Emerging Mechanisms of Physical Exercise Benefits in Adjuvant and Neoadjuvant Cancer Immunotherapy
Abstract
1. Introduction
2. Boosting Cancer Immunotherapy with Exercise
2.1. The General Benefits of Exercise on the Immune System and in the Oncology Setting
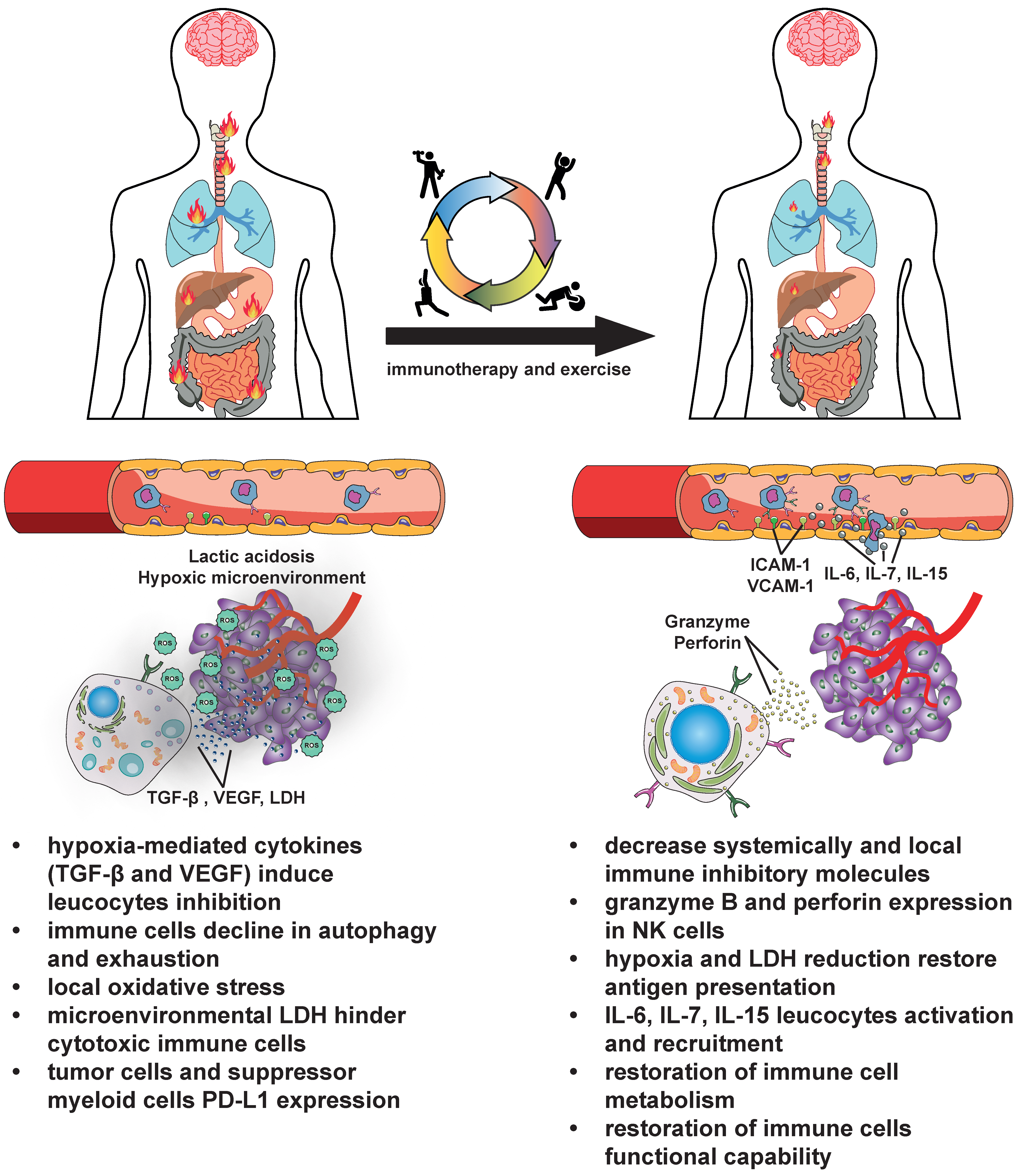
2.2. Increased Immune Cell Trafficking, Endothelial Stabilization, and Therapy Sensitization
2.3. Positive Modulation of Immune Metabolism
2.4. Systemic Action of Exercise That Benefits Immune Fitness
2.5. The Sometimes Contradictory Evidence of In Vivo Studies
3. Discussion
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Status Report on Noncommunicable Diseases 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Tran, K.B.; Lang, J.J.; Compton, K.; Xu, R.; Acheson, A.R.; Henrikson, H.J.; Kocarnik, J.M.; Penberthy, L.; Aali, A.; Abbas, Q.; et al. The global burden of cancer attributable to risk factors, 2010–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Davis, K.; Breitbart, W.; Curt, G.; Fatigue, C. Cancer-related fatigue: Prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J. Clin. Oncol. 2001, 19, 3385–3391. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Couch, M.E.; Bonetto, A. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr. Opin. Support. Palliat. Care 2018, 12, 420–426. [Google Scholar] [CrossRef]
- Pin, F.; Barreto, R.; Couch, M.E.; Bonetto, A.; O’Connell, T.M. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J. Cachexia Sarcopenia Muscle 2019, 10, 140–154. [Google Scholar] [CrossRef]
- Klassen, O.; Schmidt, M.E.; Ulrich, C.M.; Schneeweiss, A.; Potthoff, K.; Steindorf, K.; Wiskemann, J. Muscle strength in breast cancer patients receiving different treatment regimes. J. Cachexia Sarcopenia Muscle 2017, 8, 305–316. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: A patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022, 23, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Park, M.A.; Whelan, C.J.; Ahmed, S.; Boeringer, T.; Brown, J.; Crowder, S.L.; Gage, K.; Gregg, C.; Jeong, D.K.; Jim, H.S.L.; et al. Defining and Addressing Research Priorities in Cancer Cachexia through Transdisciplinary Collaboration. Cancers 2024, 16, 2364. [Google Scholar] [CrossRef]
- Goede, V. Frailty and Cancer: Current Perspectives on Assessment and Monitoring. Clin. Interv. Aging 2023, 18, 505–521. [Google Scholar] [CrossRef]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef]
- Garcia-Hermoso, A.; Lopez-Gil, J.F.; Ramirez-Velez, R.; Alonso-Martinez, A.M.; Izquierdo, M.; Ezzatvar, Y. Adherence to aerobic and muscle-strengthening activities guidelines: A systematic review and meta-analysis of 3.3 million participants across 32 countries. Br. J. Sports Med. 2023, 57, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.C.; Lee, I.M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; Berrington de Gonzalez, A.; Hartge, P.; et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef]
- Christensen, J.F.; Simonsen, C.; Hojman, P. Exercise Training in Cancer Control and Treatment. Compr. Physiol. 2018, 9, 165–205. [Google Scholar] [CrossRef]
- Rao, R.; Cruz, V.; Peng, Y.; Harker-Murray, A.; Haley, B.B.; Zhao, H.; Xie, X.J.; Euhus, D. Bootcamp during neoadjuvant chemotherapy for breast cancer: A randomized pilot trial. Breast Cancer 2012, 6, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Loughney, L.A.; West, M.A.; Kemp, G.J.; Grocott, M.P.; Jack, S. Exercise interventions for people undergoing multimodal cancer treatment that includes surgery. Cochrane Database Syst. Rev. 2018, 12, CD012280. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Alfano, C.M. Exercise-oncology research: Past, present, and future. Acta Oncol. 2013, 52, 195–215. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvao, D.A.; Alfano, C.M.; et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef]
- Maio, M.; Blank, C.; Necchi, A.; Di Giacomo, A.M.; Ibrahim, R.; Lahn, M.; Fox, B.A.; Bell, R.B.; Tortora, G.; Eggermont, A.M.M. Neoadjuvant immunotherapy is reshaping cancer management across multiple tumour types: The future is now! Eur. J. Cancer 2021, 152, 155–164. [Google Scholar] [CrossRef]
- Farhangnia, P.; Ghomi, S.M.; Akbarpour, M.; Delbandi, A.A. Bispecific antibodies targeting CTLA-4: Game-changer troopers in cancer immunotherapy. Front. Immunol. 2023, 14, 1155778. [Google Scholar] [CrossRef] [PubMed]
- Utkarsh, K.; Srivastava, N.; Kumar, S.; Khan, A.; Dagar, G.; Kumar, M.; Singh, M.; Haque, S. CAR-T cell therapy: A game-changer in cancer treatment and beyond. Clin. Transl. Oncol. 2024, 26, 1300–1318. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune checkpoint therapy-current perspectives and future directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. Cancer Immunology and Immunotherapy Showcased in the AACR Cancer Progress Report 2023. Cancer Immunol. Res. 2023, 11, 1298–1299. [Google Scholar] [CrossRef]
- Zebertavage, L.; Schopf, A.; Nielsen, M.; Matthews, J.; Erbe, A.K.; Aiken, T.J.; Katz, S.; Sun, C.; Witt, C.M.; Rakhmilevich, A.L.; et al. Evaluation of a Combinatorial Immunotherapy Regimen That Can Cure Mice Bearing MYCN-Driven High-Risk Neuroblastoma That Resists Current Clinical Therapy. J. Clin. Med. 2024, 13, 2561. [Google Scholar] [CrossRef]
- Garate-Soraluze, E.; Serrano-Mendioroz, I.; Rodriguez-Ruiz, M. Methods to assess radiation induced abscopal responses in mice. Methods Cell Biol. 2023, 180, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Spicer, J.D.; Cascone, T.; Wynes, M.W.; Ahn, M.J.; Dacic, S.; Felip, E.; Forde, P.M.; Higgins, K.A.; Kris, M.G.; Mitsudomi, T.; et al. Neoadjuvant and Adjuvant Treatments for Early Stage Resectable NSCLC: Consensus Recommendations From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2024, 19, 1373–1414. [Google Scholar] [CrossRef]
- Aredo, J.V.; Wakelee, H.A. Top advances of the year: Perioperative therapy for lung cancer. Cancer 2024, 130, 2897–2903. [Google Scholar] [CrossRef]
- Kakish, H.; Xu, K.; Ahmed, F.A.; Loftus, A.W.; Elshami, M.; Hoehn, R.S.; Ammori, J.B.; Mangla, A.; Rothermel, L.D. Preoperative therapy in melanoma: Evolving perspectives in clinical trials. Crit. Rev. Oncol. Hematol. 2024, 193, 104193. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Dissecting the mechanisms of immune checkpoint therapy. Nat. Rev. Immunol. 2020, 20, 75–76. [Google Scholar] [CrossRef]
- Leko, V.; Rosenberg, S.A. Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell 2020, 38, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Siddiqui, B.A.; Anandhan, S.; Yadav, S.S.; Subudhi, S.K.; Gao, J.; Goswami, S.; Allison, J.P. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021, 11, 838–857. [Google Scholar] [CrossRef] [PubMed]
- Klobuch, S.; Seijkens, T.T.P.; Schumacher, T.N.; Haanen, J. Tumour-infiltrating lymphocyte therapy for patients with advanced-stage melanoma. Nat. Rev. Clin. Oncol. 2024, 21, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Ugai, T.; Yao, Q.; Ugai, S.; Ogino, S. Advancing precision oncology: Insights into the tumor microenvironment and immunotherapy outcomes. Innovation 2024, 5, 100656. [Google Scholar] [CrossRef]
- Cantoni, C.; Falco, M.; Vitale, M.; Pietra, G.; Munari, E.; Pende, D.; Mingari, M.C.; Sivori, S.; Moretta, L. Human NK cells and cancer. Oncoimmunology 2024, 13, 2378520. [Google Scholar] [CrossRef]
- Said, S.S.; Ibrahim, W.N. Cancer Resistance to Immunotherapy: Comprehensive Insights with Future Perspectives. Pharmaceutics 2023, 15, 1143. [Google Scholar] [CrossRef]
- Perez-Ruiz, E.; Melero, I.; Kopecka, J.; Sarmento-Ribeiro, A.B.; Garcia-Aranda, M.; De Las Rivas, J. Cancer immunotherapy resistance based on immune checkpoints inhibitors: Targets, biomarkers, and remedies. Drug Resist. Updat. 2020, 53, 100718. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Hodgman, C.F.; Hunt, R.M.; Crane, J.C.; Elzayat, M.T.; LaVoy, E.C. A Scoping Review on the Effects of Physical Exercise and Fitness on Peripheral Leukocyte Energy Metabolism in Humans. Exerc. Immunol. Rev. 2023, 29, 54–87. [Google Scholar]
- Remskar, M.; Western, M.J.; Osborne, E.L.; Maynard, O.M.; Ainsworth, B. Effects of combining physical activity with mindfulness on mental health and wellbeing: Systematic review of complex interventions. Ment. Health Phys. Act. 2024, 26, 100575. [Google Scholar] [CrossRef]
- Weiss, J.A.; Jain, S. Neoadjuvant and adjuvant therapy in esophageal cancer. J. Gastrointest. Oncol. 2023, 14, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Fernandez-Garcia, B.; Lehmann, H.I.; Li, G.; Kroemer, G.; Lopez-Otin, C.; Xiao, J. Exercise sustains the hallmarks of health. J. Sport Health Sci. 2023, 12, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Llavero, F.; Alejo, L.B.; Fiuza-Luces, C.; Lopez Soto, A.; Valenzuela, P.L.; Castillo-Garcia, A.; Morales, J.S.; Fernandez, D.; Aldazabal, I.P.; Ramirez, M.; et al. Exercise training effects on natural killer cells: A preliminary proteomics and systems biology approach. Exerc. Immunol. Rev. 2021, 27, 125–141. [Google Scholar] [PubMed]
- Toffoli, E.C.; Sweegers, M.G.; Bontkes, H.J.; Altenburg, T.M.; Verheul, H.M.W.; van der Vliet, H.J.; de Gruijl, T.D.; Buffart, L.M. Effects of physical exercise on natural killer cell activity during (neo)adjuvant chemotherapy: A randomized pilot study. Physiol. Rep. 2021, 9, e14919. [Google Scholar] [CrossRef]
- MacDonald, G.; Sitlinger, A.; Deal, M.A.; Hanson, E.D.; Ferraro, S.; Pieper, C.F.; Weinberg, J.B.; Brander, D.M.; Bartlett, D.B. A pilot study of high-intensity interval training in older adults with treatment naive chronic lymphocytic leukemia. Sci. Rep. 2021, 11, 23137. [Google Scholar] [CrossRef]
- Coletta, A.M.; Agha, N.H.; Baker, F.L.; Niemiro, G.M.; Mylabathula, P.L.; Brewster, A.M.; Bevers, T.B.; Fuentes-Mattei, E.; Basen-Engquist, K.; Gilchrist, S.C.; et al. The impact of high-intensity interval exercise training on NK-cell function and circulating myokines for breast cancer prevention among women at high risk for breast cancer. Breast Cancer Res. Treat. 2021, 187, 407–416. [Google Scholar] [CrossRef]
- Spielmann, G.; McFarlin, B.K.; O’Connor, D.P.; Smith, P.J.; Pircher, H.; Simpson, R.J. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav. Immun. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Youssef, L.; Harroum, N.; Francisco, B.A.; Johnson, L.; Arvisais, D.; Pageaux, B.; Romain, A.J.; Hayward, K.S.; Neva, J.L. Neurophysiological effects of acute aerobic exercise in young adults: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2024, 164, 105811. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, T.; Zhu, Z.; Yang, Y. The association between immune cells and breast cancer: Insights from mendelian randomization and meta-analysis. Int. J. Surg. 2024. [Google Scholar] [CrossRef]
- Betof Warner, A. Exercise as an Immune Boost: Mechanism-Driven Support for Lifestyle Interventions. Cancer Immunol. Res. 2023, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Konigstein, K.; Dipla, K.; Zafeiridis, A. Training the Vessels: Molecular and Clinical Effects of Exercise on Vascular Health-A Narrative Review. Cells 2023, 12, 2544. [Google Scholar] [CrossRef] [PubMed]
- McCullough, D.J.; Stabley, J.N.; Siemann, D.W.; Behnke, B.J. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J. Natl. Cancer Inst. 2014, 106, dju036. [Google Scholar] [CrossRef] [PubMed]
- Vaccarezza, M.; Vitale, M. Tumor chemosensitization by physical exercise? Insights from an anidmal model. Futur. Oncol. 2015, 11, 885–887. [Google Scholar] [CrossRef]
- Vaccarezza, M. Physical exercise as chemosensitizer. Clin. Exp. Med. 2015, 15, 427. [Google Scholar] [CrossRef][Green Version]
- Simpson, R.J.; Bigley, A.B.; Agha, N.; Hanley, P.J.; Bollard, C.M. Mobilizing Immune Cells With Exercise for Cancer Immunotherapy. Exerc. Sport Sci. Rev. 2017, 45, 163–172. [Google Scholar] [CrossRef]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar] [CrossRef]
- Evans, E.S.; Hackney, A.C.; McMurray, R.G.; Randell, S.H.; Muss, H.B.; Deal, A.M.; Battaglini, C.L. Impact of Acute Intermittent Exercise on Natural Killer Cells in Breast Cancer Survivors. Integr. Cancer Ther. 2015, 14, 436–445. [Google Scholar] [CrossRef]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Ashcroft, S.P.; Stocks, B.; Egan, B.; Zierath, J.R. Exercise induces tissue-specific adaptations to enhance cardiometabolic health. Cell Metab. 2024, 36, 278–300. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.T.; Chen, Q.; Skitzki, J.J.; Muhitch, J.B.; Zhou, L.; Appenheimer, M.M.; Vardam, T.D.; Weis, E.L.; Passanese, J.; Wang, W.C.; et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J. Clin. Investig. 2011, 121, 3846–3859. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.; Cooper, M.A. Metabolic regulation of NK cell function: Implications for immunotherapy. Immunometabolism 2023, 5, e00020. [Google Scholar] [CrossRef]
- Marcais, A.; Cherfils-Vicini, J.; Viant, C.; Degouve, S.; Viel, S.; Fenis, A.; Rabilloud, J.; Mayol, K.; Tavares, A.; Bienvenu, J.; et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 2014, 15, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H.; Jocken, J.W.; van Baak, M.A.; Jansen, E.H.; Saris, W.H.; Blaak, E.E. Short-term beta-adrenergic regulation of leptin, adiponectin and interleukin-6 secretion in vivo in lean and obese subjects. Diabetes Obes. Metab. 2008, 10, 1029–1038. [Google Scholar] [CrossRef]
- Kruger, K.; Lechtermann, A.; Fobker, M.; Volker, K.; Mooren, F.C. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav. Immun. 2008, 22, 324–338. [Google Scholar] [CrossRef]
- Hapuarachi, B.; Danson, S.; Wadsley, J.; Muthana, M. Exercise to transform tumours from cold to hot and improve immunotherapy responsiveness. Front. Immunol. 2023, 14, 1335256. [Google Scholar] [CrossRef]
- Djurhuus, S.S.; Simonsen, C.; Toft, B.G.; Thomsen, S.N.; Wielsoe, S.; Roder, M.A.; Hasselager, T.; Ostergren, P.B.; Jakobsen, H.; Pedersen, B.K.; et al. Exercise training to increase tumour natural killer-cell infiltration in men with localised prostate cancer: A randomised controlled trial. BJU Int. 2023, 131, 116–124. [Google Scholar] [CrossRef]
- Djurhuus, S.S.; Schauer, T.; Simonsen, C.; Toft, B.G.; Jensen, A.R.D.; Erler, J.T.; Roder, M.A.; Hojman, P.; Brasso, K.; Christensen, J.F. Effects of acute exercise training on tumor outcomes in men with localized prostate cancer: A randomized controlled trial. Physiol. Rep. 2022, 10, e15408. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Garcia, C.; Campbell, J.P.; Taaffe, D.R.; Peddle-McIntyre, C.J.; Jeffery, E.; Galvao, D.A.; Redfern, A.D.; Newton, R.U. Unleashing anti-tumour immunity: Dietary restriction and exercise interventions adjunct to chemotherapy for cancer patients. Exerc. Immunol. Rev. 2024, 30, 26–48. [Google Scholar] [PubMed]
- Kruger, K.; Mooren, F.C. T cell homing and exercise. Exerc. Immunol. Rev. 2007, 13, 37–54. [Google Scholar]
- Gustafson, M.P.; Wheatley-Guy, C.M.; Rosenthal, A.C.; Gastineau, D.A.; Katsanis, E.; Johnson, B.D.; Simpson, R.J. Exercise and the immune system: Taking steps to improve responses to cancer immunotherapy. J. Immunother. Cancer 2021, 9, e001872. [Google Scholar] [CrossRef]
- Idorn, M.; Hojman, P. Exercise-Dependent Regulation of NK Cells in Cancer Protection. Trends Mol. Med. 2016, 22, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Simpson, R.J.; Bosslau, T.K.; Weyh, C.; Niemiro, G.M.; Batatinha, H.; Smith, K.A.; Kruger, K. Exercise and adrenergic regulation of immunity. Brain Behav. Immun. 2021, 97, 303–318. [Google Scholar] [CrossRef]
- Graff, R.M.; Kunz, H.E.; Agha, N.H.; Baker, F.L.; Laughlin, M.; Bigley, A.B.; Markofski, M.M.; LaVoy, E.C.; Katsanis, E.; Bond, R.A.; et al. beta(2)-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav. Immun. 2018, 74, 143–153. [Google Scholar] [CrossRef]
- Aveseh, M.; Nikooie, R.; Aminaie, M. Exercise-induced changes in tumour LDH-B and MCT1 expression are modulated by oestrogen-related receptor alpha in breast cancer-bearing BALB/c mice. J. Physiol. 2015, 593, 2635–2648. [Google Scholar] [CrossRef]
- Hersey, P.; Watts, R.N.; Zhang, X.D.; Hackett, J. Metabolic approaches to treatment of melanoma. Clin. Cancer Res. 2009, 15, 6490–6494. [Google Scholar] [CrossRef]
- Babar, M.U.; Nassar, A.F.; Nie, X.; Zhang, T.; He, J.; Yeung, J.; Norris, P.; Ogura, H.; Muldoon, A.; Chen, L.; et al. Is Lipid Metabolism of Value in Cancer Research and Treatment? Part II: Role of Specialized Pro-Resolving Mediators in Inflammation, Infections, and Cancer. Metabolites 2024, 14, 314. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.L.; Indra, A.K. Oxidative Stress in Melanoma: Beneficial Antioxidant and Pro-Oxidant Therapeutic Strategies. Cancers 2023, 15, 3038. [Google Scholar] [CrossRef]
- Vina, J.; Sanchis-Gomar, F.; Martinez-Bello, V.; Gomez-Cabrera, M.C. Exercise acts as a drug; the pharmacological benefits of exercise. Br. J. Pharmacol. 2012, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ziemys, A.; Simic, V.; Milosevic, M.; Kojic, M.; Liu, Y.T.; Yokoi, K. Attenuated Microcirculation in Small Metastatic Tumors in Murine Liver. Pharmaceutics 2021, 13, 703. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Vaupel, P. Hypoxia Compromises Anti-Cancer Immune Responses. Adv. Exp. Med. Biol. 2020, 1232, 131–143. [Google Scholar] [CrossRef]
- Ferrari, D.; Vuerich, M.; Casciano, F.; Longhi, M.S.; Melloni, E.; Secchiero, P.; Zech, A.; Robson, S.C.; Muller, T.; Idzko, M. Eosinophils and Purinergic Signaling in Health and Disease. Front. Immunol. 2020, 11, 1339. [Google Scholar] [CrossRef]
- Ferrari, D.; la Sala, A.; Milani, D.; Celeghini, C.; Casciano, F. Purinergic Signaling in Controlling Macrophage and T Cell Functions During Atherosclerosis Development. Front. Immunol. 2020, 11, 617804. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting intratumoral hypoxia to enhance anti-tumor immunity. Semin. Cancer Biol. 2023, 96, 5–10. [Google Scholar] [CrossRef]
- Semenza, G.L. Intratumoral Hypoxia and Mechanisms of Immune Evasion Mediated by Hypoxia-Inducible Factors. Physiology 2021, 36, 73–83. [Google Scholar] [CrossRef]
- Finger, E.C.; Giaccia, A.J. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010, 29, 285–293. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, I.B.; Smallwood, C.A.; Siemens, D.R.; Graham, C.H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014, 74, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Angelin, A.; Gil-de-Gomez, L.; Dahiya, S.; Jiao, J.; Guo, L.; Levine, M.H.; Wang, Z.; Quinn, W.J., 3rd; Kopinski, P.K.; Wang, L.; et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab. 2017, 25, 1282–1293.e1287. [Google Scholar] [CrossRef]
- Quinn, W.J., 3rd; Jiao, J.; TeSlaa, T.; Stadanlick, J.; Wang, Z.; Wang, L.; Akimova, T.; Angelin, A.; Schafer, P.M.; Cully, M.D.; et al. Lactate Limits T Cell Proliferation via the NAD(H) Redox State. Cell Rep. 2020, 33, 108500. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Huang, X.; Yang, M.; Zhou, S.; Li, Z.; Fang, Z.; Tang, Y.; Chen, Q.; Hou, H.; et al. Lactate in the tumor microenvironment: A rising star for targeted tumor therapy. Front. Nutr. 2023, 10, 1113739. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Budhu, S.; Serganova, I.; Dong, L.; Mangarin, L.M.; Khan, J.F.; Bah, M.A.; Assouvie, A.; Marouf, Y.; Schulze, I.; et al. Pharmacologic LDH inhibition redirects intratumoral glucose uptake and improves antitumor immunity in solid tumor models. J. Clin. Investig. 2024, 134. [Google Scholar] [CrossRef]
- Mirchandani, A.S.; Sanchez-Garcia, M.A.; Walmsley, S.R. How oxygenation shapes immune responses: Emerging roles for physioxia and pathological hypoxia. Nat. Rev. Immunol. 2024. [Google Scholar] [CrossRef]
- Vivier, E.; Rebuffet, L.; Narni-Mancinelli, E.; Cornen, S.; Igarashi, R.Y.; Fantin, V.R. Natural killer cell therapies. Nature 2024, 626, 727–736. [Google Scholar] [CrossRef]
- Fenis, A.; Demaria, O.; Gauthier, L.; Vivier, E.; Narni-Mancinelli, E. New immune cell engagers for cancer immunotherapy. Nat. Rev. Immunol. 2024, 24, 471–486. [Google Scholar] [CrossRef]
- Ferrari, D.; Casciano, F.; Secchiero, P.; Reali, E. Purinergic Signaling and Inflammasome Activation in Psoriasis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9449. [Google Scholar] [CrossRef]
- Rehman, J.; Mills, P.J.; Carter, S.M.; Chou, J.; Thomas, J.; Maisel, A.S. Dynamic exercise leads to an increase in circulating ICAM-1: Further evidence for adrenergic modulation of cell adhesion. Brain Behav. Immun. 1997, 11, 343–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Y.; Liao, Q.; Qian, Y.; Zhu, L.; Yu, D.G.; Xu, Y.; Lu, X.; Kim, I.; Song, W. Silver oxide decorated urchin-like microporous organic polymer composites as versatile antibacterial organic coating materials. J. Mater. Chem. B 2024, 12, 2054–2069. [Google Scholar] [CrossRef]
- Liao, Q.; Kim, E.J.; Tang, Y.; Xu, H.; Yu, D.G.; Song, W.; Kim, B.J. Rational design of hyper-crosslinked polymers for biomedical applications. J. Polym. Sci. 2023, 62, 1517–1535. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Raimondo, T.M.; Reed, K.; Shi, D.; Langer, R.; Anderson, D.G. Delivering the next generation of cancer immunotherapies with RNA. Cell 2023, 186, 1535–1540. [Google Scholar] [CrossRef]
- Cao, Y.; Langer, R.; Ferrara, N. Targeting angiogenesis in oncology, ophthalmology and beyond. Nat. Rev. Drug Discov. 2023, 22, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Peng, W.B.; Zhang, P.; Yang, X.P.; Zhou, Q. Lactate in the tumour microenvironment: From immune modulation to therapy. EBioMedicine 2021, 73, 103627. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, H.; Liu, G.; Wu, J.; Yuan, Y.; Shang, A. Tumor Microenvironment: Lactic Acid Promotes Tumor Development. J. Immunol. Res. 2022, 2022, 3119375. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef]
- Xia, H.; Green, D.R.; Zou, W. Autophagy in tumour immunity and therapy. Nat. Rev. Cancer 2021, 21, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.S. The Lactate and the Lactate Dehydrogenase in Inflammatory Diseases and Major Risk Factors in COVID-19 Patients. Inflammation 2022, 45, 2091–2123. [Google Scholar] [CrossRef] [PubMed]
| Immunotherapy | |
| [24,25,26,27,28,29,30,42] |
| [28,29,30,43] |
| Exercise | |
| Enhance leukocyte metabolism and activity | [40,44,45,46,47,48,49] |
| Increase overall quality of life | [11,12,13,50] |
| Modulate tumor vessel blood flow | [51,52,53,54,55,56,57,58] |
| Induce hyperthermia with vessel expansion and tumor growth reduction | [59,60] |
| Induce release of polarizing cytokines (IL-6, IL-15) | [51,61,62,63,64,65,66,67,68,69] |
| Mitigate side effects of cancer treatments | [17] |
| Mobilize immune cells from lymphoid tissues to cancer sites | [70,71,72,73,74,75] |
| Mobilize immune cells via adrenergic and epinephrine signaling | [59,68,69,76,77,78,79] |
| Reduce intratumoral lactate | [40,80,81,82,83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casciano, F.; Caruso, L.; Zauli, E.; Gonelli, A.; Zauli, G.; Vaccarezza, M. Emerging Mechanisms of Physical Exercise Benefits in Adjuvant and Neoadjuvant Cancer Immunotherapy. Biomedicines 2024, 12, 2528. https://doi.org/10.3390/biomedicines12112528
Casciano F, Caruso L, Zauli E, Gonelli A, Zauli G, Vaccarezza M. Emerging Mechanisms of Physical Exercise Benefits in Adjuvant and Neoadjuvant Cancer Immunotherapy. Biomedicines. 2024; 12(11):2528. https://doi.org/10.3390/biomedicines12112528
Chicago/Turabian StyleCasciano, Fabio, Lorenzo Caruso, Enrico Zauli, Arianna Gonelli, Giorgio Zauli, and Mauro Vaccarezza. 2024. "Emerging Mechanisms of Physical Exercise Benefits in Adjuvant and Neoadjuvant Cancer Immunotherapy" Biomedicines 12, no. 11: 2528. https://doi.org/10.3390/biomedicines12112528
APA StyleCasciano, F., Caruso, L., Zauli, E., Gonelli, A., Zauli, G., & Vaccarezza, M. (2024). Emerging Mechanisms of Physical Exercise Benefits in Adjuvant and Neoadjuvant Cancer Immunotherapy. Biomedicines, 12(11), 2528. https://doi.org/10.3390/biomedicines12112528







