The Role of Probiotics in Modulating Myocardial Infarction and Depression-like Symptoms: A Study on Sex-Specific Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design
2.3. Probiotics Regimen
2.4. Surgical Procedures
2.5. Infarct Size Measurement
2.6. Ex Vivo Intestinal Resistance
2.7. Plasma Levels of Estradiol and CRP
2.8. Behavioural Measures
2.9. Social Interaction Test
2.10. Forced Swim Test
2.11. Statistical Analysis
3. Results
3.1. Myocardial Infarct Size
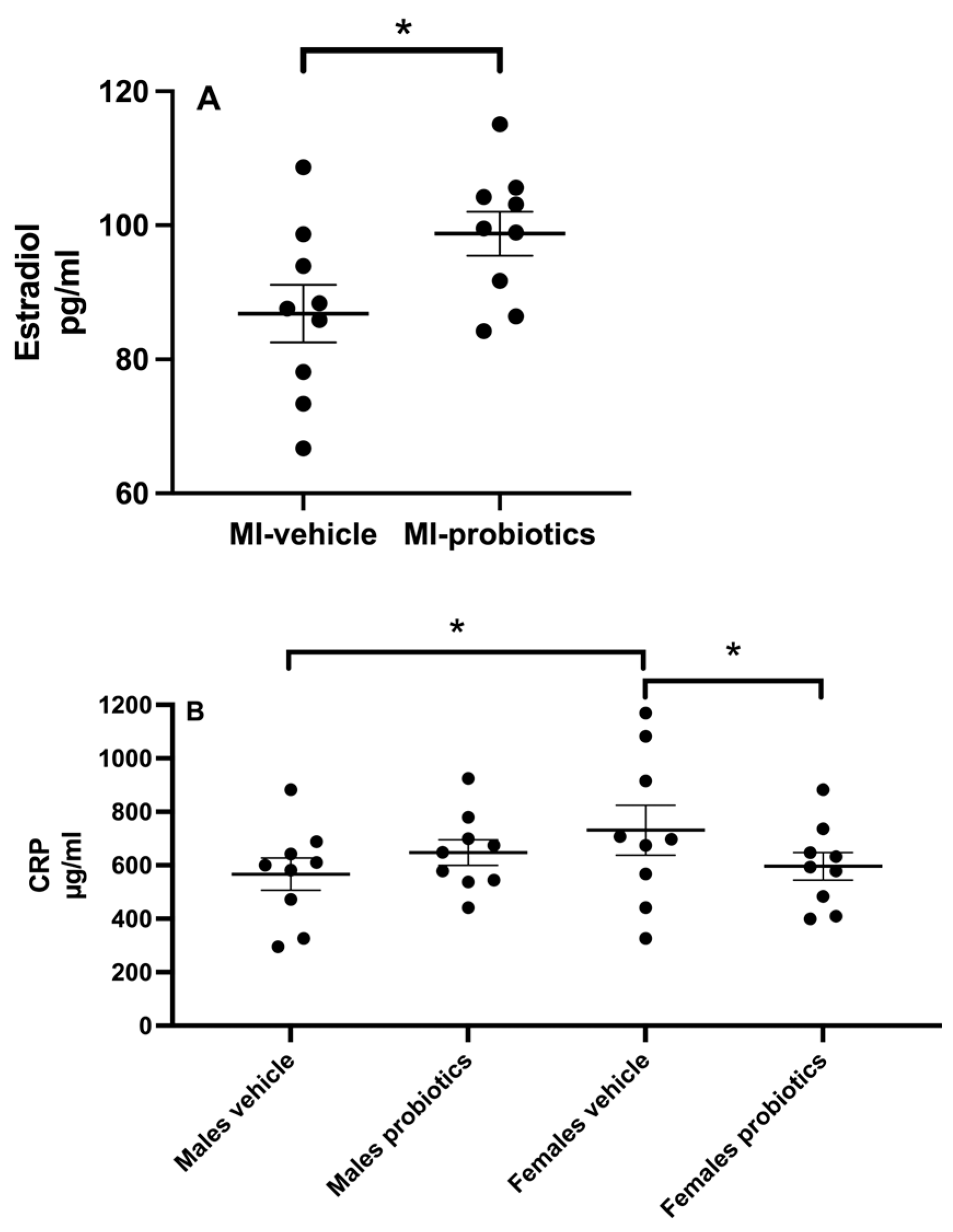
3.2. Estradiol
3.3. CRP
3.4. Intestinal Resistance
3.5. Scar Extent
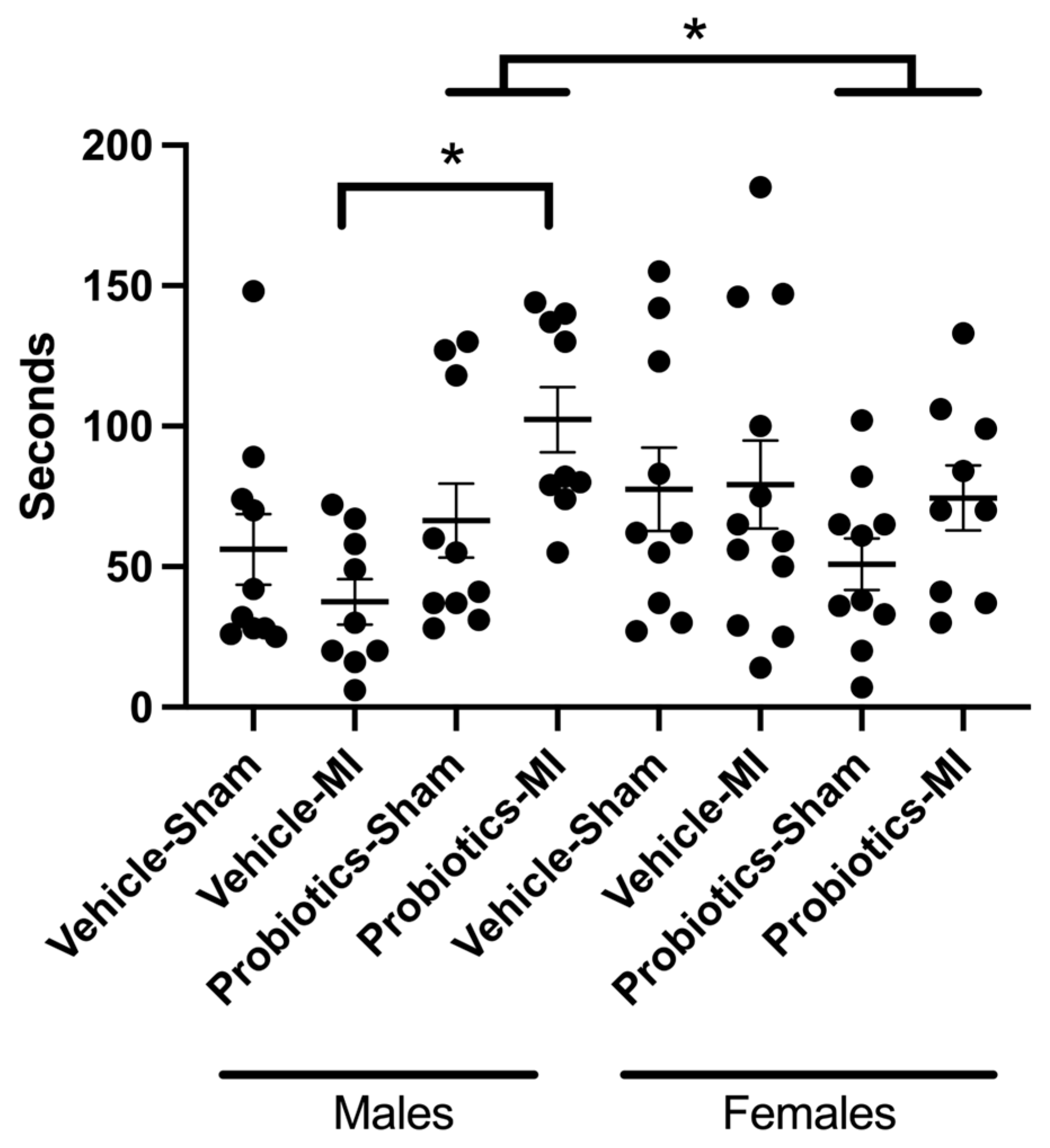
3.6. Social Interactions
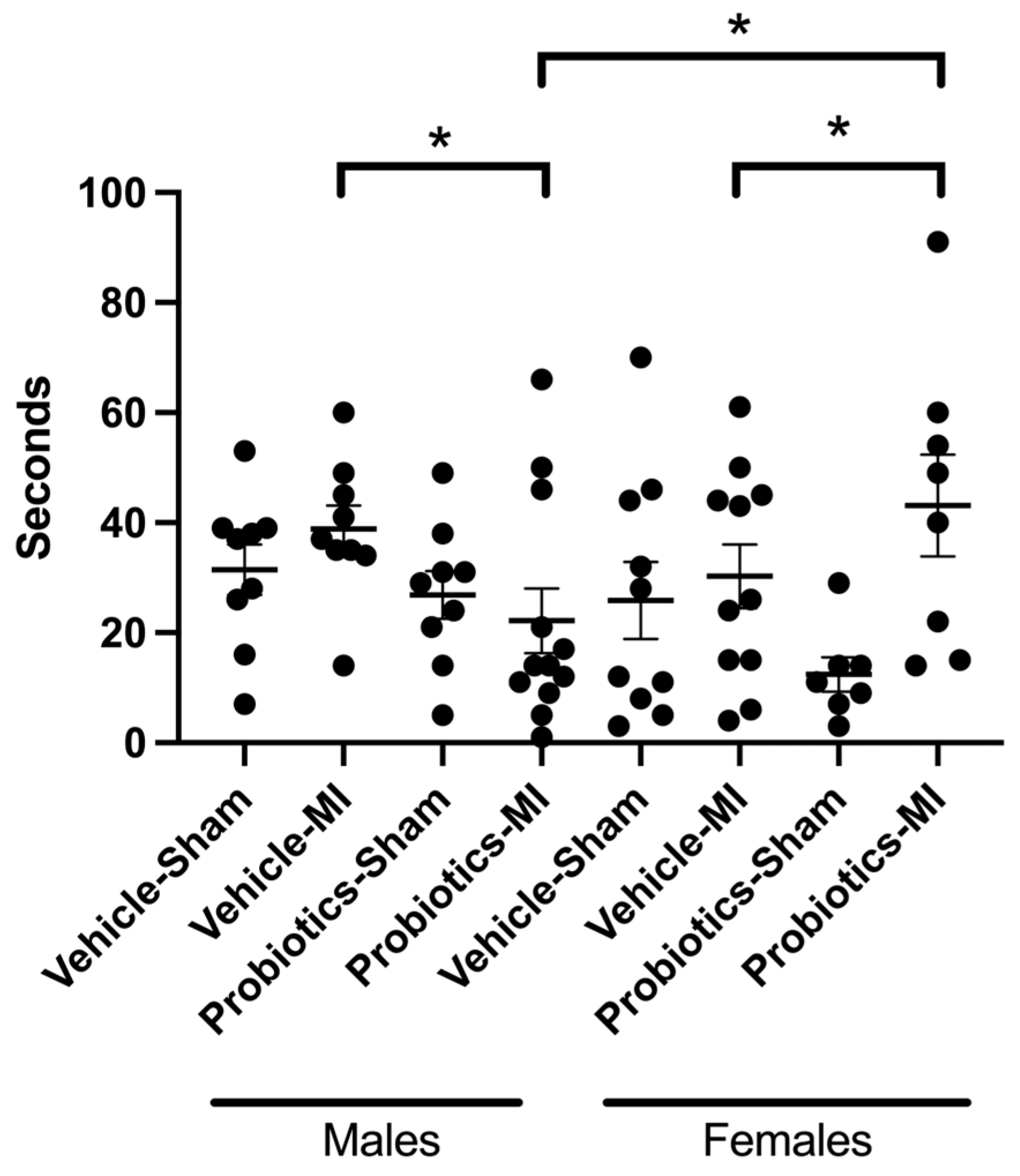
3.7. Forced Swim
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gul, S.; Durante-Mangoni, E. Unraveling the Puzzle: Health Benefits of Probiotics—A Comprehensive Review. J. Clin. Med. 2024, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.A.; Roy, T.; D’Adamo, C.R.; Wieland, L.S. Probiotics and gastrointestinal conditions: An overview of evidence from the Cochrane Collaboration. Nutrition 2018, 45, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Haller, D.; Antoine, J.-M.; Bengmark, S.; Enck, P.; Rijkers, G.T.; Lenoir-Wijnkoop, I. Guidance for Substantiating the Evidence for Beneficial Effects of Probiotics: Probiotics in Chronic Inflammatory Bowel Disease and the Functional Disorder Irritable Bowel Syndrome1–3. J. Nutr. 2010, 140, 690S–697S. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, G. Gut-Brain Axis and Mood Disorder. Front. Psychiatry 2018, 9, 223. [Google Scholar] [CrossRef]
- Ross, S.M. Gut-Brain Axis and Stress Regulation: Effects of Probiotics on Neurocognition. Holist. Nurs. Pract. 2019, 33, 312–315. [Google Scholar] [CrossRef]
- Mörkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef]
- Rajanala, K.; Kumar, N.; Chamallamudi, M.R. Modulation of Gut-Brain Axis by probiotics: A promising anti-depressant approach. Curr. Neuropharmacol. 2021, 19, 990–1006. [Google Scholar] [CrossRef]
- Arseneault-Breard, J.; Rondeau, I.; Gilbert, K.; Girard, S.A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012, 107, 1793–1799. [Google Scholar] [CrossRef]
- Trudeau, F.; Gilbert, K.; Tremblay, A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Bifidobacterium longum R0175 attenuates post-myocardial infarction depressive-like behaviour in rats. PLoS ONE 2019, 14, e0215101. [Google Scholar] [CrossRef]
- Malick, M.; Gilbert, K.; Daniel, J.; Arseneault-Breard, J.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Vagotomy prevents the effect of probiotics on caspase activity in a model of postmyocardial infarction depression. Neurogastroenterol. Motil. 2015, 27, 663–671. [Google Scholar] [CrossRef]
- Gagné, M.A.; Barbeau, C.; Frégeau, G.; Gilbert, K.; Mathieu, O.; Auger, J.; Tompkins, T.A.; Charbonney, E.; Godbout, R.; Rousseau, G. Dysbiotic microbiota contributes to the extent of acute myocardial infarction in rats. Sci. Rep. 2022, 12, 16517. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Xu, X.; Li, Y.; Li, X.; Yang, X.; Chen, H.; Zhu, Y.; Lu, N.; He, C. Sex-specific association between the gut microbiome and high-fat diet-induced metabolic disorders in mice. Biol. Sex Differ. 2020, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, S.; Nadatani, Y.; Higashimori, A.; Otani, K.; Fujimoto, K.; Nagata, Y.; Ominami, M.; Fukunaga, S.; Hosomi, S.; Kamata, N.; et al. Ovariectomy-Induced Dysbiosis May Have a Minor Effect on Bone in Mice. Microorganisms 2021, 9, 2563. [Google Scholar] [CrossRef]
- Lephart, E.D.; Naftolin, F. Estrogen Action and Gut Microbiome Metabolism in Dermal Health. Dermatol. Ther. 2022, 12, 1535–1550. [Google Scholar] [CrossRef]
- Kim, C.-S.; Jung, M.H.; Choi, E.Y.; Shin, D.-M. Probiotic supplementation has sex-dependent effects on immune responses in association with the gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Res. Pract. 2023, 17, 883. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Theodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus Synergistically Suppress Stress-related Visceral Hypersensitivity Through Hypothalamic-Pituitary-Adrenal Axis Modulation. J. Neurogastroenterol. Motil. 2018, 24, 138–146. [Google Scholar] [CrossRef]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Girard, S.-A.; Bah, T.M.; Kaloustian, S.; Lada-Moldovan, L.; Rondeau, I.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Lactobacillus helveticus and Bifidobacterium longum taken in combination reduce the apoptosis propensity in the limbic system after myocardial infarction in a rat model. Br. J. Nutr. 2009, 102, 1420–1425. [Google Scholar] [CrossRef]
- Wang, M.; Smith, K.; Yu, Q.; Miller, C.; Singh, K.; Sen, C.K. Mitochondrial connexin 43 in sex-dependent myocardial responses and estrogen-mediated cardiac protection following acute ischemia/reperfusion injury. Basic Res. Cardiol. 2019, 115, 1. [Google Scholar] [CrossRef] [PubMed]
- Booth, E.A.; Flint, R.R.; Lucas, K.L.; Knittel, A.K.; Lucchesi, B.R. Estrogen protects the heart from ischemia-reperfusion injury via COX-2-derived PGI2. J. Cardiovasc. Pharmacol. 2008, 52, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Booth, E.A.; Marchesi, M.; Knittel, A.K.; Kilbourne, E.J.; Lucchesi, B.R. The pathway-selective estrogen receptor ligand WAY-169916 reduces infarct size after myocardial ischemia and reperfusion by an estrogen receptor dependent mechanism. J. Cardiovasc. Pharmacol. 2007, 49, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Ornatsky, O.; Stewart, D.J.; Picard, P.; Dawood, F.; Wen, W.H.; Liu, P.P.; Webb, D.J.; Monge, J.C. Effects of estrogen replacement on infarct size, cardiac remodeling, and the endothelin system after myocardial infarction in ovariectomized rats. Circulation 2000, 102, 2983–2989. [Google Scholar] [CrossRef]
- Bopassa, J.C.; Eghbali, M.; Toro, L.; Stefani, E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H16–H23. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Pulkkinen, M.O.; Hämäläinen, E.K.; Korpela, J.T. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J. Steroid Biochem. 1984, 20, 217–229. [Google Scholar] [CrossRef]
- Kamimura, I.; Watarai, A.; Takamura, T.; Takeo, A.; Miura, K.; Morita, H.; Mogi, K.; Kikusui, T. Gonadal steroid hormone secretion during the juvenile period depends on host-specific microbiota and contributes to the development of odor preference. Dev. Psychobiol. 2019, 61, 670–678. [Google Scholar] [CrossRef]
- Shen, R.L.; Dang, X.Y.; Dong, J.L.; Hu, X.Z. Effects of oat β-glucan and barley β-glucan on fecal characteristics, intestinal microflora, and intestinal bacterial metabolites in rats. J. Agric. Food Chem. 2012, 60, 11301–11308. [Google Scholar] [CrossRef]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocrinol. 2018, 51, 80–101. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Ferns, G.A.; Vatanparast, H. Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients 2017, 9, 20. [Google Scholar] [CrossRef]
- Mohammadi, G.; Dargahi, L.; Peymani, A.; Mirzanejad, Y.; Alizadeh, S.A.; Naserpour, T.; Nassiri-Asl, M. The Effects of Probiotic Formulation Pretreatment (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) on a Lipopolysaccharide Rat Model. J. Am. Coll. Nutr. 2019, 38, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Amdekar, S.; Singh, V.; Singh, R.; Sharma, P.; Keshav, P.; Kumar, A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines: Lactobacillus casei: COX-2 inhibitor. J. Clin. Immunol. 2011, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Alipour, B.; Homayouni-Rad, A.; Vaghef-Mehrabany, E.; Sharif, S.K.; Vaghef-Mehrabany, L.; Asghari-Jafarabadi, M.; Nakhjavani, M.R.; Mohtadi-Nia, J. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: A randomized double-blind clinical trial. Int. J. Rheum. Dis. 2014, 17, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Graziani, C.; Laterza, L.; Talocco, C.; Pizzoferrato, M.; Di Simone, N.; D’Ippolito, S.; Ricci, C.; Gervasoni, J.; Persichilli, S.; Del Chierico, F.; et al. Intestinal Permeability and Dysbiosis in Female Patients with Recurrent Cystitis: A Pilot Study. J. Pers. Med. 2022, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Rudyk, M.; Hurmach, Y.; Serhiichuk, T.; Akulenko, I.; Skivka, L.; Berehova, T.; Ostapchenko, L. Multi-probiotic consumption sex-dependently interferes with MSG-induced obesity and concomitant phagocyte pro-inflammatory polarization in rats: Food for thought about personalized nutrition. Heliyon 2023, 9, e13381. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef]
- Wann, B.P.; Bah, T.M.; Kaloustian, S.; Boucher, M.; Dufort, A.M.; Le Marec, N.; Godbout, R.; Rousseau, G. Behavioural signs of depression and apoptosis in the limbic system following myocardial infarction: Effects of sertraline. J. Psychopharmacol. 2009, 23, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Frasure-Smith, N.; Lespérance, F. Depression and Cardiac Risk: Present Status and Future Directions. Heart 2010, 86, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Carrier, N.; Kabbaj, M. Sex differences in social interaction behaviors in rats are mediated by extracellular signal-regulated kinase 2 expression in the medial prefrontal cortex. Neuroscience 2012, 212, 86–92. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagné, M.-A.; Frégeau, G.; Godbout, R.; Rousseau, G. The Role of Probiotics in Modulating Myocardial Infarction and Depression-like Symptoms: A Study on Sex-Specific Responses. Biomedicines 2024, 12, 2511. https://doi.org/10.3390/biomedicines12112511
Gagné M-A, Frégeau G, Godbout R, Rousseau G. The Role of Probiotics in Modulating Myocardial Infarction and Depression-like Symptoms: A Study on Sex-Specific Responses. Biomedicines. 2024; 12(11):2511. https://doi.org/10.3390/biomedicines12112511
Chicago/Turabian StyleGagné, Marc-André, Geneviève Frégeau, Roger Godbout, and Guy Rousseau. 2024. "The Role of Probiotics in Modulating Myocardial Infarction and Depression-like Symptoms: A Study on Sex-Specific Responses" Biomedicines 12, no. 11: 2511. https://doi.org/10.3390/biomedicines12112511
APA StyleGagné, M.-A., Frégeau, G., Godbout, R., & Rousseau, G. (2024). The Role of Probiotics in Modulating Myocardial Infarction and Depression-like Symptoms: A Study on Sex-Specific Responses. Biomedicines, 12(11), 2511. https://doi.org/10.3390/biomedicines12112511








