The Potential of Mesenchymal Stem Cells in Treating Spinocerebellar Ataxia: Advances and Future Directions
Abstract
1. Introduction
2. Overview of SCAs
3. The Essentials of MSCs and Their Characteristics
3.1. Basics of Stem Cell Therapy
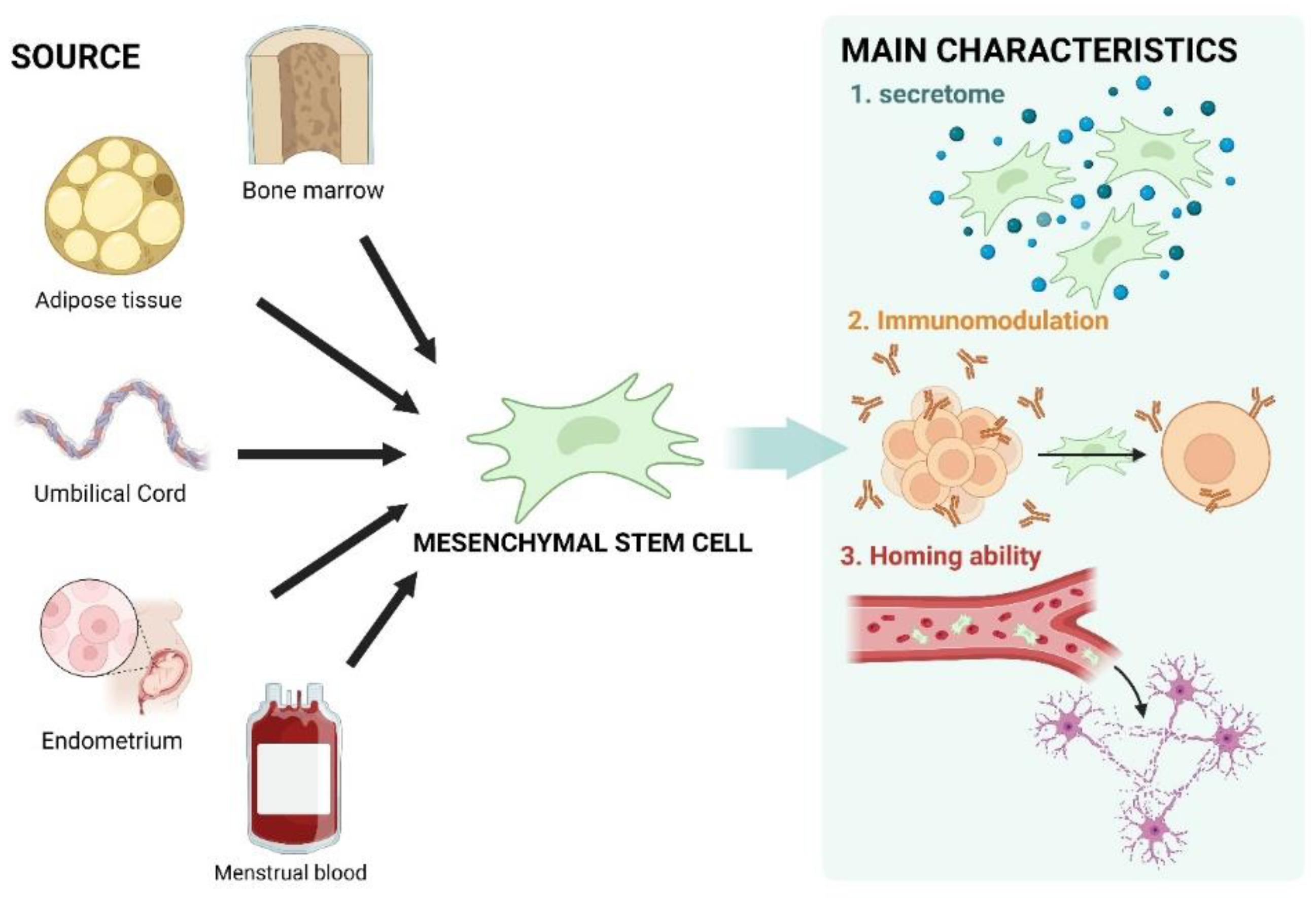
3.2. Characteristics of MSCs
4. Preclinical Research on MSC Treatments for CA
4.1. The Role of MSCs in Addressing Neurological Diseases
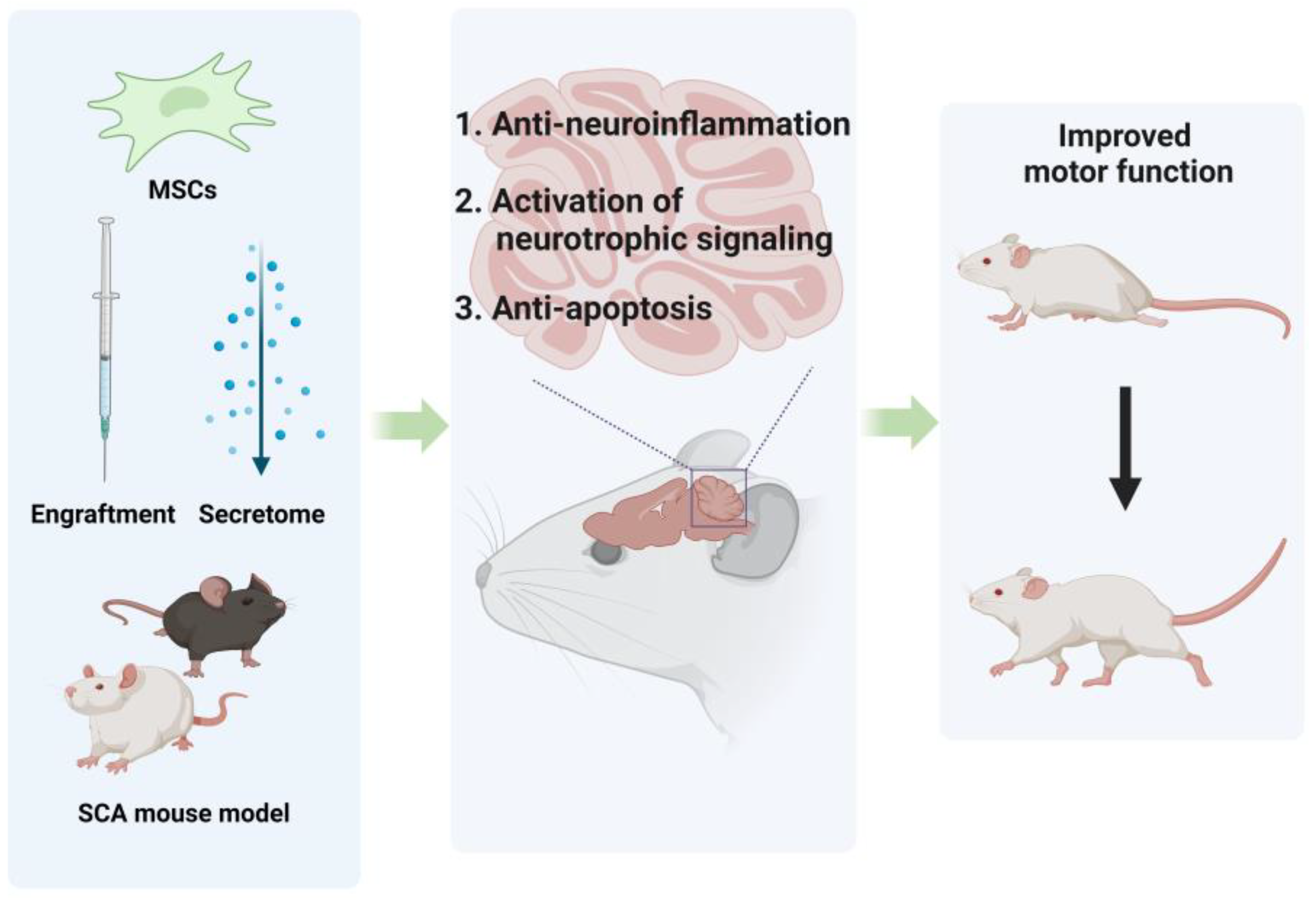
4.2. MSCs for SCA
4.2.1. Administration of MSCs Engraftment
4.2.2. Administration of MSCs-Derived Subcellular Components
5. Clinical Trials for MSC-Based SCA Therapy
5.1. Review of Previous Clinical Trials
5.2. Current and Upcoming Clinical Trials
5.3. Future Directions for MSC-Based Treatments
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manto, M.; Marmolino, D. Animal models of human cerebellar ataxias: A cornerstone for the therapies of the twenty-first century. Cerebellum 2009, 8, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.H. Ataxia. Contin. Lifelong Learn. Neurol. 2019, 25, 1036–1054. [Google Scholar] [CrossRef] [PubMed]
- Coarelli, G.; Coutelier, M.; Durr, A. Autosomal dominant cerebellar ataxias: New genes and progress towards treatments. Lancet Neurol. 2023, 22, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.T.; Mao, Z.T.; Yang, R.; Li, J.J.; Jia, S.S.; Zhao, J.L.; Zhong, F.T.; Yu, P.; Dong, M. Spinocerebellar ataxias: From pathogenesis to recent therapeutic advances. Front. Neurosci. 2024, 18, 1422442. [Google Scholar] [CrossRef]
- Tsai, P.J.; Yeh, C.C.; Huang, W.J.; Min, M.Y.; Huang, T.H.; Ko, T.L.; Huang, P.Y.; Chen, T.H.; Hsu, S.P.C.; Soong, B.W.; et al. Xenografting of human umbilical mesenchymal stem cells from Wharton’s jelly ameliorates mouse spinocerebellar ataxia type 1. Transl. Neurodegener. 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Suto, N.; Mieda, T.; Iizuka, A.; Nakamura, K.; Hirai, H. Morphological and Functional Attenuation of Degeneration of Peripheral Neurons by Mesenchymal Stem Cell-Conditioned Medium in Spinocerebellar Ataxia Type 1-Knock-in Mice. CNS Neurosci. Ther. 2016, 22, 670–676. [Google Scholar] [CrossRef]
- Correia, J.S.; Neves-Carvalho, A.; Mendes-Pinheiro, B.; Pires, J.; Teixeira, F.G.; Lima, R.; Monteiro, S.; Silva, N.A.; Soares-Cunha, C.; Serra, S.C.; et al. Preclinical Assessment of Mesenchymal-Stem-Cell-Based Therapies in Spinocerebellar Ataxia Type 3. Biomedicines 2021, 9, 1754. [Google Scholar] [CrossRef]
- Oliveira Miranda, C.; Marcelo, A.; Silva, T.P.; Barata, J.; Vasconcelos-Ferreira, A.; Pereira, D.; Nóbrega, C.; Duarte, S.; Barros, I.; Alves, J.; et al. Repeated Mesenchymal Stromal Cell Treatment Sustainably Alleviates Machado-Joseph Disease. Mol. Ther. 2018, 26, 2131–2151. [Google Scholar] [CrossRef]
- You, H.J.; Fang, S.B.; Wu, T.T.; Zhang, H.; Feng, Y.K.; Li, X.J.; Yang, H.H.; Li, G.; Li, X.H.; Wu, C.; et al. Mesenchymal stem cell-derived exosomes improve motor function and attenuate neuropathology in a mouse model of Machado-Joseph disease. Stem Cell Res. Ther. 2020, 11, 222. [Google Scholar] [CrossRef]
- Akbar, U.; Ashizawa, T. Ataxia. Neurol. Clin. 2015, 33, 225–248. [Google Scholar] [CrossRef]
- Klockgether, T. The clinical diagnosis of autosomal dominant spinocerebellar ataxias. Cerebellum 2008, 7, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Klockgether, T. Sporadic ataxia with adult onset: Classification and diagnostic criteria. Lancet Neurol. 2010, 9, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.D.; El Haddad, L.; Sethi, R.; Huston, M.L.; Lai, E.; Abdelbarr, M.M.; Mhandire, D.Z.; ElMallah, M.K. Neuro-respiratory pathology in spinocerebellar ataxia. J. Neurol. Sci. 2022, 443, 120493. [Google Scholar] [CrossRef] [PubMed]
- Bodranghien, F.; Bastian, A.; Casali, C.; Hallett, M.; Louis, E.D.; Manto, M.; Mariën, P.; Nowak, D.A.; Schmahmann, J.D.; Serrao, M.; et al. Consensus Paper: Revisiting the Symptoms and Signs of Cerebellar Syndrome. Cerebellum 2016, 15, 369–391. [Google Scholar] [CrossRef]
- Lo, R.Y.; Figueroa, K.P.; Pulst, S.M.; Perlman, S.; Wilmot, G.; Gomez, C.; Schmahmann, J.; Paulson, H.; Shakkottai, V.G.; Ying, S.; et al. Depression and clinical progression in spinocerebellar ataxias. Park. Relat. Disord. 2016, 22, 87–92. [Google Scholar] [CrossRef]
- Campuzano, V.; Montermini, L.; Moltò, M.D.; Pianese, L.; Cossée, M.; Cavalcanti, F.; Monros, E.; Rodius, F.; Duclos, F.; Monticelli, A.; et al. Friedreich’s ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996, 271, 1423–1427. [Google Scholar] [CrossRef]
- Vicente-Acosta, A.; Herranz-Martín, S.; Pazos, M.R.; Galán-Cruz, J.; Amores, M.; Loria, F.; Díaz-Nido, J. Glial cell activation precedes neurodegeneration in the cerebellar cortex of the YG8-800 murine model of Friedreich ataxia. Neurobiol. Dis. 2024, 200, 106631. [Google Scholar] [CrossRef]
- Hoffman-Zacharska, D.; Sulek, A. The New Face of Dynamic Mutation-The CAA [CAG]n CAA CAG Motif as a Mutable Unit in the TBP Gene Causative for Spino-Cerebellar Ataxia Type 17. Int. J. Mol. Sci. 2024, 25, 8190. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Oliveira, J.; Santos, M.; Costa, S.; Silva, L.; Lemos, C.; Barros, J.; Sequeiros, J.; Damásio, J. Spinocerebellar Ataxias: Phenotypic Spectrum of PolyQ versus Non-Repeat Expansion Forms. Cerebellum 2024. [Google Scholar] [CrossRef]
- Tejwani, L.; Lim, J. Pathogenic mechanisms underlying spinocerebellar ataxia type 1. Cell Mol. Life Sci. 2020, 77, 4015–4029. [Google Scholar] [CrossRef]
- Drobotenko, M.I.; Lyasota, O.M.; Hernandez-Caceres, J.L.; Labrada, R.R.; Svidlov, A.A.; Dorohova, A.A.; Baryshev, M.G.; Nechipurenko, Y.D.; Pérez, L.V.; Dzhimak, S.S. Abnormal open states patterns in the ATXN2 DNA sequence depends on the CAG repeats length. Int. J. Biol. Macromol. 2024, 276, 133849. [Google Scholar] [CrossRef] [PubMed]
- Saucier, J.; Al-Qadi, M.; Amor, M.B.; Ishikawa, K.; Chamard-Witkowski, L. Spinocerebellar ataxia type 31: A clinical and radiological literature review. J. Neurol. Sci. 2023, 444, 120527. [Google Scholar] [CrossRef] [PubMed]
- Bastian, A.J. Mechanisms of ataxia. Phys. Ther. 1997, 77, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Del Bondio, A.; Longo, F.; De Ritis, D.; Spirito, E.; Podini, P.; Brais, B.; Bachi, A.; Quattrini, A.; Maltecca, F. Restoring calcium homeostasis in Purkinje cells arrests neurodegeneration and neuroinflammation in the ARSACS mouse model. JCI Insight 2023, 8, e163576. [Google Scholar] [CrossRef]
- Zhu, J.; Qiu, W.; Wei, F.; Zhang, J.; Yuan, Y.; Liu, L.; Cheng, M.; Xiong, H.; Xu, R. Toll-like receptor 4 deficiency in Purkinje neurons drives cerebellar ataxia by impairing the BK channel-mediated after-hyperpolarization and cytosolic calcium homeostasis. Cell Death Dis. 2024, 15, 594. [Google Scholar] [CrossRef]
- Yang, H.; Liu, S.; He, W.T.; Zhao, J.; Jiang, L.L.; Hu, H.Y. Aggregation of Polyglutamine-expanded Ataxin 7 Protein Specifically Sequesters Ubiquitin-specific Protease 22 and Deteriorates Its Deubiquitinating Function in the Spt-Ada-Gcn5-Acetyltransferase (SAGA) Complex. J. Biol. Chem. 2015, 290, 21996–22004. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Liu, Y.J.; Zhang, X.L.; Liu, Y.H.; Jiang, L.L.; Hu, H.Y. PolyQ-expanded ataxin-2 aggregation impairs cellular processing-body homeostasis via sequestering the RNA helicase DDX6. J. Biol. Chem. 2024, 300, 107413. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. The Role of Protein Quantity Control in Polyglutamine Spinocerebellar Ataxias. Cerebellum 2024. [Google Scholar] [CrossRef]
- Manto, M. Toxic agents causing cerebellar ataxias. Handb. Clin. Neurol. 2012, 103, 201–213. [Google Scholar] [CrossRef]
- Swinnen, B.; Robberecht, W.; Van Den Bosch, L. RNA toxicity in non-coding repeat expansion disorders. EMBO J. 2020, 39, e101112. [Google Scholar] [CrossRef]
- Perez, B.A.; Shorrock, H.K.; Banez-Coronel, M.; Zu, T.; Romano, L.E.; Laboissonniere, L.A.; Reid, T.; Ikeda, Y.; Reddy, K.; Gomez, C.M.; et al. CCG•CGG interruptions in high-penetrance SCA8 families increase RAN translation and protein toxicity. EMBO Mol. Med. 2021, 13, e14095. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, H.B.; Jahanbakhsh, F.; Deng, L.; Wu, B.; Ying, M.; Margolis, R.L.; Li, P.P. Bidirectional Transcription at the PPP2R2B Gene Locus in Spinocerebellar Ataxia Type 12. Mov. Disord. 2023, 38, 2230–2240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Muraleetharan, A.; Langiu, M.; Gregory, K.J.; Hellyer, S.D. SCA44- and SCAR13-associated GRM1 mutations affect metabotropic glutamate receptor 1 function through distinct mechanisms. Br. J. Pharmacol. 2024, 181, 4514–4530. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Hutchins, R. Embryonic stem cells. Stem Cells Dev. 2007, 16, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.; Rao, M.S. Human embryonic stem cells. Neurosurg. Clin. N. Am. 2007, 18, 1–14. [Google Scholar] [CrossRef]
- Levenberg, S.; Zoldan, J.; Basevitch, Y.; Langer, R. Endothelial potential of human embryonic stem cells. Blood 2007, 110, 806–814. [Google Scholar] [CrossRef]
- Chen, G.; Yin, S.; Zeng, H.; Li, H.; Wan, X. Regulation of Embryonic Stem Cell Self-Renewal. Life 2022, 12, 1151. [Google Scholar] [CrossRef]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis and embryonic stem cells. Chem. Biol. Interact. 2022, 352, 109783. [Google Scholar] [CrossRef]
- Kristiansen, C.K.; Chen, A.; Høyland, L.E.; Ziegler, M.; Sullivan, G.J.; Bindoff, L.A.; Liang, K.X. Comparing the mitochondrial signatures in ESCs and iPSCs and their neural derivations. Cell Cycle 2022, 21, 2206–2221. [Google Scholar] [CrossRef]
- Sharkis, S.J.; Jones, R.J.; Civin, C.; Jang, Y.Y. Pluripotent stem cell-based cancer therapy: Promise and challenges. Sci. Transl. Med. 2012, 4, 127ps9. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Chaudhari, P.; Jang, Y.Y. Applications of patient-specific induced pluripotent stem cells; focused on disease modeling, drug screening and therapeutic potentials for liver disease. Int. J. Biol. Sci. 2010, 6, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Romeo, F.; Costanzo, F.; Agostini, M. Embryonic stem cells and inducible pluripotent stem cells: Two faces of the same coin? Aging (Albany NY) 2012, 4, 878–886. [Google Scholar] [CrossRef]
- Doss, M.X.; Sachinidis, A. Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef]
- Chang, C.Y.; Ting, H.C.; Liu, C.A.; Su, H.L.; Chiou, T.W.; Lin, S.Z.; Harn, H.J.; Ho, T.J. Induced Pluripotent Stem Cell (iPSC)-Based Neurodegenerative Disease Models for Phenotype Recapitulation and Drug Screening. Molecules 2020, 25, 2000. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Santostefano, K.E.; Yachnis, A.T.; Terada, N. A pathologist’s perspective on induced pluripotent stem cells. Lab. Investig. 2017, 97, 1126–1132. [Google Scholar] [CrossRef]
- Almalki, S.G.; Agrawal, D.K. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 2016, 92, 41–51. [Google Scholar] [CrossRef]
- Krampera, M.; Le Blanc, K. Mesenchymal stromal cells: Putative microenvironmental modulators become cell therapy. Cell Stem Cell 2021, 28, 1708–1725. [Google Scholar] [CrossRef]
- Gartner, S.; Kaplan, H.S. Long-term culture of human bone marrow cells. Proc. Natl. Acad. Sci. USA 1980, 77, 4756–4759. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transpl. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells 2010, 28, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor. Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef]
- Fričová, D.; Korchak, J.A.; Zubair, A.C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson’s disease. NPJ Regen. Med. 2020, 5, 20. [Google Scholar] [CrossRef]
- Bindhya, S.; Sidhanth, C.; Shabna, A.; Krishnapriya, S.; Garg, M.; Ganesan, T.S. Induced pluripotent stem cells: A new strategy to model human cancer. Int. J. Biochem. Cell Biol. 2019, 107, 62–68. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J. From embryonic stem cells to induced pluripotent stem cells-Ready for clinical therapy? Clin. Transpl. 2019, 33, e13573. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Y.; Su, X.; He, J.; Bai, W.; He, X. MSCs-Derived Exosomes and Neuroinflammation, Neurogenesis and Therapy of Traumatic Brain Injury. Front. Cell. Neurosci. 2017, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, R.; Li, H.; Li, J.; Han, Q.; Zhao, R.C. Mesenchymal stem cells and immune disorders: From basic science to clinical transition. Front. Med. 2019, 13, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yin, Z.; Chen, F.; Lei, P. Mesenchymal stem cell-derived exosome: A promising alternative in the therapy of Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.M.; Jorgensen, C.; Khoury, M. Editorial: The Role of Metabolism in MSC-Mediated Immunomodulation. Front. Immunol. 2021, 12, 751865. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006, 24, 74–85. [Google Scholar] [CrossRef]
- Mehdipour, F.; Razmkhah, M.; Rezaeifard, S.; Bagheri, M.; Talei, A.R.; Khalatbari, B.; Ghaderi, A. Mesenchymal stem cells induced anti-inflammatory features in B cells from breast tumor draining lymph nodes. Cell Biol. Int. 2018, 42, 1658–1669. [Google Scholar] [CrossRef]
- Gronthos, S.; Zannettino, A.C.; Hay, S.J.; Shi, S.; Graves, S.E.; Kortesidis, A.; Simmons, P.J. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J. Cell Sci. 2003, 116, 1827–1835. [Google Scholar] [CrossRef]
- Yao, P.; Zhou, L.; Zhu, L.; Zhou, B.; Yu, Q. Mesenchymal Stem Cells: A Potential Therapeutic Strategy for Neurodegenerative Diseases. Eur. Neurol. 2020, 83, 235–241. [Google Scholar] [CrossRef]
- de Vernejoul, M.C.; Pointillart, A.; Bourdeau, A.; Morieux, C.; Modrowski, D.; Miravet, L.; Caulin, F. Effect of calcitonin administration on young pig trabecular bone remodeling. Bone 1990, 11, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, M.H.; Schulz, J.B.; Giunti, P. Co-enzyme Q10 and idebenone use in Friedreich’s ataxia. J. Neurochem. 2013, 126 (Suppl. S1), 125–141. [Google Scholar] [CrossRef] [PubMed]
- Stephen, C.D.; Brizzi, K.T.; Bouffard, M.A.; Gomery, P.; Sullivan, S.L.; Mello, J.; MacLean, J.; Schmahmann, J.D. The Comprehensive Management of Cerebellar Ataxia in Adults. Curr. Treat. Options Neurol. 2019, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Gazulla, J.; Berciano, J. Potential Clinical Benefit of Very Long Chain Fatty Acid Supplementation in Spinocerebellar Ataxia Type 34. Cerebellum 2024, 23, 2193–2196. [Google Scholar] [CrossRef]
- Hotz, I.; Mildner, S.; Stampfer-Kountchev, M.; Slamik, B.; Blättner, C.; Türtscher, E.; Kübler, F.; Höfer, C.; Panzl, J.; Rücker, M.; et al. Robot-assisted gait training in patients with various neurological diseases: A mixed methods feasibility study. PLoS ONE 2024, 19, e0307434. [Google Scholar] [CrossRef]
- Pilotto, F.; Chellapandi, D.M.; Puccio, H. Omaveloxolone: A groundbreaking milestone as the first FDA-approved drug for Friedreich ataxia. Trends Mol. Med. 2024, 30, 117–125. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Huang, Y.; Ma, Z.; Gao, W.; Zhang, T.; Deng, J.; Cheng, X.; Liu, Y.; Wang, B.; et al. Trehalose prevents the formation of aggregates of mutant ataxin-3 and reduces soluble ataxin-3 protein levels in an SCA3 cell model. Neuroscience 2024, 555, 76–82. [Google Scholar] [CrossRef]
- Hernández, A.E.; García, E. Mesenchymal Stem Cell Therapy for Alzheimer’s Disease. Stem Cells Int. 2021, 2021, 7834421. [Google Scholar] [CrossRef]
- Alipour, M.; Nabavi, S.M.; Arab, L.; Vosough, M.; Pakdaman, H.; Ehsani, E.; Shahpasand, K. Stem cell therapy in Alzheimer’s disease: Possible benefits and limiting drawbacks. Mol. Biol. Rep. 2019, 46, 1425–1446. [Google Scholar] [CrossRef]
- Bhatt, A.; Bhardwaj, H.; Srivastava, P. Mesenchymal stem cell therapy for Alzheimer’s disease: A novel therapeutic approach for neurodegenerative diseases. Neuroscience 2024, 555, 52–68. [Google Scholar] [CrossRef]
- Kaniowska, D.; Wenk, K.; Rademacher, P.; Weiss, R.; Fabian, C.; Schulz, I.; Guthardt, M.; Lange, F.; Greiser, S.; Schmidt, M.; et al. Extracellular Vesicles of Mesenchymal Stromal Cells Can be Taken Up by Microglial Cells and Partially Prevent the Stimulation Induced by β-amyloid. Stem Cell Rev. Rep. 2022, 18, 1113–1126. [Google Scholar] [CrossRef]
- Santamaria, G.; Brandi, E.; Vitola, P.; Grandi, F.; Ferrara, G.; Pischiutta, F.; Vegliante, G.; Zanier, E.R.; Re, F.; Uccelli, A.; et al. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer’s mice. Cell Death Differ. 2021, 28, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Wu, J.; Mou, F.F.; Xie, W.H.; Wang, F.B.; Wang, Q.L.; Fang, J.; Xu, Y.W.; Dong, Y.R.; Liu, J.R.; et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. Faseb J. 2018, 32, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Oyebode, O.D.; Tulay, P. Mesenchymal Stem Cells Applications in Alzheimer’s Disease. Glob. Med. Genet. 2023, 10, 382–387. [Google Scholar] [CrossRef]
- Chang, J.; Feng, Z.; Li, Y.; Lv, H.; Liu, S.; Luo, Y.; Hao, N.; Zhao, L.; Liu, J. Mesenchymal stem cell-derived extracellular vesicles: A novel promising neuroprotective agent for Alzheimer’s disease. Biochem. Pharmacol. 2024, 222, 116064. [Google Scholar] [CrossRef]
- Ye, Y.; Gao, M.; Shi, W.; Gao, Y.; Li, Y.; Yang, W.; Zheng, X.; Lu, X. The immunomodulatory effects of mesenchymal stem cell-derived extracellular vesicles in Alzheimer’s disease. Front. Immunol. 2023, 14, 1325530. [Google Scholar] [CrossRef]
- Qin, C.; Li, Y.; Wang, K. Functional Mechanism of Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Animal Models with Alzheimer’s Disease: Inhibition of Neuroinflammation. J. Inflamm. Res. 2021, 14, 4761–4775. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Z.; Wei, L.; Yang, H.; Yang, S.; Zhu, Z.; Wang, P.; Zhao, C.; Bi, J. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem Cell Res. Ther. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ma, T.; Gong, K.; Ao, Q.; Zhang, X.; Gong, Y. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regen. Res. 2014, 9, 798–805. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.H.; Kim, J.H.; Lee, D.; Jeon, H.B.; Kwon, S.J.; Kim, S.M.; Yoo, Y.J.; Lee, E.H.; Choi, S.J.; et al. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell Death Differ. 2012, 19, 680–691. [Google Scholar] [CrossRef]
- Sha, S.; Shen, X.; Cao, Y.; Qu, L. Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer’s disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/β-catenin pathway. Aging (Albany NY) 2021, 13, 15285–15306. [Google Scholar] [CrossRef] [PubMed]
- Nabil, M.; Kassem, D.H.; Ali, A.A.; El-Mesallamy, H.O. Adipose tissue-derived mesenchymal stem cells ameliorate cognitive impairment in Alzheimer’s disease rat model: Emerging role of SIRT1. Biofactors 2023, 49, 1121–1142. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Lee, D.; Choi, W.K.; Choi, S.J.; Oh, W.; Kim, D.H. Galectin-3 Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Reduces Aberrant Tau Phosphorylation in an Alzheimer Disease Model. Stem Cells Int. 2020, 2020, 8878412. [Google Scholar] [CrossRef]
- Xiao, Z.; Lei, T.; Liu, Y.; Yang, Y.; Bi, W.; Du, H. The potential therapy with dental tissue-derived mesenchymal stem cells in Parkinson’s disease. Stem Cell Res. Ther. 2021, 12, 5. [Google Scholar] [CrossRef]
- Li, B.; Dettmer, U. Interactions of alpha-synuclein with membranes in Parkinson’s disease: Mechanisms and therapeutic strategies. Neurobiol. Dis. 2024, 201, 106646. [Google Scholar] [CrossRef]
- Heris, R.M.; Shirvaliloo, M.; Abbaspour-Aghdam, S.; Hazrati, A.; Shariati, A.; Youshanlouei, H.R.; Niaragh, F.J.; Valizadeh, H.; Ahmadi, M. The potential use of mesenchymal stem cells and their exosomes in Parkinson’s disease treatment. Stem Cell Res. Ther. 2022, 13, 371. [Google Scholar] [CrossRef]
- Liu, S.F.; Li, L.Y.; Zhuang, J.L.; Li, M.M.; Ye, L.C.; Chen, X.R.; Lin, S.; Chen, C.N. Update on the application of mesenchymal stem cell-derived exosomes in the treatment of Parkinson’s disease: A systematic review. Front. Neurol. 2022, 13, 950715. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.H.; Salem, A.M.; Atta, H.M.; Eskandar, E.F.; Farrag, A.R.; Ghazy, M.A.; Salem, N.A.; Aglan, H.A. Updates in the pathophysiological mechanisms of Parkinson’s disease: Emerging role of bone marrow mesenchymal stem cells. World J. Stem Cells 2016, 8, 106–117. [Google Scholar] [CrossRef]
- Mostafavi, H.; Ghassemifard, L.; Rostami, A.; Alipour, M.; Nadri, S. Trabecular meshwork mesenchymal stem cell transplantation improve motor symptoms of parkinsonian rat model. Biologicals 2019, 61, 61–67. [Google Scholar] [CrossRef]
- Park, H.J.; Shin, J.Y.; Kim, H.N.; Oh, S.H.; Lee, P.H. Neuroprotective effects of mesenchymal stem cells through autophagy modulation in a parkinsonian model. Neurobiol. Aging 2014, 35, 1920–1928. [Google Scholar] [CrossRef]
- Chen, H.X.; Liang, F.C.; Gu, P.; Xu, B.L.; Xu, H.J.; Wang, W.T.; Hou, J.Y.; Xie, D.X.; Chai, X.Q.; An, S.J. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Kim, D.Y.; Lee, J.; Shin, Y.J.; Kim, Y.S.; Lee, P.H. Priming mesenchymal stem cells with α-synuclein enhances neuroprotective properties through induction of autophagy in Parkinsonian models. Stem Cell Res. Ther. 2022, 13, 483. [Google Scholar] [CrossRef]
- Blandini, F.; Cova, L.; Armentero, M.T.; Zennaro, E.; Levandis, G.; Bossolasco, P.; Calzarossa, C.; Mellone, M.; Giuseppe, B.; Deliliers, G.L.; et al. Transplantation of undifferentiated human mesenchymal stem cells protects against 6-hydroxydopamine neurotoxicity in the rat. Cell Transpl. 2010, 19, 203–217. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Panchalingam, K.M.; Rodrigues, A.J.; Mendes-Pinheiro, B.; Anjo, S.; Manadas, B.; Behie, L.A.; Sousa, N.; Salgado, A.J. Impact of the Secretome of Human Mesenchymal Stem Cells on Brain Structure and Animal Behavior in a Rat Model of Parkinson’s Disease. Stem Cells Transl. Med. 2017, 6, 634–646. [Google Scholar] [CrossRef]
- d’Angelo, M.; Cimini, A.; Castelli, V. Insights into the Effects of Mesenchymal Stem Cell-Derived Secretome in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 5241. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Chen, H.; Zhu, M. VEGF-Expressing Mesenchymal Stem Cell Therapy for Safe and Effective Treatment of Pain in Parkinson’s Disease. Cell Transpl. 2023, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Goetz, C.G.; Kordower, J.H.; Stoessl, A.J.; Sossi, V.; Brin, M.F.; Shannon, K.M.; Nauert, G.M.; Perl, D.P.; Godbold, J.; et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003, 54, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Rahimi Darehbagh, R.; Seyedoshohadaei, S.A.; Ramezani, R.; Rezaei, N. Stem cell therapies for neurological disorders: Current progress, challenges, and future perspectives. Eur. J. Med. Res. 2024, 29, 386. [Google Scholar] [CrossRef]
- Madadi, S.; Shiri, E.; Pasbakhsh, P.; Tahmasebi, F.; Kazemzadeh, S.; Zibara, K.; Kashani, I.R. Combination Therapy of Mesenchymal Stem Cell Transplantation and Astrocyte Ablation Improve Remyelination in a Cuprizone-Induced Demyelination Mouse Model. Mol. Neurobiol. 2022, 59, 7278–7292. [Google Scholar] [CrossRef]
- Fujiwara, M.; Raheja, R.; Garo, L.P.; Ajay, A.K.; Kadowaki-Saga, R.; Karandikar, S.H.; Gabriely, G.; Krishnan, R.; Beynon, V.; Paul, A.; et al. microRNA-92a promotes CNS autoimmunity by modulating the regulatory and inflammatory T cell balance. J. Clin. Investig. 2022, 132, e155693. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, J.; Wang, H.; Chen, C.; Han, M.; Gao, L.; Tang, C.; Sun, P.; Zhao, X.; Guo, F.; et al. Trojan Horse Nanocapsule Enabled In Situ Modulation of the Phenotypic Conversion of Th17 Cells to Treg Cells for the Treatment of Multiple Sclerosis in Mice. Adv. Mater. 2023, 35, e2210262. [Google Scholar] [CrossRef] [PubMed]
- Allegretta, C.; D’Amico, E.; Manuti, V.; Avolio, C.; Conese, M. Mesenchymal Stem Cell-Derived Extracellular Vesicles and Their Therapeutic Use in Central Nervous System Demyelinating Disorders. Int. J. Mol. Sci. 2022, 23, 3829. [Google Scholar] [CrossRef]
- Hu, H.; Li, H.; Li, R.; Liu, P.; Liu, H. Re-establishing immune tolerance in multiple sclerosis: Focusing on novel mechanisms of mesenchymal stem cell regulation of Th17/Treg balance. J. Transl. Med. 2024, 22, 663. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Lahouty, M.; Charkhian, H.; Ghafour, A.A.; Moazzendizaji, S.; Rezaei, J.; Alipour, S.; Irannejad, V.S.; Ansari, M.H.K. Human umbilical cord mesenchymal stem cell-derived exosomes alleviate the severity of experimental autoimmune encephalomyelitis and enhance lag-3 expression on foxp3 + CD4 + T cells. Mol. Biol. Rep. 2024, 51, 522. [Google Scholar] [CrossRef] [PubMed]
- Zargarani, S.; Tavaf, M.J.; Soltanmohammadi, A.; Yazdanpanah, E.; Baharlou, R.; Yousefi, B.; Sadighimoghaddam, B.; Esmaeili, S.A.; Haghmorad, D. Adipose-derived mesenchymal stem cells ameliorates experimental autoimmune encephalomyelitis via modulation of Th1/Th17 and expansion of Th2/Treg responses. Cell Biol. Int. 2024, 48, 1124–1137. [Google Scholar] [CrossRef]
- Sadeghnejad, A.; Pazoki, A.; Yazdanpanah, E.; Esmaeili, S.A.; Yousefi, B.; Sadighi-Moghaddam, B.; Baharlou, R.; Haghmorad, D. Exploring the role of mesenchymal stem cells in modulating immune responses via Treg and Th2 cell activation: Insights from mouse model of multiple sclerosis. Apmis 2024, 132, 888–899. [Google Scholar] [CrossRef]
- Jafarinia, M.; Alsahebfosoul, F.; Salehi, H.; Eskandari, N.; Azimzadeh, M.; Mahmoodi, M.; Asgary, S.; Ganjalikhani Hakemi, M. Therapeutic effects of extracellular vesicles from human adipose-derived mesenchymal stem cells on chronic experimental autoimmune encephalomyelitis. J. Cell. Physiol. 2020, 235, 8779–8790. [Google Scholar] [CrossRef] [PubMed]
- Laso-García, F.; Ramos-Cejudo, J.; Carrillo-Salinas, F.J.; Otero-Ortega, L.; Feliú, A.; Gómez-de Frutos, M.; Mecha, M.; Díez-Tejedor, E.; Guaza, C.; Gutiérrez-Fernández, M. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS ONE 2018, 13, e0202590. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; He, X.; Yang, X.; Shan, F.; Feng, J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int. Immunopharmacol. 2019, 67, 268–280. [Google Scholar] [CrossRef]
- Alavi, O.; Alizadeh, A.; Dehghani, F.; Alipour, H.; Tanideh, N. Anti-inflammatory Effects of Umbilical Cord Mesenchymal Stem Cell and Autologous Conditioned Serum on Oligodendrocyte, Astrocyte, and Microglial Specific Gene in Cuprizone Animal Model. Curr. Stem Cell Res. Ther. 2024, 19, 71–82. [Google Scholar] [CrossRef]
- Mojaverrostami, S.; Khadivi, F.; Zarini, D.; Mohammadi, A. Combination effects of mesenchymal stem cells transplantation and anodal transcranial direct current stimulation on a cuprizone-induced mouse model of multiple sclerosis. J. Mol. Histol. 2022, 53, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Mieda, T.; Suto, N.; Iizuka, A.; Matsuura, S.; Iizuka, H.; Takagishi, K.; Nakamura, K.; Hirai, H. Mesenchymal stem cells attenuate peripheral neuronal degeneration in spinocerebellar ataxia type 1 knockin mice. J. Neurosci. Res. 2016, 94, 246–252. [Google Scholar] [CrossRef]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, D.; Danzi, M.C.; Renaud, M.; Houlden, H.; Synofzik, M.; Zuchner, S.; Brais, B. Spinocerebellar ataxia 27B: A novel, frequent and potentially treatable ataxia. Clin. Transl. Med. 2024, 14, e1504. [Google Scholar] [CrossRef]
- Marsili, L.; Sharma, J.; Outeiro, T.F.; Colosimo, C. Stem Cell Therapies in Movement Disorders: Lessons from Clinical Trials. Biomedicines 2023, 11, 505. [Google Scholar] [CrossRef]
- Zhang, X.; Kuang, Q.; Xu, J.; Lin, Q.; Chi, H.; Yu, D. MSC-Based Cell Therapy in Neurological Diseases: A Concise Review of the Literature in Pre-Clinical and Clinical Research. Biomolecules 2024, 14, 538. [Google Scholar] [CrossRef]
- Kim, H.J.; Seo, S.W.; Chang, J.W.; Lee, J.I.; Kim, C.H.; Chin, J.; Choi, S.J.; Kwon, H.; Yun, H.J.; Lee, J.M.; et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase 1 clinical trial. Alzheimer’s Dement. 2015, 1, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Karussis, D.; Karageorgiou, C.; Vaknin-Dembinsky, A.; Gowda-Kurkalli, B.; Gomori, J.M.; Kassis, I.; Bulte, J.W.; Petrou, P.; Ben-Hur, T.; Abramsky, O.; et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010, 67, 1187–1194. [Google Scholar] [CrossRef]
- Appelt, P.A.; Comella, K.; de Souza, L.; Luvizutto, G.J. Effect of stem cell treatment on functional recovery of spinocerebellar ataxia: Systematic review and meta-analysis. Cerebellum Ataxias 2021, 8, 8. [Google Scholar] [CrossRef]
- Tsai, Y.A.; Liu, R.S.; Lirng, J.F.; Yang, B.H.; Chang, C.H.; Wang, Y.C.; Wu, Y.S.; Ho, J.H.; Lee, O.K.; Soong, B.W. Treatment of Spinocerebellar Ataxia With Mesenchymal Stem Cells: A Phase I/IIa Clinical Study. Cell Transpl. 2017, 26, 503–512. [Google Scholar] [CrossRef]
- Schmitz-Hübsch, T.; du Montcel, S.T.; Baliko, L.; Berciano, J.; Boesch, S.; Depondt, C.; Giunti, P.; Globas, C.; Infante, J.; Kang, J.S.; et al. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 2006, 66, 1717–1720. [Google Scholar] [CrossRef] [PubMed]
- Dongmei, H.; Jing, L.; Mei, X.; Ling, Z.; Hongmin, Y.; Zhidong, W.; Li, D.; Zikuan, G.; Hengxiang, W. Clinical analysis of the treatment of spinocerebellar ataxia and multiple system atrophy-cerebellar type with umbilical cord mesenchymal stromal cells. Cytotherapy 2011, 13, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.L.; Liu, Z.; Lu, Z.J.; Guan, D.N.; Wang, C.; Chen, Z.B.; Zhang, J.; Zhang, W.Y.; Wu, J.Y.; Xu, Y. Safety and efficacy of umbilical cord mesenchymal stem cell therapy in hereditary spinocerebellar ataxia. Curr. Neurovasc Res. 2013, 10, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Huda, F.; Fan, Y.; Suzuki, M.; Konno, A.; Matsuzaki, Y.; Takahashi, N.; Chan, J.K.; Hirai, H. Fusion of Human Fetal Mesenchymal Stem Cells with "Degenerating" Cerebellar Neurons in Spinocerebellar Ataxia Type 1 Model Mice. PLoS ONE 2016, 11, e0164202. [Google Scholar] [CrossRef]
- Klockgether, T.; Lüdtke, R.; Kramer, B.; Abele, M.; Bürk, K.; Schöls, L.; Riess, O.; Laccone, F.; Boesch, S.; Lopes-Cendes, I.; et al. The natural history of degenerative ataxia: A retrospective study in 466 patients. Brain 1998, 121 Pt 4, 589–600. [Google Scholar] [CrossRef]
- Conaty, P.; Sherman, L.S.; Naaldijk, Y.; Ulrich, H.; Stolzing, A.; Rameshwar, P. Methods of Mesenchymal Stem Cell Homing to the Blood-Brain Barrier. Methods Mol. Biol. 2018, 1842, 81–91. [Google Scholar] [CrossRef]
- Sang, X.; Tang, L.; Zhao, L.; Xu, N.; Liu, F.; Shen, Y.; Wei, W.; Cheng, Y.; Huang, W.; Liu, Y.; et al. Umbilical cord mesenchymal stem cell-derived exosomes promote axon regeneration during optic nerve injury through microRNA-dependent mTORC1 signalling. Clin. Transl. Med. 2023, 13, e1319. [Google Scholar] [CrossRef]
- Donega, V.; Nijboer, C.H.; van Tilborg, G.; Dijkhuizen, R.M.; Kavelaars, A.; Heijnen, C.J. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp. Neurol. 2014, 261, 53–64. [Google Scholar] [CrossRef]
- Jung, J.W.; Kwon, M.; Choi, J.C.; Shin, J.W.; Park, I.W.; Choi, B.W.; Kim, J.Y. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med. J. 2013, 54, 1293–1296. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Sato, Y.; Kitase, Y.; Suzuki, T.; Kondo, T.; Mikrogeorgiou, A.; Horinouchi, A.; Maruyama, S.; Shimoyama, Y.; Tsuji, M.; et al. Intravenous Administration of Bone Marrow-Derived Mesenchymal Stem Cell, but not Adipose Tissue-Derived Stem Cell, Ameliorated the Neonatal Hypoxic-Ischemic Brain Injury by Changing Cerebral Inflammatory State in Rat. Front. Neurol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Rodriguez-Pallares, J.; Garcia-Garrote, M.; Parga, J.A.; Labandeira-Garcia, J.L. Dose-dependent effect of mesenchymal stromal cells co-grafted with dopaminergic neurons in a Parkinson’s disease rat model. J. Cell Mol. Med. 2021, 25, 9884–9889. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Shi, B.; Chang, H.; Su, X.; Zhang, L.; Bi, C.; Shuai, Y.; Du, X.; Deng, Z.; Jin, Y. Heparin improves BMSC cell therapy: Anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics 2017, 7, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Ghareghani, M.; Arneaud, A.; Rivest, S. The evolution of mesenchymal stem cell-derived neural progenitor therapy for Multiple Sclerosis: From concept to clinic. Front. Cell. Neurosci. 2024, 18, 1428652. [Google Scholar] [CrossRef] [PubMed]
- Tee, C.A.; Roxby, D.N.; Othman, R.; Denslin, V.; Bhat, K.S.; Yang, Z.; Han, J.; Tucker-Kellogg, L.; Boyer, L.A. Metabolic modulation to improve MSC expansion and therapeutic potential for articular cartilage repair. Stem Cell Res. Ther. 2024, 15, 308. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Velez, M.; Enam, S.F.; Mehta, N.; Lyon, J.G.; LaPlaca, M.C.; Bellamkonda, R.V. Immuno-suppressive hydrogels enhance allogeneic MSC survival after transplantation in the injured brain. Biomaterials 2021, 266, 120419. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef]
- Fang, S.B.; Zhang, H.Y.; Wang, C.; He, B.X.; Liu, X.Q.; Meng, X.C.; Peng, Y.Q.; Xu, Z.B.; Fan, X.L.; Wu, Z.J.; et al. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. J. Extracell. Vesicles 2020, 9, 1723260. [Google Scholar] [CrossRef]
| ClinicalTrials.gov ID | NCT03378414 | NCT06397274 |
|---|---|---|
| Study Start | 31 December 2024 | 1 June 2025 |
| Phase | Phase 2 | Phase 2 |
| Details | Randomized, open label, and parallel controlled experiment; Follow-up visit by doctors 1, 2, 3, 6, and 12 months after treatment, and efficacy evaluation employed. | Randomized, double-blind, placebo-controlled, single-center study. |
| Enrollment | 45 | 20 |
| Ages Eligible for Study | 16 years to 60 years (child, adult) | 20 years to 70 years (adult, older adult) |
| Inclusion Criteria | Spinocerebellar ataxias (SCA); SARA 1 scores of 2–5; Can complete 8 m walking test; No stem cell treatment in 6 months; Signed the consent form based on the experiment process and statement. | Genotypically confirmed SCA3; SARA 1 scores of 5–15; Female subjects of child-bearing potential and are capable of conception must be post-menopausal; Male subjects must use a medically accepted form of contraception during the study period; Signed informed consent. |
| MSC source | Human umbilical cord mesenchymal stem cells. | Stemchymal® (allogeneic adipose-derived mesenchymal stem cells). |
| Participant Group | Intravenous infusion group: 2 × 107 cells (30 mL); Intrathecal injection group: 2 × 107 cells (1 mL); Control groups. | Experimental: Stemchymal® through intravenous infusion; Placebo comparator: placebo through intravenous infusion. |
| Outcome Measures | Primary: SARA 1 score; Secondary: MRI plain scan of brain, INAS 2 score, cerebrospinal fluid routine. | SARA 1 scores from baseline (week 0) to 6 months (week 24). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.B.; Park, S.M.; Jung, U.J.; Kim, S.R. The Potential of Mesenchymal Stem Cells in Treating Spinocerebellar Ataxia: Advances and Future Directions. Biomedicines 2024, 12, 2507. https://doi.org/10.3390/biomedicines12112507
Lee GB, Park SM, Jung UJ, Kim SR. The Potential of Mesenchymal Stem Cells in Treating Spinocerebellar Ataxia: Advances and Future Directions. Biomedicines. 2024; 12(11):2507. https://doi.org/10.3390/biomedicines12112507
Chicago/Turabian StyleLee, Gi Beom, Se Min Park, Un Ju Jung, and Sang Ryong Kim. 2024. "The Potential of Mesenchymal Stem Cells in Treating Spinocerebellar Ataxia: Advances and Future Directions" Biomedicines 12, no. 11: 2507. https://doi.org/10.3390/biomedicines12112507
APA StyleLee, G. B., Park, S. M., Jung, U. J., & Kim, S. R. (2024). The Potential of Mesenchymal Stem Cells in Treating Spinocerebellar Ataxia: Advances and Future Directions. Biomedicines, 12(11), 2507. https://doi.org/10.3390/biomedicines12112507









