Cefiderocol in Combating Carbapenem-Resistant Acinetobacter baumannii: Action and Resistance
Abstract
:1. Multidrug and Pandrug Resistant Acinetobacter baumannii
2. Monotherapy and Combination Therapy of Acinetobacter baumannii
3. The Effect and Role of Beta-Lactamase Inhibitor on Cefiderocol
4. Mechanisms of Reduced Susceptibility to Cefiderocol
5. Mechanism of Action and Bacterial Resistance to Cefiderocol
Mechanism of Action
6. Susceptibility Profiles Against Cefiderocol and Comparators
7. Cefiderocol for Therapy in Carbapenem-Resistant Acinetobacter baumannii
8. Cefiderocol and Its Implications in the Management of A. baumannii Infections
9. Therapy of Infection-Related Ventilator-Associated Complications (IVACs)
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alnomani, Y.; Ghasemian, A.; Memariani, M.; Eslami, M.; Sabokrouh, A.; Abbas, A.F.; Shafiei, M. The association of acetylacetate (Acr AB-Tol C) and QepA genes with multiple antibiotic resistance among Escherichia coli clinical isolates. Rev. Med. Microbiol. 2020, 31, 159–164. [Google Scholar] [CrossRef]
- Andersen, C.T.; Langendorf, C.; Garba, S.; Sayinzonga-Makombe, N.; Mambula, C.; Mouniaman, I.; Hanson, K.E.; Grais, R.F.; Isanaka, S. Risk of community-and hospital-acquired bacteremia and profile of antibiotic resistance in children hospitalized with severe acute malnutrition in Niger. Int. J. Infect. Dis. 2022, 119, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Razo-Gutierrez, C.; Le, C.; Courville, R.; Pimentel, C.; Liu, C.; Fung, S.E.; Tuttobene, M.R.; Phan, K.; Vila, A.J. Cerebrospinal fluid (CSF) augments metabolism and virulence expression factors in Acinetobacter baumannii. Sci. Rep. 2021, 11, 4737. [Google Scholar] [CrossRef] [PubMed]
- Nodari, C.S.; Cayô, R.; Streling, A.P.; Lei, F.; Wille, J.; Almeida, M.S.; De Paula, A.I.; Pignatari, A.C.C.; Seifert, H.; Higgins, P.G. Genomic analysis of carbapenem-resistant Acinetobacter baumannii isolates belonging to major endemic clones in South America. Front. Microbiol. 2020, 11, 584603. [Google Scholar] [CrossRef] [PubMed]
- Wareth, G.; Linde, J.; Hammer, P.; Nguyen, N.H.; Nguyen, T.N.; Splettstoesser, W.D.; Makarewicz, O.; Neubauer, H.; Sprague, L.D.; Pletz, M.W. Phenotypic and WGS-derived antimicrobial resistance profiles of clinical and non-clinical Acinetobacter baumannii isolates from Germany and Vietnam. Int. J. Antimicrob. Agents 2020, 56, 106127. [Google Scholar] [CrossRef]
- Bandy, A.; Tantry, B. ESBL Activity, MDR, and Carbapenem Resistance among Predominant Enterobacterales Isolated in 2019. Antibiotics 2021, 10, 744. [Google Scholar] [CrossRef]
- Nocera, F.P.; Attili, A.R.; De Martino, L. Acinetobacter baumannii: Its Clinical Significance in Human and Veterinary Medicine. Pathogens 2021, 10, 127. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Bartal, C.; Rolston, K.V.I.; Nesher, L. Carbapenem-resistant Acinetobacter baumannii: Colonization, Infection and Current Treatment Options. Infect. Dis. Ther. 2022, 11, 683–694. [Google Scholar] [CrossRef]
- Zhang, S.; Di, L.; Qi, Y.; Qian, X.; Wang, S. Treatment of infections caused by carbapenem-resistant Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 2024, 14, 1395260. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; Van Duin, D.; Clancy, C.J. Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2021, 72, e169–e183. [Google Scholar] [PubMed]
- Dimopoulos, G.; Almyroudi, M.; Kapralos, I.; Apostolopoulou, O.; Flevari, A.; Nicolau, D.; Dokoumetzidis, A. Intrapulmonary pharmacokinetics of high doses of tigecycline in patients with ventilator-associated pneumonia. Int. J. Antimicrob. Agents 2022, 59, 106487. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Bassetti, M.; Bellelli, V.; Bianchi, L.; Marincola Cattaneo, F.; Mazzocchetti, S.; Paciacconi, E.; Cottini, F.; Schiattarella, A.; Tufaro, G. Efficacy of a fosfomycin-containing regimen for treatment of severe pneumonia caused by multidrug-resistant Acinetobacter baumannii: A prospective, observational study. Infect. Dis. Ther. 2021, 10, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, I.; Olesky, M.; Hawser, S.; Lob, S.H.; Karlowsky, J.A.; Corey, G.R.; Bassetti, M.; Fyfe, C. In vitro activity of eravacycline against Gram-negative bacilli isolated in clinical laboratories worldwide from 2013 to 2017. Antimicrob. Agents Chemother. 2020, 64, e01699-19. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, J.H.; Kim, H.J.; Han, S.H.; Jeong, S.H.; Byun, M.K. Colistin monotherapy versus colistin/rifampicin combination therapy in pneumonia caused by colistin-resistant Acinetobacter baumannii: A randomised controlled trial. J. Glob. Antimicrob. Resist. 2019, 17, 66–71. [Google Scholar] [CrossRef]
- Cheng, J.; Yan, J.; Reyna, Z.; Slarve, M.; Lu, P.; Spellberg, B.; Luna, B. Synergistic rifabutin and colistin reduce emergence of resistance when treating Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e02204-20. [Google Scholar] [CrossRef]
- Ong’uti, S.; Czech, M.; Robilotti, E.; Holubar, M. Cefiderocol: A New Cephalosporin Stratagem against Multidrug-Resistant Gram-Negative Bacteria. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 74, 1303–1312. [Google Scholar] [CrossRef]
- Ito, A.; Nishikawa, T.; Matsumoto, S.; Yoshizawa, H.; Sato, T.; Nakamura, R.; Tsuji, M.; Yamano, Y. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 7396–7401. [Google Scholar] [CrossRef]
- Viale, P.; Sandrock, C.E.; Ramirez, P.; Rossolini, G.M.; Lodise, T.P. Treatment of critically ill patients with cefiderocol for infections caused by multidrug-resistant pathogens: Review of the evidence. Ann. Intensive Care 2023, 13, 52. [Google Scholar] [CrossRef]
- Rahman, M.S.; Koh, Y.-S. A novel antibiotic agent, cefiderocol, for multidrug-resistant Gram-negative bacteria. J. Bacteriol. Virol. 2020, 50, 218–226. [Google Scholar] [CrossRef]
- Mezcord, V.; Wong, O.; Pasteran, F.; Corso, A.; Tolmasky, M.E.; Bonomo, R.A.; Ramirez, M.S. Role of β-lactamase inhibitors on cefiderocol activity against carbapenem-resistant Acinetobacter species. Int. J. Antimicrob. Agents 2023, 61, 106700. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.M.; Abdelraouf, K.; Oota, M.; Nakamura, R.; Kuroiwa, M.; Ishioka, Y.; Takemura, M.; Yamano, Y.; Nicolau, D.P. Assessment of sustained efficacy and resistance emergence under human-simulated exposure of cefiderocol against Acinetobacter baumannii using in vitro chemostat and in vivo murine infection models. JAC-Antimicrob. Resist. 2022, 4, dlac047. [Google Scholar] [CrossRef] [PubMed]
- Cook-Libin, S.; Sykes, E.M.; Kornelsen, V.; Kumar, A. Iron Acquisition Mechanisms and Their Role in the Virulence of Acinetobacter baumannii. Infect. Immun. 2022, 90, e00223-22. [Google Scholar] [CrossRef] [PubMed]
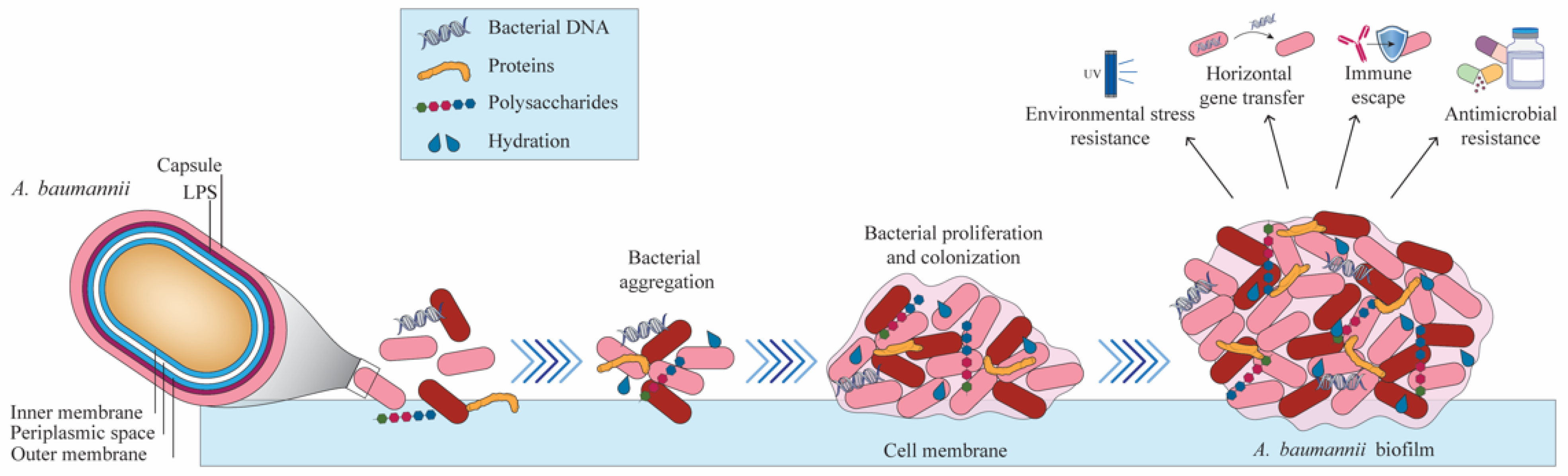
- Gedefie, A.; Demsis, W.; Ashagrie, M.; Kassa, Y.; Tesfaye, M.; Tilahun, M.; Bisetegn, H.; Sahle, Z. Acinetobacter baumannii Biofilm Formation and Its Role in Disease Pathogenesis: A Review. Infect. Drug Resist. 2021, 14, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Mendes, S.G.; Combo, S.I.; Allain, T.; Domingues, S.; Buret, A.G.; Da Silva, G.J. Co-regulation of biofilm formation and antimicrobial resistance in Acinetobacter baumannii: From mechanisms to therapeutic strategies. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1405–1423. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, N.Y.; Ko, S.Y.; Park, S.Y.; Oh, M.H.; Shin, M.S.; Lee, Y.C.; Lee, J.C. Complementary Regulation of BfmRS Two-Component and AbaIR Quorum Sensing Systems to Express Virulence-Associated Genes in Acinetobacter baumannii. Int. J. Mol. Sci. 2022, 23, 13136. [Google Scholar] [CrossRef]
- Jean, S.S.; Hsueh, S.C.; Lee, W.S.; Hsueh, P.R. Cefiderocol: A promising antibiotic against multidrug-resistant Gram-negative bacteria. Expert Rev. Anti-Infect. Ther. 2019, 17, 307–309. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhao, J.; Guan, J.; Ni, W.; Gao, Z. Susceptibility of cefiderocol and other antibiotics against carbapenem-resistant, Gram-negative bacteria. Ann. Transl. Med. 2022, 10, 261. [Google Scholar] [CrossRef]
- Liu, X.; Lei, T.; Yang, Y.; Zhang, L.; Liu, H.; Leptihn, S.; Yu, Y.; Hua, X. Structural Basis of PER-1-Mediated Cefiderocol Resistance and Synergistic Inhibition of PER-1 by Cefiderocol in Combination with Avibactam or Durlobactam in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2022, 66, e00828-22. [Google Scholar] [CrossRef]
- Domingues, S.; Lima, T.; Saavedra, M.J.; Da Silva, G.J. An Overview of Cefiderocol’s Therapeutic Potential and Underlying Resistance Mechanisms. Life 2023, 13, 1427. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Shields, R.K.; Doi, Y.; Takemura, M.; Echols, R.; Matsunaga, Y.; Yamano, Y. Mechanisms of reduced susceptibility to cefiderocol among isolates from the CREDIBLE-CR and APEKS-NP clinical trials. Microb. Drug Resist. 2022, 28, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Yamano, Y.; Ishibashi, N.; Kuroiwa, M.; Takemura, M.; Sheng, W.-H.; Hsueh, P.-R. Characterisation of cefiderocol-non-susceptible Acinetobacter baumannii isolates from Taiwan. J. Glob. Antimicrob. Resist. 2022, 28, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Peerayeh, S.N.; Rostami, E.; Eslami, M.; Rezaee, M.A. High frequency of extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli isolates from male patients’ Urine. Arch. Clin. Infect. Dis. 2016, 11, e60127. [Google Scholar]
- Peerayeh, S.N.; Eslami, M.; Memariani, M.; Siadat, S.D. High prevalence of blaCTX-M-1 group extended-spectrum β-lactamase genes in Escherichia coli isolates from Tehran. Jundishapur J. Microbiol. 2013, 6, e6863. [Google Scholar]
- Bryan, E.J.; Qiao, Q.; Wang, Y.; Roberge, J.Y.; LaVoie, E.J.; Pilch, D.S. A FtsZ Inhibitor That Can Utilize Siderophore-Ferric Iron Uptake Transporter Systems for Activity against Gram-Negative Bacterial Pathogens. Antibiotics 2024, 13, 209. [Google Scholar] [CrossRef]
- Pybus, C.A.; Felder-Scott, C.; Obuekwe, V.; Greenberg, D.E. Cefiderocol Retains Antibiofilm Activity in Multidrug-Resistant Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2021, 65, e01194-20. [Google Scholar] [CrossRef]
- Russo, C.; Mesini, A.; Mariani, M.; Tavella, E.; Sette, C.; Ugolotti, E.; Bartalucci, C.; Palmero, C.; Bandettini, R.; Castagnola, E. Reduce susceptibility to cefiderocol in gram negative bacteria in children: Is hope already lost before it’s even arrived? J. Infect. Public Health 2024, 17, 624–631. [Google Scholar] [CrossRef]
- Lodise, T.P.; Bassetti, M.; Ferrer, R.; Naas, T.; Niki, Y.; Paterson, D.L.; Zeitlinger, M.; Echols, R. All-cause mortality rates in adults with carbapenem-resistant Gram-negative bacterial infections: A comprehensive review of pathogen-focused, prospective, randomized, interventional clinical studies. Expert Rev. Anti-Infect. Ther. 2022, 20, 707–719. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Heil, E.L.; Tamma, P.D. Cefiderocol: The Trojan horse has arrived but will Troy fall? Lancet Infect. Dis. 2021, 21, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-Y.; Ko, W.-C.; Lee, W.-S.; Lu, P.-L.; Chen, Y.-H.; Cheng, S.-H.; Lu, M.-C.; Lin, C.-Y.; Wu, T.-S.; Yen, M.-Y.; et al. In vitro activity of cefiderocol, cefepime/enmetazobactam, cefepime/zidebactam, eravacycline, omadacycline, and other comparative agents against carbapenem-non-susceptible Pseudomonas aeruginosa and Acinetobacter baumannii isolates associated from bloodstream infection in Taiwan between 2018–2020. J. Microbiol. Immunol. Infect. 2022, 55, 888–895. [Google Scholar] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Kanj, S.S.; Bassetti, M.; Kiratisin, P.; Rodrigues, C.; Villegas, M.V.; Yu, Y.; van Duin, D. Clinical data from studies involving novel antibiotics to treat multidrug-resistant Gram-negative bacterial infections. Int. J. Antimicrob. Agents 2022, 60, 106633. [Google Scholar] [CrossRef]
- Shahin, N.P.; Majid, E.; Amin, T.B.A.; Bita, B. Host characteristics and virulence typing of Escherichia coli isolated from diabetic patients. Gene Rep. 2019, 15, 100371. [Google Scholar] [CrossRef]
- Nichols, W.W.; Newell, P.; Critchley, I.A.; Riccobene, T.; Das, S. Avibactam pharmacokinetic/pharmacodynamic targets. Antimicrob. Agents Chemother. 2018, 62, e02446-17. [Google Scholar] [CrossRef]
- Nguyen, M.; Joshi, S. Carbapenem resistance in Acinetobacter baumannii, and their importance in hospital-acquired infections: A scientific review. J. Appl. Microbiol. 2021, 131, 2715–2738. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Oinuma, K.I.; Suzuki, M.; Sakiyama, A.; Tsubouchi, T.; Saeki, K.; Sato, K.; Niki, M.; Yamada, K.; Shibayama, K.; Kakeya, H.; et al. Genomic characterization of triple-carbapenemase-producing Acinetobacter baumannii. JAC Antimicrob. Resist. 2021, 3, dlab191. [Google Scholar] [CrossRef]
- Jean, S.-S.; Chang, Y.-C.; Lin, W.-C.; Lee, W.-S.; Hsueh, P.-R.; Hsu, C.-W. Epidemiology, treatment, and prevention of nosocomial bacterial pneumonia. J. Clin. Med. 2020, 9, 275. [Google Scholar] [CrossRef]
- Feng, W.; Huang, Q.; Wang, Y.; Yuan, Q.; Li, X.; Xia, P.; Sun, F. Changes in the resistance and epidemiological characteristics of Pseudomonas aeruginosa during a ten-year period. J. Microbiol. Immunol. Infect. 2021, 54, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Perencevich, E.; Goto, M.; Alexander, B.; Nair, R.; Puig-Asensio, M.; Ernst, E.; Livorsi, D.J. A comprehensive assessment of carbapenem use across 90 Veterans Health Administration hospitals with defined stewardship strategies for carbapenems. J. Antimicrob. Chemother. 2021, 76, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-D.; Shih, M.-C.; Chuang, Y.-C.; Wang, J.-T.; Sheng, W.-H. Comparative efficacy of doripenem versus meropenem for hospital-acquired and ventilator-associated pneumonia. J. Microbiol. Immunol. Infect. 2019, 52, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, S.; Yousefi, B.; Abdolshahi, A.; Emadi, A.; Eslami, M. Determination of genetic relationship between environmental Escherichia coli with PFGE and investigation of IS element in blaCTX-M gene of these isolates. Microb. Pathog. 2021, 159, 105154. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, B.; Pakdel, A.; Hasanpour, S.; Abdolshahi, A.; Emadi, A.; Pahlevan, D.; Dadashpour, M.; Eslami, M. CTX-M gene and presence of insertion elements in patients with septicemia caused by Escherichia coli. Rev. Res. Med. Microbiol. 2023, 34, 140–148. [Google Scholar]
- Simner, P.J.; Patel, R. Cefiderocol antimicrobial susceptibility testing considerations: The Achilles’ heel of the Trojan horse? J. Clin. Microbiol. 2020, 59, e00951-20. [Google Scholar] [CrossRef]
- Aoki, T.; Yoshizawa, H.; Yamawaki, K.; Yokoo, K.; Sato, J.; Hisakawa, S.; Hasegawa, Y.; Kusano, H.; Sano, M.; Sugimoto, H. Cefiderocol (S-649266), a new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: Structure activity relationship. Eur. J. Med. Chem. 2018, 155, 847–868. [Google Scholar] [CrossRef]
- Simner, P.J.; Beisken, S.; Bergman, Y.; Ante, M.; Posch, A.E.; Tamma, P.D. Defining Baseline Mechanisms of Cefiderocol Resistance in the Enterobacterales. Microb. Drug Resist. 2022, 28, 161–170. [Google Scholar] [CrossRef]
- Pimentel, C.; Le, C.; Tuttobene, M.R.; Subils, T.; Martinez, J.; Sieira, R.; Papp-Wallace, K.M.; Keppetipola, N.; Bonomo, R.A.; Actis, L.A.; et al. Human Pleural Fluid and Human Serum Albumin Modulate the Behavior of a Hypervirulent and Multidrug-Resistant (MDR) Acinetobacter baumannii Representative Strain. Pathogens 2021, 10, 471. [Google Scholar] [CrossRef]
- Meschiari, M.; Volpi, S.; Faltoni, M.; Dolci, G.; Orlando, G.; Franceschini, E.; Menozzi, M.; Sarti, M.; Del Fabro, G.; Fumarola, B.; et al. Real-life experience with compassionate use of cefiderocol for difficult-to-treat resistant Pseudomonas aeruginosa (DTR-P) infections. JAC Antimicrob. Resist. 2021, 3, dlab188. [Google Scholar] [CrossRef] [PubMed]
- Kufel, W.D.; Abouelhassan, Y.; Steele, J.M.; Gutierrez, R.L.; Perwez, T.; Bourdages, G.; Nicolau, D.P. Plasma and cerebrospinal fluid concentrations of cefiderocol during successful treatment of carbapenem-resistant Acinetobacter baumannii meningitis. J. Antimicrob. Chemother. 2022, 77, 2737–2741. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, B.; Escalante, J.; Tuttobene, M.R.; Subils, T.; Mezcord, V.; Pimentel, C.; Georgeos, N.; Pasteran, F.; Rodriguez, C.; Sieira, R.; et al. Acinetobacter baumannii response to cefiderocol challenge in human urine. Sci. Rep. 2022, 12, 8763. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, B.; Escalante, J.; Mezcord, V.; Tuttobene, M.R.; Subils, T.; Actis, L.A.; Pasteran, F.; Tolmasky, M.E.; Bonomo, R.A.; Ramirez, M.S. HSA-induced modifications of Ton-B dependent receptors expression in cefiderocol-exposed carbapenem-resistant Acinetobacter baumannii (CRAB). Int. J. Antimicrob. Agents 2023, 62, 106950. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, H.; Saqib, M.; Khan, W.; Ismail, S.M.; Sohail, H.; Muneeb, M.; Sheikh, S.S. Ventilator associated pneumonia in intensive care unit patients: A systematic review. Ann. Med. Surg. 2023, 85, 2932–2939. [Google Scholar] [CrossRef]
- Alberti, C.; Brun-Buisson, C.; Burchardi, H.; Martin, C.; Goodman, S.; Artigas, A.; Sicignano, A.; Palazzo, M.; Moreno, R.; Boulmé, R.; et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002, 28, 108–121. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E.; Vena, A.; Graziano, E.; Russo, A.; Peghin, M. Risk stratification and treatment of ICU-acquired pneumonia caused by multidrug- resistant/extensively drug-resistant/pandrug-resistant bacteria. Curr. Opin. Crit. Care 2018, 24, 385–393. [Google Scholar] [CrossRef]
- Kidd, J.M.; Kuti, J.L.; Nicolau, D.P. Novel pharmacotherapy for the treatment of hospital-acquired and ventilator-associated pneumonia caused by resistant gram-negative bacteria. Expert Opin. Pharmacother. 2018, 19, 397–408. [Google Scholar] [CrossRef]
- YarMohammadi, M.A.; Eslami, M.; Amirmozafari, N. Investigating the presence of qacA/B and mecA genes in Staphylococcus aureus strains isolated from metro stations in Tehran city of Iran. Rev. Res. Med. Microbiol. 2019, 30, 212–216. [Google Scholar] [CrossRef]
- Timsit, J.F.; Paul, M.; Shields, R.K.; Echols, R.; Baba, T.; Yamano, Y.; Portsmouth, S. Cefiderocol for the treatment of infections due to metallo-B-lactamase–producing pathogens in the CREDIBLE-CR and APEKS-NP phase 3 randomized studies. Clin. Infect. Dis. 2022, 75, 1081–1084. [Google Scholar] [CrossRef]
- Trecarichi, E.M.; Quirino, A.; Scaglione, V.; Longhini, F.; Garofalo, E.; Bruni, A.; Biamonte, E.; Lionello, R.; Serapide, F.; Mazzitelli, M.; et al. Successful treatment with cefiderocol for compassionate use in a critically ill patient with XDR Acinetobacter baumannii and KPC-producing Klebsiella pneumoniae: A case report. J. Antimicrob. Chemother. 2019, 74, 3399–3401. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Cojutti, P.G.; Bartoletti, M.; Tonetti, T.; Bianchini, A.; Ramirez, S.; Pizzilli, G.; Ambretti, S.; Giannella, M.; Mancini, R.; et al. Expert clinical pharmacological advice may make an antimicrobial TDM program for emerging candidates more clinically useful in tailoring therapy of critically ill patients. Crit. Care 2022, 26, 178. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Viaggi, B.; Rossolini, G.M.; Pea, F.; Viale, P. An Evidence-Based Multidisciplinary Approach Focused on Creating Algorithms for Targeted Therapy of Infection-Related Ventilator-Associated Complications (IVACs) Caused by Pseudomonas aeruginosa and Acinetobacter baumannii in Critically Ill Adult Patients. Antibiotics 2021, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Antonini, M.V.; Sica, A.; Dell’Amore, C.; Martino, C.; Gamberini, E.; Bissoni, L.; Circelli, A.; Bolondi, G.; Santonastaso, D.P.; et al. Infection-Related Ventilator-Associated Complications in Critically Ill Patients with Trauma: A Retrospective Analysis. Antibiotics 2023, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, S.; Bala, Y.; Armstrong, T.; García-Castillo, M.; Burnham, C.A.; Wallace, M.A.; Hardy, D.; Zambardi, G.; Cantón, R. Multicenter Evaluation of the New Etest Gradient Diffusion Method for Piperacillin-Tazobactam Susceptibility Testing of Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii Complex. J. Clin. Microbiol. 2020, 58, e01042-19. [Google Scholar] [CrossRef]
- Russo, A.; Bruni, A.; Gullì, S.; Borrazzo, C.; Quirino, A.; Lionello, R.; Serapide, F.; Garofalo, E.; Serraino, R.; Romeo, F.; et al. Efficacy of cefiderocol- vs. colistin-containing regimen for treatment of bacteraemic ventilator-associated pneumonia caused by carbapenem-resistant Acinetobacter baumannii in patients with COVID-19. Int. J. Antimicrob. Agents 2023, 62, 106825. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Karamouzos, V.; Eleftheriotis, G.; Lagadinou, M.; Bartzavali, C.; Kolonitsiou, F.; Paliogianni, F.; Fligou, F.; Marangos, M. Efficacy of Fosfomycin-Containing Regimens for Treatment of Bacteremia Due to Pan-Drug Resistant Acinetobacter baumannii in Critically Ill Patients: A Case Series Study. Pathogens 2023, 12, 286. [Google Scholar] [CrossRef]



| Antibiotic Class | Examples | Mechanism of Action | Resistance Mechanisms | Resistance Status |
|---|---|---|---|---|
| Carbapenems | Imipenem, Meropenem | Inhibit cell wall synthesis by binding to PBPs. |
| High minimum inhibitory concentration (MIC) values in most isolates. |
| Cephalosporins | Ceftazidime, Cefepime | Inhibit cell wall synthesis via PBPs. |
| Widespread resistance, particularly in third-generation. |
| Aminoglycosides | Amikacin, Gentamicin | Bind to bacterial ribosomes and inhibit protein synthesis. |
| Over 72% resistance in isolates. |
| Polymyxins | Polymyxin B, Colistin | Disrupt the outer membrane of bacteria, leading to cell death. |
| Resistance noted, but polymyxin B may show better efficacy. |
| Fluoroquinolones | Ciprofloxacin | Inhibit DNA replication by targeting DNA gyrase and topoisomerase IV. |
| High resistance observed in many isolates. |
| Tetracyclines | Minocycline, Tigecycline | Inhibit protein synthesis by binding to the 30S ribosomal subunit. |
| Minocycline is effective against all isolates; tigecycline variable. |
| Siderophore-Cephalosporins | Cefiderocol | Inhibits cell wall synthesis by binding to PBPs, utilizes bacterial iron transport mechanisms to enter cells |
| Resistance observed in some isolates. |
| Aspect | Details |
|---|---|
| Prevalence of IVACs | Account for approximately 33% of HAPs; the most common mechanical ventilator-associated infection in critically ill patients. |
| Mortality Rate | Often exceeds 50%. |
| Associated Morbidity | Significant morbidity and increasing healthcare costs. |
| Common Pathogens |
|
| Challenges | Emergence of antimicrobial resistance complicates treatment options and increases adverse outcomes; resistant strains lead to severe clinical presentations and poorer prognoses. |
| First-Line Treatment for CRAB | Continuous intravenous infusion of cefiderocol:
|
| Mechanism of Action of Cefiderocol | Novel siderophore cephalosporin that binds to iron receptors on bacterial surfaces, enhancing penetration into bacterial cell walls. |
| Second-Line Treatment Options |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefi, B.; Kashanipoor, S.; Mazaheri, P.; Alibabaei, F.; Babaeizad, A.; Asli, S.; Mohammadi, S.; Gorgin, A.H.; Alipour, T.; Oksenych, V.; et al. Cefiderocol in Combating Carbapenem-Resistant Acinetobacter baumannii: Action and Resistance. Biomedicines 2024, 12, 2532. https://doi.org/10.3390/biomedicines12112532
Yousefi B, Kashanipoor S, Mazaheri P, Alibabaei F, Babaeizad A, Asli S, Mohammadi S, Gorgin AH, Alipour T, Oksenych V, et al. Cefiderocol in Combating Carbapenem-Resistant Acinetobacter baumannii: Action and Resistance. Biomedicines. 2024; 12(11):2532. https://doi.org/10.3390/biomedicines12112532
Chicago/Turabian StyleYousefi, Bahman, Setayesh Kashanipoor, Payman Mazaheri, Farnaz Alibabaei, Ali Babaeizad, Shima Asli, Sina Mohammadi, Amir Hosein Gorgin, Tahereh Alipour, Valentyn Oksenych, and et al. 2024. "Cefiderocol in Combating Carbapenem-Resistant Acinetobacter baumannii: Action and Resistance" Biomedicines 12, no. 11: 2532. https://doi.org/10.3390/biomedicines12112532
APA StyleYousefi, B., Kashanipoor, S., Mazaheri, P., Alibabaei, F., Babaeizad, A., Asli, S., Mohammadi, S., Gorgin, A. H., Alipour, T., Oksenych, V., & Eslami, M. (2024). Cefiderocol in Combating Carbapenem-Resistant Acinetobacter baumannii: Action and Resistance. Biomedicines, 12(11), 2532. https://doi.org/10.3390/biomedicines12112532








