S.A.; Vieira, V.J.; Woods, J.A.; et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci. 2010, 30, 5368–5375. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Kumar, H.; Cho, D.Y.; Yun, Y.S.; Choi, D.K. Toxin-Induced Experimental Models of Learning and Memory Impairment. Int. J. Mol. Sci. 2016, 17, 1447. [Google Scholar] [CrossRef] [PubMed]
- Enokido, Y. New aspects of neuronal cell death: The molecular basis of neurodevelopmental and neurodegenerative disorders. Jpn. J. Psychopharmacol. 2012, 32, 31–35. [Google Scholar]
- Ibrahim, A.M.; Chauhan, L.; Bhardwaj, A.; Sharma, A.; Fayaz, F.; Kumar, B.; Alhashmi, M.; AlHajri, N.; Alam, M.S.; Pottoo, F.H. Brain-Derived Neurotropic Factor in Neurodegenerative Disorders. Biomedicines 2022, 10, 1143. [Google Scholar] [CrossRef]
- Pepeu, G.; Giovannini, M.G.; Bracco, L. Effect of cholinesterase inhibitors on attention. Chem. Biol. Interact. 2013, 203, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Mosimann, U.P.; McKeith, I.G. Role of cholinesterase inhibitors in Parkinson’s disease and dementia with Lewy bodies. J. Geriatr. Psychiatry Neurol. 2004, 17, 164–171. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean III, R.L.; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Dawson, G.R.; Heyes, C.M.; Iversen, S.D. Pharmacological mechanisms and animal models of cognition. Behav. Pharmacol. 1992, 3, 285–297. [Google Scholar] [CrossRef]
- Molchan, S.E.; Martinez, R.A.; Hill, J.L.; Weingartner, H.J.; Thompson, K.; Vitiello, B.; Sunderland, T. Increased cognitive sensitivity to scopolamine with age and a perspective on the scopolamine model. Brain Res. Rev. 1992, 17, 215–226. [Google Scholar] [CrossRef]
- Iversen, S.D. Behavioural evaluation of cholinergic drugs. Life Sci. 1997, 60, 1145–1152. [Google Scholar] [CrossRef]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 2006, Cd005593. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Sathya, S.; Manogari, B.G.; Thamaraiselvi, K.; Vaidevi, S.; Ruckmani, K.; Devi, K.P. Phytol loaded PLGA nanoparticles ameliorate scopolamine-induced cognitive dysfunction by attenuating cholinesterase activity, oxidative stress and apoptosis in Wistar rat. Nutr. Neurosci. 2022, 25, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Demirci, K.; Nazıroğlu, M.; Övey, İ.S.; Balaban, H. Selenium attenuates apoptosis, inflammation and oxidative stress in the blood and brain of aged rats with scopolamine-induced dementia. Metab. Brain Dis. 2017, 32, 321–329. [Google Scholar] [CrossRef]
- Belardo, C.; Boccella, S.; Perrone, M.; Fusco, A.; Morace, A.M.; Ricciardi, F.; Bonsale, R.; Elbini-Dhouib, I.; Guida, F.; Luongo, L.; et al. Scopolamine-Induced Memory Impairment in Mice: Effects of PEA-OXA on Memory Retrieval and Hippocampal LTP. Int. J. Mol. Sci. 2023, 24, 14399. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, H.-G.; Lee, H.-W.; Han, J.-M.; Lee, S.-K.; Kim, D.-W.; Saravanakumar, A.; Son, C.-G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, W.S.; Suo, D.Q.; Li, Y.; Peng, L.; Xu, L.X.; Zeng, K.Y.; Ren, T.; Wang, Y.; Zhou, Y.; et al. Moringa oleifera Seed Extract Alleviates Scopolamine-Induced Learning and Memory Impairment in Mice. Front. Pharmacol. 2018, 9, 389. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, C.; Qu, S.; Dong, S.; Ma, Q.; Hao, Y.; Liu, Z.; Wang, S.; Zhao, H.; Shi, Y. Chinese Herbal Extracts Exert Neuroprotective Effect in Alzheimer’s Disease Mouse Through the Dopaminergic Synapse/Apoptosis Signaling Pathway. Front. Pharmacol. 2022, 13, 817213. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, E.B.; Jang, H.H.; Cha, Y.S.; Park, Y.S.; Lee, S.H. Allium hookeri Extracts Improve Scopolamine-Induced Cognitive Impairment via Activation of the Cholinergic System and Anti-Neuroinflammation in Mice. Nutrients 2021, 13, 2890. [Google Scholar] [CrossRef]
- Rahimzadegan, M.; Soodi, M. Comparison of Memory Impairment and Oxidative Stress Following Single or Repeated Doses Administration of Scopolamine in Rat Hippocampus. BCN 2018, 9, 5–14. [Google Scholar] [CrossRef]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Gavrilova, S.I.; Alvarez, A. Cerebrolysin in the therapy of mild cognitive impairment and dementia due to Alzheimer’s disease: 30 years of clinical use. Med. Res. Rev. 2021, 41, 2775–2803. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Kim, H.G.; Choi, J.G.; Oh, H.; Lee, P.K.; Ha, S.K.; Kim, S.Y.; Park, Y.; Huh, Y.; Oh, M.S. 6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Biochem. Biophys. Res. Commun. 2014, 449, 8–13. [Google Scholar] [CrossRef]
- Puangmalai, N.; Thangnipon, W.; Soi-Ampornkul, R.; Suwanna, N.; Tuchinda, P.; Nobsathian, S. Neuroprotection of N-benzylcinnamide on scopolamine-induced cholinergic dysfunction in human SH-SY5Y neuroblastoma cells. Neural. Regen. Res. 2017, 12, 1492–1498. [Google Scholar] [PubMed]
- Hsieh, M.T.; Hsieh, C.L.; Lin, L.W.; Wu, C.R.; Huang, G.S. Differential gene expression of scopolamine-treated rat hippocampus-application of cDNA microarray technology. Life Sci. 2003, 73, 1007–1016. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Arciniega-Martínez, I.M.; García-Marín, I.D.; Correa-Basurto, J.; Rosales-Hernández, M.C. Chronic Administration of Scopolamine Increased GSK3βP9, Beta Secretase, Amyloid Beta, and Oxidative Stress in the Hippocampus of Wistar Rats. Mol. Neurobiol. 2020, 57, 3979–3988. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef]
- Pang, Y.; Shi, M. Repetitive Transcranial Magnetic Stimulation Improves Mild Cognitive Impairment Associated with Alzheimer’s Disease in Mice by Modulating the miR-567/NEUROD2/PSD95 Axis. Neuropsychiatr. Dis. Treat. 2021, 17, 2151–2161. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Kim, S.K.; Kwon, D.A.; Kim, Y.S.; Lee, H.S.; Kim, H.K.; Kim, W.K. Standardized Extract (HemoHIM) Protects against Scopolamine-Induced Amnesia in a Murine Model. Evid. Based Complement. Altern. Med. 2021, 2021, 8884243. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Seo, S.G.; Choi, B.R.; Han, J.S.; Lee, K.W.; Kim, J. Sulforaphane alleviates scopolamine-induced memory impairment in mice. Pharmacol. Res. 2014, 85, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Yadang, F.S.A.; Nguezeye, Y.; Kom, C.W.; Betote, P.H.D.; Mamat, A.; Tchokouaha, L.R.Y.; Taiwé, G.S.; Agbor, G.A.; Bum, E.N. Scopolamine-Induced Memory Impairment in Mice: Neuroprotective Effects of Carissa edulis (Forssk.) Valh (Apocynaceae) Aqueous Extract. Int. J. Alzheimer’s Dis. 2020, 2020, 6372059. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More Insight into BDNF against Neurodegeneration: Anti-Apoptosis, Anti-Oxidation, and Suppression of Autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef]
- Leal, G.; Bramham, C.R.; Duarte, C.B. BDNF and Hippocampal Synaptic Plasticity. Vitam. Horm. 2017, 104, 153–195. [Google Scholar]
- Phillips, H.S.; Hains, J.M.; Armanini, M.; Laramee, G.R.; Johnson, S.A.; Winslow, J.W. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 1991, 7, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Fukumoto, H.; Orne, J.; Klucken, J.; Raju, S.; Vanderburg, C.R.; Irizarry, M.C.; Hyman, B.T.; Ingelsson, M. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp. Neurol. 2005, 194, 91–96. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Dawbarn, D. The neurotrophins and their role in Alzheimer’s disease. Curr. Neuropharmacol. 2011, 9, 559–573. [Google Scholar] [CrossRef]
- Yang, S.; Fan, L.; Zhang, R.; Song, C.; Shi, J.; Wang, J.; Zhang, P.; Wang, H.; Zhang, Y. Smilagenin induces expression and epigenetic remodeling of BDNF in alzheimer’s disease. Phytomedicine 2023, 118, 154956. [Google Scholar] [CrossRef]
- Karthivashan, G.; Park, S.Y.; Kim, J.S.; Cho, D.Y.; Ganesan, P.; Choi, D.K. Comparative Studies on Behavioral, Cognitive and Biomolecular Profiling of ICR, C57BL/6 and Its Sub-Strains Suitable for Scopolamine-Induced Amnesic Models. Int. J. Mol. Sci. 2017, 18, 1735. [Google Scholar] [CrossRef]
- Kellner, Y.; Gödecke, N.; Dierkes, T.; Thieme, N.; Zagrebelsky, M.; Korte, M. The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Front. Synaptic Neurosci. 2014, 6, 5. [Google Scholar] [CrossRef]
- Parikh, V.; Howe, W.M.; Welchko, R.M.; Naughton, S.X.; D’Amore, D.E.; Han, D.H.; Deo, M.; Turner, D.L.; Sarter, M. Diminished trkA receptor signaling reveals cholinergic-attentional vulnerability of aging. Eur. J. Neurosci. 2013, 37, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Faldu, K.G.; Patel, S.S.; Shah, J.S. Celastrus paniculatus oil ameliorates NF-KB mediated neuroinflammation and synaptic plasticity in the scopolamine-induced cognitive impairment rat model. Metab. Brain. Dis. 2023, 38, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.; Lim, H.S.; Kim, Y.J.; Kim, B.Y.; Kim, J.H.; Jeong, S.J. Elaeagnus glabra f. oxyphylla Attenuates Scopolamine-Induced Learning and Memory Impairments in Mice by Improving Cholinergic Transmission via Activation of CREB/NGF Signaling. Nutrients 2019, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Muneoka, K.T.; Shirayama, Y.; Yamamoto, A.; Kawahara, R. A study of a dendritic marker, microtubule-associated protein 2 (MAP-2), in rats neonatally treated neurosteroids, pregnenolone and dehydroepiandrosterone (DHEA). Neurosci. Lett. 2005, 386, 145–149. [Google Scholar] [CrossRef][Green Version]
- Spina, M.G.; Grecksch, G.; Kovar, K.A.; Wolf, G.; Putzke, J. Microtubule-associated protein 2 (MAP2) and c-fos expression in the rat prefrontal cortex following subchronic treatment with substituted amphetamines. Ann. N. Y. Acad. Sci. 2000, 914, 65–70. [Google Scholar] [CrossRef]
- Khuchua, Z.; Wozniak, D.F.; Bardgett, M.E.; Yue, Z.; McDonald, M.; Boero, J.; Hartman, R.E.; Sims, H.; Strauss, A.W. Deletion of the N-terminus of murine map2 by gene targeting disrupts hippocampal ca1 neuron architecture and alters contextual memory. Neuroscience 2003, 119, 101–111. [Google Scholar] [CrossRef]
- Johnson, G.V.; Jope, R.S. The role of microtubule-associated protein 2 (MAP-2) in neuronal growth, plasticity, and degeneration. J. Neurosci. Res. 1992, 33, 505–512. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142, 111–121. [Google Scholar] [CrossRef]
- Singh, R.; Sharad, S.; Kapur, S. Free Radicals and Oxidative Stress in Neurodegenerative Diseases: Relevance of Dietary Antioxidants. J. Indian Acad. Clin. Med. 2004, 5, 218–225. [Google Scholar]
- Biesmans, S.; Meert, T.F.; Bouwknecht, J.A.; Acton, P.D.; Davoodi, N.; De Haes, P.; Kuijlaars, J.; Langlois, X.; Matthews, L.J.; Ver Donck, L.; et al. Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediat. Inflamm. 2013, 2013, 271359. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.V.; Williams, C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef] [PubMed]
- Wong-Guerra, M.; Jiménez-Martin, J.; Fonseca-Fonseca, L.A.; Ramírez-Sánchez, J.; Montano-Peguero, Y.; Rocha, J.B.; D’ Avila, F.; de Assis, A.M.; Souza, D.O.; Pardo-Andreu, G.L.; et al. JM-20 protects memory acquisition and consolidation on scopolamine model of cognitive impairment. Neurol. Res. 2019, 41, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, J.H.; Chung, H.S.; Song, J.H.; Ha, J.; Bae, H. Neuroprotective Effects of AMP-Activated Protein Kinase on Scopolamine Induced Memory Impairment. Korean J. Physiol. Pharmacol. 2013, 17, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Ali, N.; Almehmadi, M.; Ahmad, M.; Khalil, A.A.K.; Jamal, S.B.; Ahmad, H.; et al. Attenuation of Scopolamine-Induced Amnesia via Cholinergic Modulation in Mice by Synthetic Curcumin Analogs. Molecules 2022, 27, 2468. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Xu, Y.J.; Yang, C.; Tang, Y.; Li, L.; Cai, H.B.; Hou, B.N.; Chen, H.F.; Wang, Q.; Shi, X.G.; et al. Sodium Tanshinone IIA Sulfonate Attenuates Scopolamine-Induced Cognitive Dysfunctions via Improving Cholinergic System. BioMed Res. Int. 2016, 2016, 9852536. [Google Scholar] [CrossRef]
- Suthprasertporn, N.; Mingchinda, N.; Fukunaga, K.; Thangnipon, W. Neuroprotection of SAK3 on scopolamine-induced cholinergic dysfunction in human neuroblastoma SH-SY5Y cells. Cytotechnology 2020, 72, 155–164. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Yu, R.; Yang, Y.; Cui, Z.; Zheng, L.; Zeng, Z.; Zhang, H. Novel peptide VIP-TAT with higher affinity for PAC1 inhibited scopolamine induced amnesia. Peptides 2014, 60, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.Q.; Wu, D.W.; Zhang, C.X.; Yan, R.; Yang, C.; Rong, C.P.; Zhang, L.; Chang, X.; Su, R.Y.; Zhang, S.J.; et al. Bushen-Yizhi formula ameliorates cognition deficits and attenuates oxidative stress-related neuronal apoptosis in scopolamine-induced senescence in mice. Int. J. Mol. Med. 2014, 34, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Hu, J.; Li, J.; Yang, Z.; Xin, X.; Wang, J.; Ding, J.; Geng, M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci. Lett. 2005, 374, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ajami, M.; Eghtesadi, S.; Habibey, R.; Mirzay Razaz, J.; Peyrovi, H.; Zarrindast, M.; Pazoki-Toroudi, H. Effect of short and long-term treatment with omega-3 Fatty acids on scopolamine-induced amnesia. Iran. J. Pharm. Res. 2012, 11, 533–540. [Google Scholar]
- Giridharan, V.V.; Thandavarayan, R.A.; Sato, S.; Ko, K.M.; Konishi, T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free. Radic. Res. 2011, 45, 950–958. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, J.; Yang, H.; Gao, J.; Chen, H.; Liu, C.; Gao, W. Prunella vulgaris L. an Edible and Medicinal Plant, Attenuates Scopolamine-Induced Memory Impairment in Rats. J. Agric. Food Chem. 2017, 65, 291–300. [Google Scholar] [CrossRef]
- Smith, C.D.; Carney, J.M.; Starke-Reed, P.E.; Oliver, C.N.; Stadtman, E.R.; Floyd, R.A.; Markesbery, W.R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1991, 88, 10540–10543. [Google Scholar] [CrossRef]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Biebl, M.; Wilhelm, D.; Li, M.; Friedlander, R.M.; Winkler, J. Increased generation of granule cells in adult Bcl-2-overexpressing mice: A role for cell death during continued hippocampal neurogenesis. Eur. J. Neurosci. 2005, 22, 1907–1915. [Google Scholar] [CrossRef]
- Sun, X.Q.; Xu, Z.P.; Zhang, S.; Cao, X.S.; Liu, T.S. Simulated weightlessness aggravates hypergravity-induced impairment of learning and memory and neuronal apoptosis in rats. Behav. Brain Res. 2009, 199, 197–202. [Google Scholar] [CrossRef]
- Şener, G.; Karakadıoglu, G.; Ozbeyli, D.; Ede, S.; Yanardag, R.; Sacan, O.; Aykac, A. Petroselinum crispum extract ameliorates scopolamine-induced cognitive dysfunction: Role on apoptosis, inflammation and oxidative stress. Food Sci. Hum. Wellness 2022, 11, 1290–1298. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.; He, J.; Chen, L.; Li, H. Bcl-XL: A multifunctional anti-apoptotic protein. Pharmacol. Res. 2020, 151, 104547. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Gao, Y.; Ding, J.; Sun, N.; Lin, S. Antarctic krill peptides improve scopolamine-induced memory impairment in mice. Food Biosci. 2022, 49, 101987. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Dkhil, M.A.; Abdel-Gaber, R.; Zrieq, R.; Hafez, T.A.; Mubaraki, M.A.; Abdel Moneim, A.E. Myristica fragrans seed extract reverses scopolamine-induced cortical injury via stimulation of HO-1 expression in male rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 12395–12404. [Google Scholar] [CrossRef]

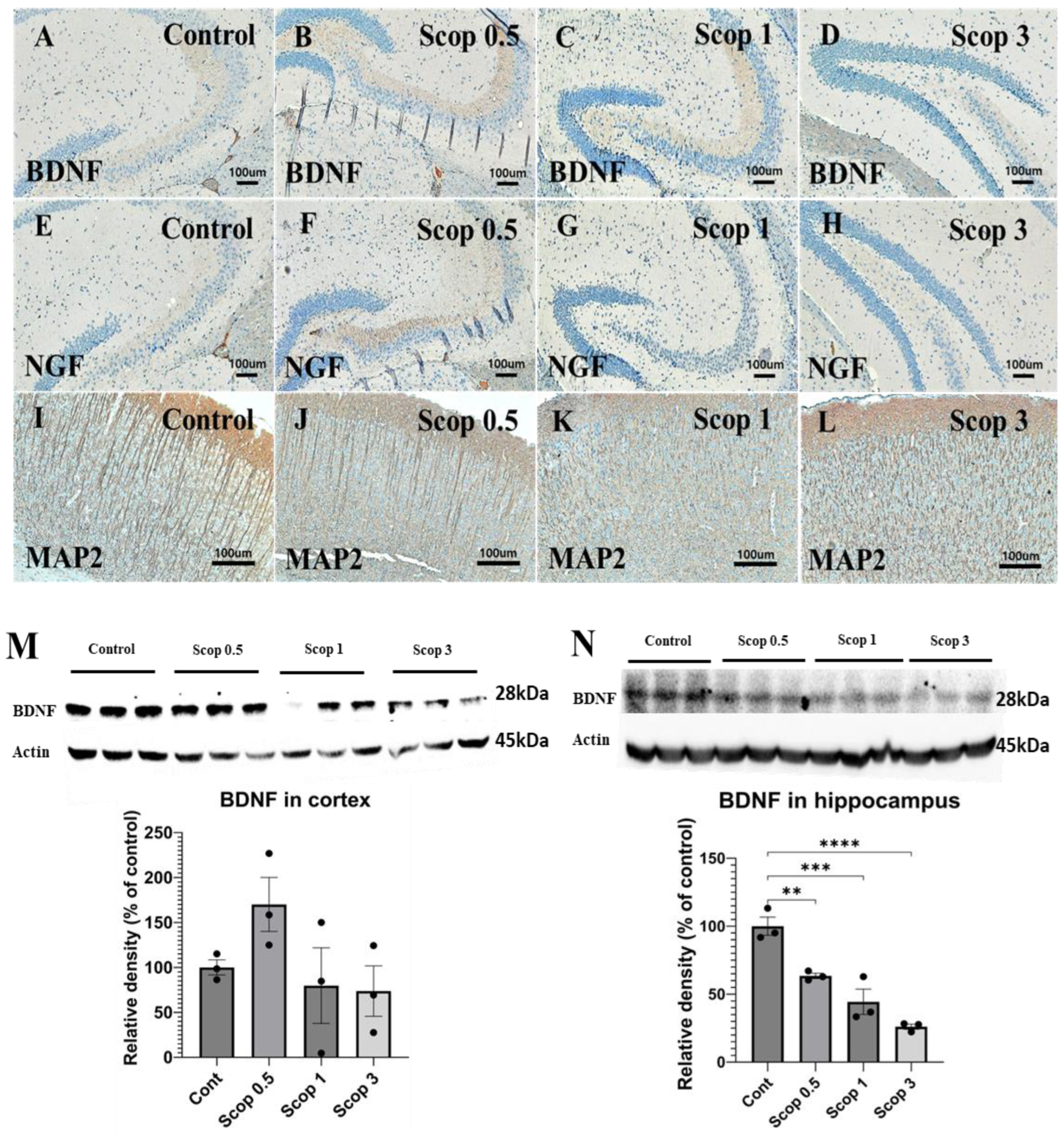
| Marker | Control | Scop 0.5 | Scop 1 | Scop 3 |
|---|---|---|---|---|
| BDNF | +++ | +++ | +++ | + |
| NGF | + | ++ | − | − |
| MAP2 | +++ | ++ | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-J.; Nam, M.-H.; Park, J.-H.; Lee, J.-M.; Hong, H.-S.; Kim, T.-W.; Lee, I.-H.; Shin, C.-H.; Lee, S.-H.; Seo, Y.-K. Comparison of Malondialdehyde, Acetylcholinesterase, and Apoptosis-Related Markers in the Cortex and Hippocampus of Cognitively Dysfunctional Mice Induced by Scopolamine. Biomedicines 2024, 12, 2475. https://doi.org/10.3390/biomedicines12112475
Park H-J, Nam M-H, Park J-H, Lee J-M, Hong H-S, Kim T-W, Lee I-H, Shin C-H, Lee S-H, Seo Y-K. Comparison of Malondialdehyde, Acetylcholinesterase, and Apoptosis-Related Markers in the Cortex and Hippocampus of Cognitively Dysfunctional Mice Induced by Scopolamine. Biomedicines. 2024; 12(11):2475. https://doi.org/10.3390/biomedicines12112475
Chicago/Turabian StylePark, Hee-Jung, Myeong-Hyun Nam, Ji-Hoon Park, Ji-Min Lee, Hye-Sun Hong, Tae-Woo Kim, In-Ho Lee, Chang-Ho Shin, Soo-Hong Lee, and Young-Kwon Seo. 2024. "Comparison of Malondialdehyde, Acetylcholinesterase, and Apoptosis-Related Markers in the Cortex and Hippocampus of Cognitively Dysfunctional Mice Induced by Scopolamine" Biomedicines 12, no. 11: 2475. https://doi.org/10.3390/biomedicines12112475
APA StylePark, H.-J., Nam, M.-H., Park, J.-H., Lee, J.-M., Hong, H.-S., Kim, T.-W., Lee, I.-H., Shin, C.-H., Lee, S.-H., & Seo, Y.-K. (2024). Comparison of Malondialdehyde, Acetylcholinesterase, and Apoptosis-Related Markers in the Cortex and Hippocampus of Cognitively Dysfunctional Mice Induced by Scopolamine. Biomedicines, 12(11), 2475. https://doi.org/10.3390/biomedicines12112475







