Characterization of Contusive Spinal Cord Injury by Monitoring Motor-Evoked Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Animal Groups
2.3. Anesthesia
- i.
- It is known that most gas anesthesia drugs, like Isoflurane, induce depression of neuronal activities and decrease the evoked response [56,57,58]. Therefore, we used a mixture of Ketamine (75 mg/kg), Xylazine (10 mg/kg), and Atropine (0.3 mg/kg) during our MEP monitoring. It was established that the intra-peritoneal (i.p.) injection of 0.1 mL of the freshly made cocktail containing Ketamine, Xylazine, and Atropine (7.0–1.0–0.5 mixture) 15 min before recording was the best choice of anesthesia for MEP monitoring. Notably, adding Atropine significantly improved MEP signals because Atropine would substantially reduce the risk of cardiac arrhythmia in anesthetized rats [52,55,59].
- ii.
2.4. Addressing Pain
2.5. Skull Implantation of Stimulating Screw Electrode
2.6. Laminectomy
2.7. Spinal Cord Injury
2.8. Post-SCI Care
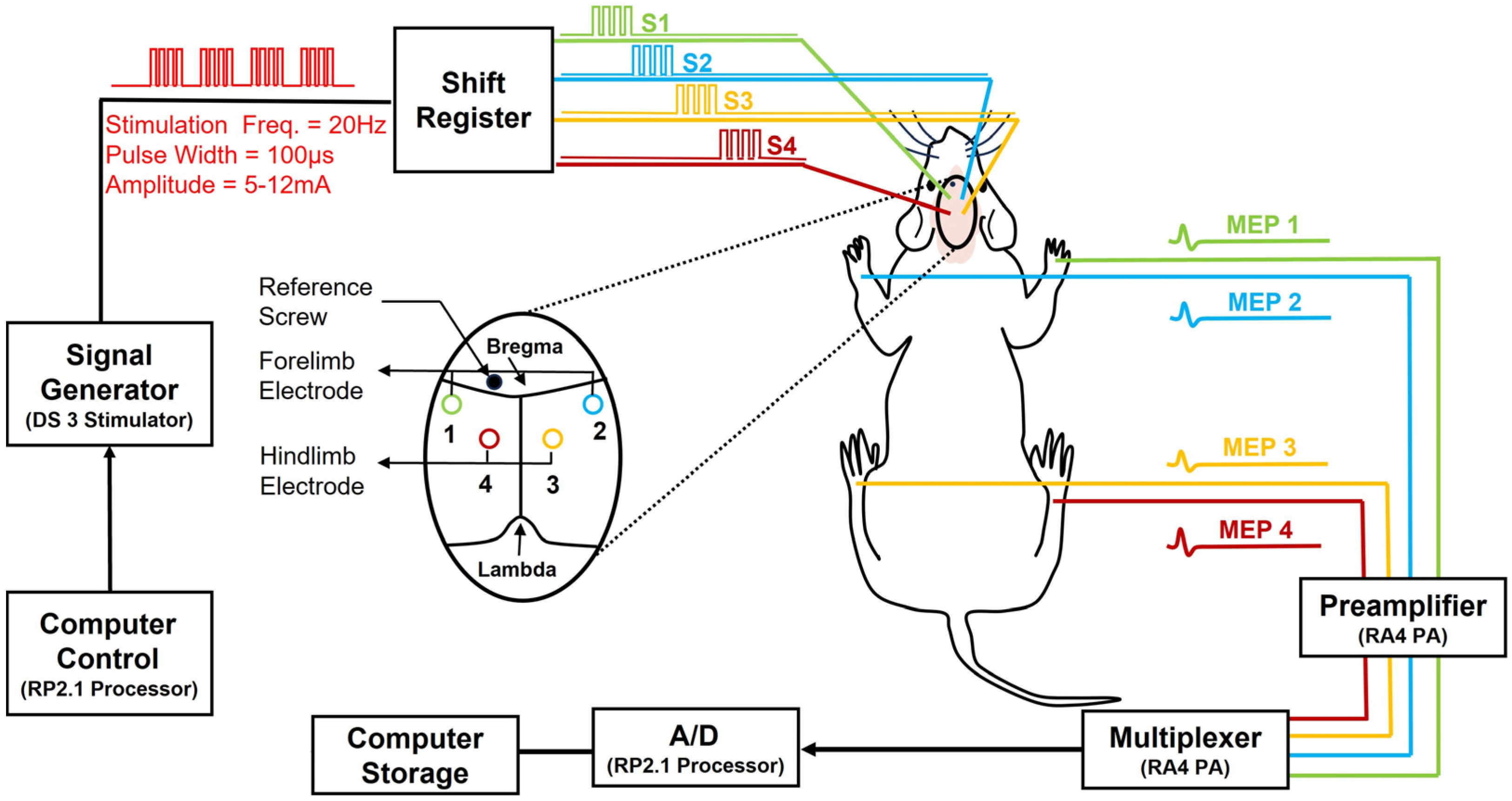
2.9. Neuroelectrophysiology Monitoring—Motor-Evoked Potentials
2.10. Stimulation (Via Skull Screw Electrodes)
2.11. Recording (Via Intra-Muscle Needle Electrodes)
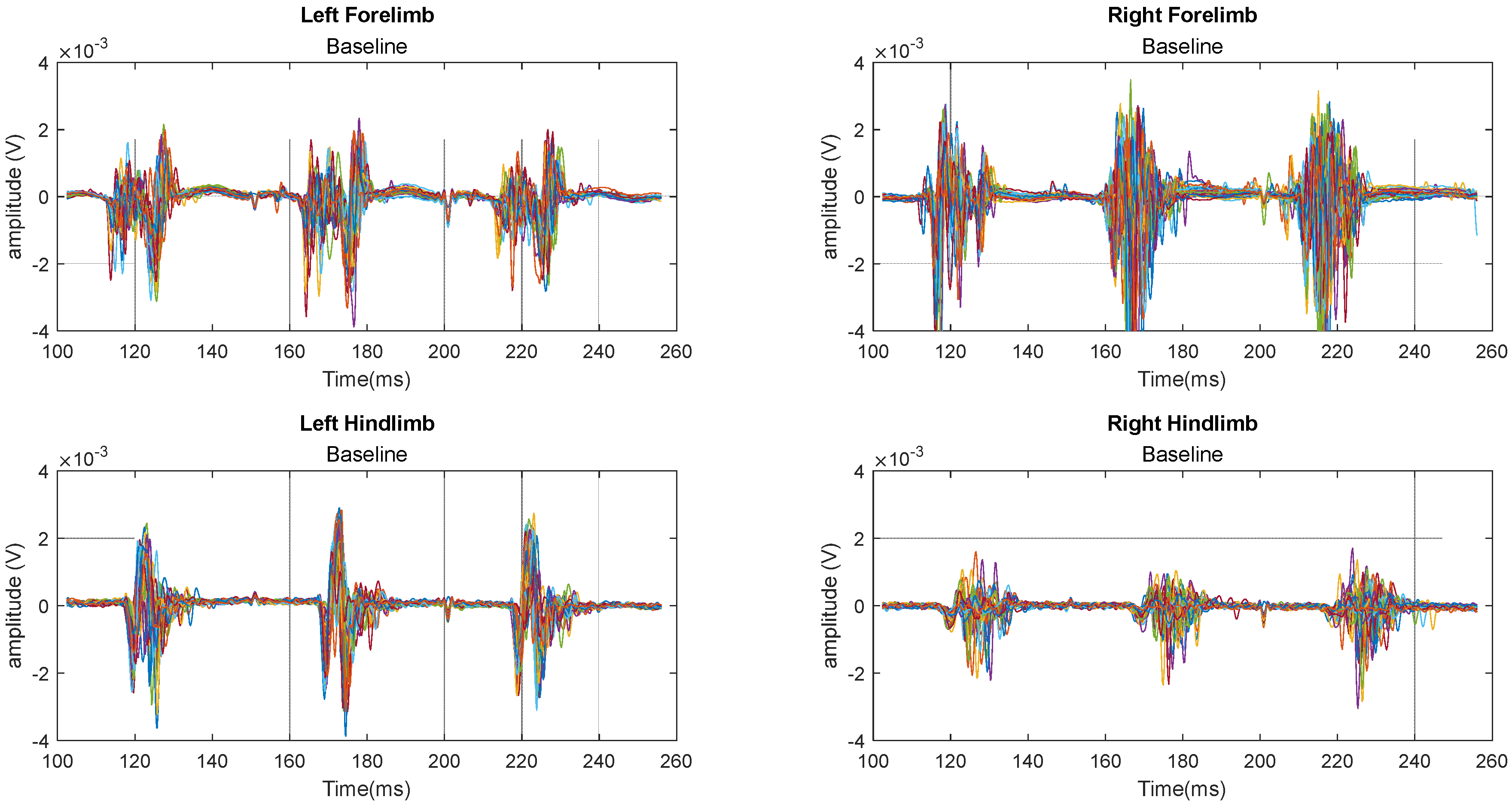
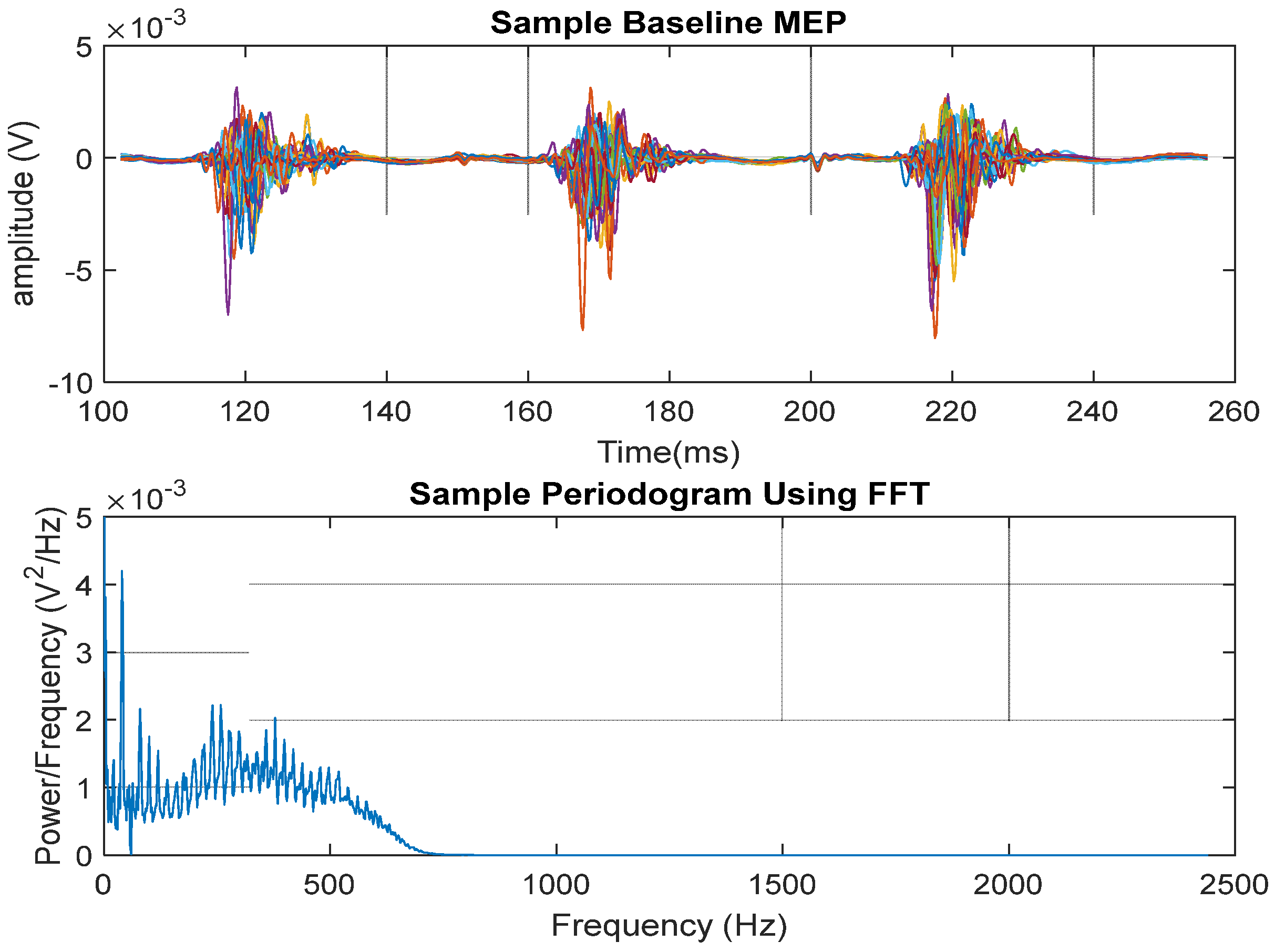
2.12. MEP Signal Presentation
2.13. Statistical Analysis
2.14. Motor Behavioral Assessment
2.15. Histological Examination
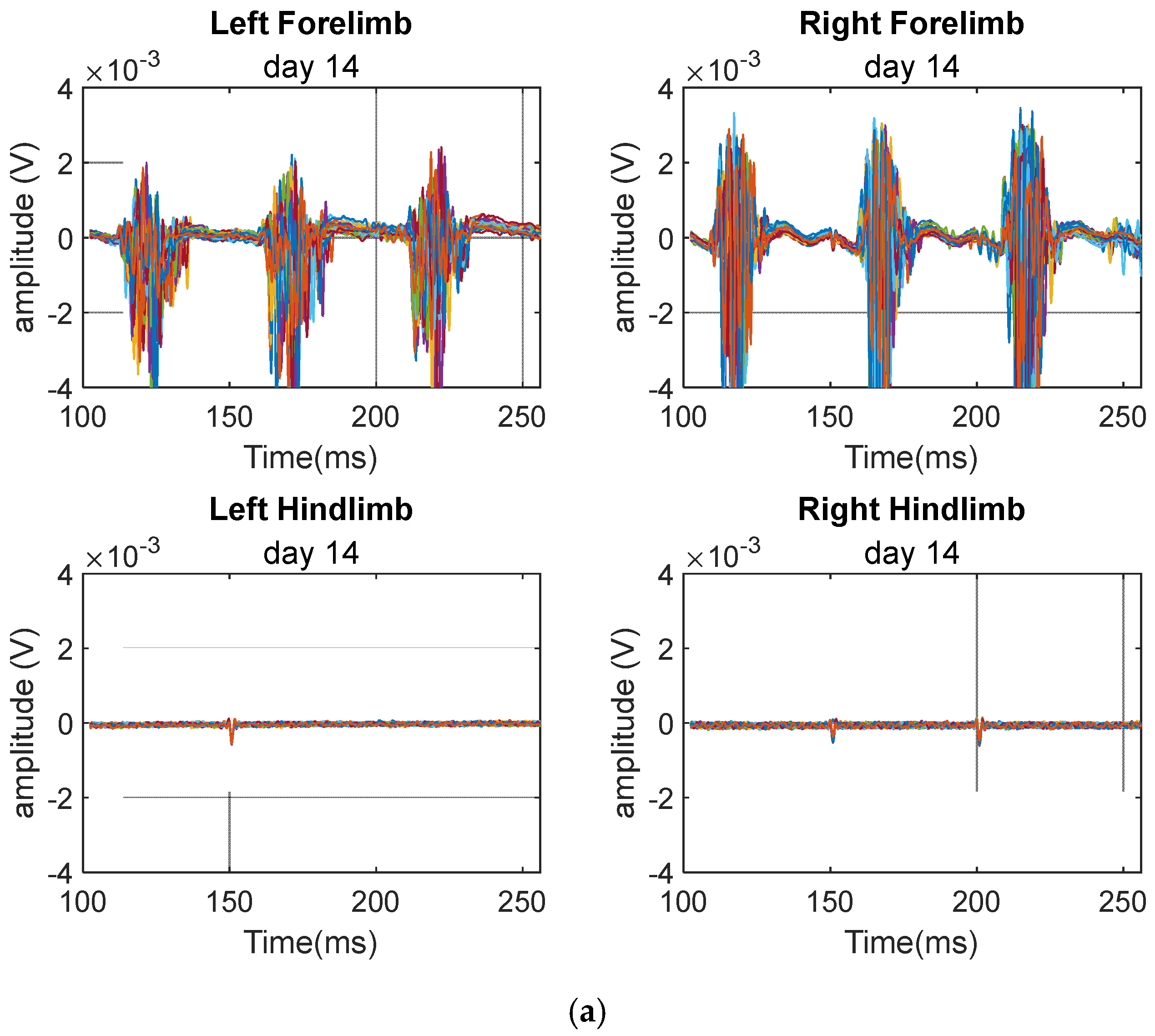
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Spinal Cord Injury (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury (accessed on 16 April 2024).
- Ding, W.; Hu, S.; Wang, P.; Kang, H.; Peng, R.; Dong, Y.; Li, F. Spinal Cord Injury: The Global Incidence, Prevalence, and Disability from the Global Burden of Disease Study 2019. Spine 2022, 47, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, H.; Golpayegani, M.; Ghodsi, Z.; Sadeghi-Naini, M.; Asgardoon, M.; Baigi, V.; Vaccaro, A.R.; Rahimi-Movaghar, V. Direct Cost of Illness for Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2022, 12, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Young, W. Spinal cord contusion models. Prog. Brain Res. 2002, 137, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Kerr, C.; Thakor, N.V.; All, A.H. Characterization of Graded Multicenter Animal Spinal Cord Injury Study Contusion Spinal Cord Injury Using Somatosensory-Evoked Potentials. Spine 2010, 35, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- All, A.H.; Al Nashash, H.; Mir, H.; Luo, S.; Liu, X. Characterization of transection spinal cord injuries by monitoring somatosensory evoked potentials and motor behavior. Brain Res. Bull. 2019, 156, 150–163. [Google Scholar] [CrossRef] [PubMed]
- All, A.H.; Al-Nashash, H. Comparative analysis of functional assessment for contusion and transection models of spinal cord injury. Spinal Cord 2021, 59, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, J.M.; Dabrowski, W.; Dąbrowska-Bouta, B.; Sulkowski, G.; Oakden, W.; Kwiecien-Delaney, C.J.; Yaron, J.R.; Zhang, L.; Schutz, L.; Marzec-Kotarska, B.; et al. Prolonged inflammation leads to ongoing damage after spinal cord injury. PLoS ONE 2020, 15, e0226584. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after spinal cord injury:a review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflam. 2021, 18, 284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, B.; Zhang, Y.; Tian, Y.; Yang, S.; Chen, Y.; Wu, H. Combination of single-cell and bulk RNA seq reveals the immune infiltration landscape and targeted therapeutic drugs in spinal cord injury. Front. Immunol. 2023, 14, 1068359. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S. Pathophysiology of blood-spinal cord barrier in traumatic injury and repair. Curr. Pharm. Des. 2005, 11, 1353–1389. [Google Scholar] [CrossRef] [PubMed]
- Maschmann, C.; Jeppesen, E.; Rubin, M.A.; Barfod, C. New clinical guidelines on the spinal stabilisation of adult trauma patients—Consensus and evidence based. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 77. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.K. Autonomic dysfunction in spinal cord injury: Clinical presentation of symptoms and signs. Prog. Brain Res. 2006, 152, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karsy, M.; Hawryluk, G. Modern Medical Management of Spinal Cord Injury. Curr. Neurol. Neurosci. Rep. 2019, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Al-Nashash, H.; Wong, K.L.; All, A.H. Hypothermia effects on neuronal plasticity post spinal cord injury. PLoS ONE 2024, 19, e0301430. [Google Scholar] [CrossRef] [PubMed]
- Al-Nashash, H.; All, A.H. Neuroprotective Role of Hypothermia in Acute Spinal Cord Injury. Biomedicines 2022, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Maybhate, A.; Hu, C.; Bazley, F.A.; Yu, Q.; Thakor, N.V.; Kerr, C.L.; All, A.H. Potential long-term benefits of acute hypothermia after spinal cord injury: Assessments with somatosensory evoked potentials. Crit. Care Med. 2012, 40, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Teh, D.B.L.; Chua, S.M.; Prasad, A.; Kakkos, I.; Jiang, W.; Yue, M.; Liu, X.; All, A.H. Neuroprotective assessment of prolonged local hypothermia post contusive spinal cord injury in rodent model. Spine J. 2018, 18, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Bazley, F.A.; Pashai, N.; Kerr, C.; Thakor, N.; All, A.H. A simple and effective semi-invasive method for inducing local hypothermia in rat spinal cord. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 2013, 6321–6324. [Google Scholar] [CrossRef] [PubMed]
- Bazley, F.A.; Pashai, N.; Kerr, C.L.; All, A.H. The Effects Long of Local and General Hypothermia on Temperature Profiles of the Central Nervous System Following Spinal Cord Injury in Rats. Ther. Hypothermia Temp. Manag. 2014, 4, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Vipin, A.; Kortelainen, J.; Al-Nashash, H.; Chua, S.M.; Thow, X.; Manivannan, J.; Astrid Thakor, N.V.; Kerr, C.L.; All, A.H. Prolonged Local Hypothermia Has No Long-Term Adverse Effect on the Spinal Cord. Ther. Hypothermia Temp. Manag. 2015, 5, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.L.; Letzen, B.S.; Hill, C.M.; Agrawal, G.; Thakor, N.V.; Sterneckert, J.L.; Gearhart, J.D.; All, A.H. Efficient Differentiation of Human Embryonic Stem Cells into Oligodendrocyte Progenitors for Application in a Rat Contusion Model of Spinal Cord Injury. Int. J. Neurosci. 2010, 120, 305–313. [Google Scholar] [CrossRef] [PubMed]
- All, A.H.; Bazley, F.A.; Gupta, S.; Pashai, N.; Hu, C.; Pourmorteza, A.; Kerr, C. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitors Aid in Functional Recovery of Sensory Pathways following Contusive Spinal Cord Injury. PLoS ONE 2012, 7, e47645. [Google Scholar] [CrossRef] [PubMed]
- All, A.H.; Gharibani, P.; Gupta, S.; Bazley, F.A.; Pashai, N.; Chou, B.K.; Shah, S.; Resar, L.M.; Cheng, L.; Gearhart, J.D.; et al. Early Intervention for Spinal Cord Injury with Human Induced Pluripotent Stem Cells Oligodendrocyte Progenitors. PLoS ONE 2015, 10, e0116933. [Google Scholar] [CrossRef] [PubMed]
- Bazley, F.A.; Pourmorteza, A.; Gupta, S.; Pashai, N.; Kerr, C.; All, A.H. DTI for assessing axonal integrity after contusive spinal cord injury and transplantation of oligodendrocyte progenitor cells. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2012, 2012, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Pashai, N.; Hao, H.; All, A.; Gupta, S.; Chaerkady, R.; De Los Angeles, A.; Gearhart, J.D.; Kerr, C.L. Genome-wide profiling of pluripotent cells reveals a unique molecular signature of human embryonic germ cells. PLoS ONE 2012, 7, e39088. [Google Scholar] [CrossRef] [PubMed]
- Bazley, F.A.; Liu, C.F.; Yuan, X.; Hao, H.; All, A.H.; De Los Angeles, A.; Zambidis, E.T.; Gearhart, J.D.; Kerr, C.L. Direct Reprogramming of Human Primordial Germ Cells into Induced Pluripotent Stem Cells: Efficient Generation of Genetically Engineered Germ Cells. Stem Cells Dev. 2015, 24, 2634–2648. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Teh, D.B.; Shah Jahan, F.R.; Manivannan, J.; Chua, S.M.; All, A.H. Direct Conversion Through Trans-Differentiation: Efficacy and Safety. Stem Cells Dev. 2017, 26, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Manivannan, J.; Loong, D.T.; Chua, S.M.; Gharibani, P.M.; All, A.H. A review of induced pluripotent stem cell, direct conversion by trans-differentiation, direct reprogramming and oligodendrocyte differentiation. Regen. Med. 2016, 11, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Walczak, P.; All, A.H.; Rumpal, N.; Gorelik, M.; Kim, H.; Maybhate, A.; Agrawal, G.; Campanelli, J.T.; Gilad, A.A.; Kerr, D.A.; et al. Human glial-restricted progenitors survive, proliferate, and preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination. Glia 2010, 59, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xu, H.; Zuo, Y.; Liu, X.; All, A.H. A Review of Functional Electrical Stimulation Treatment in Spinal Cord Injury. Neuromol. Med. 2020, 22, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Blasiak, A.; Agrawal, D.R.; Loong, D.T.B.; Thakor, N.V.; All, A.H.; Ho, J.S.; Yang, I.H. Subcellular electrical stimulation of neurons enhances the myelination of axons by oligodendrocytes. PLoS ONE 2017, 12, e0179642. [Google Scholar] [CrossRef] [PubMed]
- Rejc, E.; Angeli, C.A.; Bryant, N.; Harkema, S.J. Effects of Stand and Step Training with Epidural Stimulation on Motor Function for Standing in Chronic Complete Paraplegics. J. Neurotrauma. 2017, 34, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Al-Nashash, H.; Luo, S.; Liu, X.; All, A.H. Trading baseline with forelimbs somatosensory evoked potential for longitudinal analysis in thoracic transection spinal cord injury. J. Neurosci. Methods 2020, 343, 108858. [Google Scholar] [CrossRef] [PubMed]
- All, A.H.; Walczak, P.; Agrawal, G.; Gorelik, M.; Lee, C.; Thakor, N.V.; Bulte, J.W.; Kerr, D.A. Effect of MOG sensitization on somatosensory evoked potential in Lewis rats. J. Neurol. Sci. 2009, 284, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Sherman, D.L.; Walczak, P.; Bulte, J.W.M.; Thakor, N.V.; Kerr, D.A.; All, A.H. Shape analysis of Somatosensory Evoked Potentials to detect a focal spinal cord lesion. In Proceedings of the IEEE 35th Annual Northeast Bioengineering Conference, Cambridge, MA, USA, 3–5 April 2009; pp. 1–2. [Google Scholar] [CrossRef]
- Agrawal, G.; Sherman, D.; Thakor, N.; All, A. A novel shape analysis technique for somatosensory evoked potentials. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 4688–4691. [Google Scholar] [CrossRef] [PubMed]
- Al-Nashash, H.; Fatoo, N.A.; Mirza, N.N.; Ahmed, R.I.; Agrawal, G.; Thakor, N.V.; All, A.H. Spinal Cord Injury Detection and Monitoring Using Spectral Coherence. IEEE Trans. Biomed. Eng. 2009, 56, 1971. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Sherman, D.; Maybhate, A.; Gorelik, M.; Kerr, D.A.; Thakor, N.V.; All, A.H. Slope analysis of somatosensory evoked potentials in spinal cord injury for detecting contusion injury and focal demyelination. J. Clin. Neurosci. 2010, 17, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.L.; Wuyyuru, V.; Brooke, M.J.; Zhang, H.X.; Sepkuty, J.P.; Thakor, N.V.; Natarajan, A.; All, A.H. Spinal cord integrity monitoring by adaptive coherence measurement. J. Neurosci. Methods 2010, 193, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Vayrynen, E.; Noponen, K.; Vipin, A.; Thow, X.Y.; Al-Nashash, H.; Kortelainen, J.; All, A. Automatic Parametrization of Somatosensory Evoked Potentials With Chirp Modeling. IEEE Trans. Neural. Syst. Rehabil. Eng. 2016, 24, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Mir, H.; Al-Nashash, H.; Kortelainen, J.; All, A. Assessment of Spinal Cord Injury via Sparse Modeling of Somatosensory Evoked Potential Signals. In Proceedings of the Asia Modelling Symposium (AMS), Kota Kinabalu, Malaysia, 4–6 December 2017; pp. 13–17. [Google Scholar] [CrossRef]
- Mir, H.; Al-Nashash, H.; Kerr, D.; All, A.; Thakor, N. Using Variations of Somatosensory Evoked Potentials to Quantify Spinal Cord Injury Level. Engineering 2013, 5, 99–102. [Google Scholar] [CrossRef]
- Mir, H.; Al-Nashash, H.; Kortelainen, J.; All, A. Novel Modeling of Somatosensory Evoked Potentials for the Assessment of Spinal Cord Injury. IEEE Trans. Biomed. Eng. 2018, 65, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Mir, H.; Al-Nashash, H.; Kerr, D.; All, A.; Thakor, N. Spinal cord injury evaluation using morphological difference of somatosensory evoked potentials. In Proceedings of the 2011 5th International Conference on Bioinformatics and Biomedical Engineering, Wuhan, China, 10–12 May 2011; pp. 1–4. [Google Scholar] [CrossRef]
- Mir, H.; Al-Nashash, H.; All, A.; Thakor, N.V. Quantification of Spinal Cord Injury Level Using Somatosensory Evoked Potentials. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Mir, H.; Al-Nashash, H.; Kerr, D.; Thakor, N.; All, A. Histogram based quantification of spinal cord injury level using somatosensory evoked potentials. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2010, 2010, 4942–4945. [Google Scholar] [CrossRef] [PubMed]
- All, A.H.; Luo, S.; Liu, X.; Al-Nashash, H. Effect of thoracic spinal cord injury on forelimb somatosensory evoked potential. Brain Res. Bull. 2021, 173, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Maybhate, A.; Presacco, A.; All, A.H. Multi-limb acquisition of motor evoked potentials and its application in spinal cord injury. J. Neurosci. Methods 2010, 193, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Iyer, S.; All, A.H. A comparative study of recording procedures for motor evoked potential signals. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 2086–2089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Oweis, Y.; Mozaffari-Naeini, H.; Venkatesha, S.; Thakor, N.V.; Natarajan, A. Continuous Quantitative Motor Evoked Potentials for Spinal Cord Injury Detection. Conference Proceedings. In Proceedings of the 2nd International IEEE EMBS Conference on Neural Engineering, 2005, Arlington, VA, USA, 16–19 March 2005; pp. 430–433. [Google Scholar] [CrossRef]
- Bazley, F.A.; Hu, C.; Maybhate, A.; Pourmorteza, A.; Pashai, N.; Thakor, N.V.; Kerr, C.L.; All, A.H. Electrophysiological evaluation of sensory and motor pathways after incomplete unilateral spinal cord contusion. J. Neurosurg. Spine 2012, 16, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Sloan, T.; Jäntti, V. Anesthetic effects on evoked potentials. Handb. Clin. Neurophysiol. 2008, 8, 94–126. [Google Scholar] [CrossRef]
- Kortelainen, J.; Vipin, A.; Thow Xin Yuan Mir, H.; Thakor, N.; Al-Nashash, H.; All, A. Effect of isoflurane on somatosensory evoked potentials in a rat model. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 2014, 4286–4289. [Google Scholar] [CrossRef] [PubMed]
- Kortelainen, J.; Al-Nashash, H.; Vipin, A.; Thow, X.Y.; All, A. The effect of anesthesia on somatosensory evoked potential measurement in a rat model. Lab. Anim. 2015, 50, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; Knight, J.; Precious, S.; Simpkin, S. Ketamine alone and combined with diazepam or xylazine in laboratory animals: A 10 year experience. Lab. Anim. 1981, 15, 163–170. [Google Scholar] [CrossRef] [PubMed]
- All, A.H.; Agrawal, G.; Walczak, P.; Maybhate, A.; Bulte, J.W.; Kerr, D.A. Evoked potential and behavioral outcomes for experimental autoimmune encephalomyelitis in Lewis rats. Neurol. Sci. 2010, 31, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Thakor, N.V.; All, A.H. Evoked potential versus behavior to detect minor insult to the spinal cord in a rat model. J. Clin. Neurosci. 2009, 16, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Filipp, M.E.; Travis, B.J.; Henry, S.S.; Idzikowski, E.C.; Magnuson, S.A.; Loh, M.Y.; Hellenbrand, D.J.; Hanna, A.S. Differences in neuroplasticity after spinal cord injury in varying animal models and humans. Neural Regen. Res. 2019, 14, 7–19. [Google Scholar] [CrossRef]
- Eidelberg, E.; Woolf, B.; Kreinick, C.J.; Davis, F. Role of the dorsal funiculi in movement control. Brain Res. 1976, 114, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Loy, D.N.; Talbott, J.F.; Onifer, S.M.; Mills, M.D.; Burke, D.A.; Dennison, J.B.; Fajardo, L.C.; Magnuson, D.S.; Whittemore, S.R. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp. Neurol. 2002, 177, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Vipin, A.; Thow, X.Y.; Mir, H.; Kortelainen, J.; Manivannan, J.; Al-Nashash, H.; All, A.H. Natural Progression of Spinal Cord Transection Injury and Reorganization of Neural Pathways. J. Neurotrauma 2016, 33, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Mir, H.; Al-Nashash, H.; Thow, X.Y.; Kortelainen, J.; Soo Min, C.; Manivannan, J.; Astrid All, A.H. Assessment of bilateral SSEP signals enhancement following transectional spinal cord injury using linear modelling. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Toronto, Canada, 7–12 June 2015; p. 1219. [Google Scholar] [CrossRef]
- Bazley, F.A.; Maybhate, A.; Tan, C.S.; Thakor, N.V.; Kerr, C.; All, A.H. Enhancement of Bilateral Cortical Somatosensory Evoked Potentials to Intact Forelimb Stimulation Following Thoracic Contusion Spinal Cord Injury in Rats. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Bazley, F.A.; All, A.H.; Thakor, N.V.; Maybhate, A. Plasticity associated changes in cortical somatosensory evoked potentials following spinal cord injury in rats. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 2011, 2005–2008. [Google Scholar] [CrossRef] [PubMed]
- Teh, D.B.L.; Prasad, A.; Jiang, W.; Ariffin, M.Z.; Khanna, S.; Belorkar, A.; Wong, L.; Liu, X.; All, A.H. Transcriptome Analysis Reveals Neuroprotective aspects of Human Reactive Astrocytes induced by Interleukin 1β. Sci. Rep. 2017, 7, 13988. [Google Scholar] [CrossRef] [PubMed]







| (a) Laminectomy | ||||||
| Sum Square | DF1 | DF2 | Mean Square | F | p-Value | |
| Left forelimb | 5.8506 | 6 | 24 | 0.9751 | 0.9396 | 0.4854 |
| Left hindlimb | 4.2453 | 6 | 24 | 0.7076 | 1.5972 | 0.1912 |
| Right forelimb | 6.3737 | 6 | 24 | 1.0623 | 0.7982 | 0.5807 |
| Right hindlimb | 1.3232 | 6 | 24 | 0.2205 | 0.3172 | 0.9216 |
| (b) Injury | ||||||
| Sum Square | DF1 | DF2 | Mean Square | F | p-Value | |
| Left forelimb | 11.1982 | 6 | 36 | 1.866371 | 1.558701 | 0.187609 |
| Left hindlimb | 4.7869 | 6 | 36 | 0.797819 | 169.0465 | 7.59 × 10−25 |
| Right forelimb | 6.1898 | 6 | 36 | 1.031638 | 0.956734 | 0.467872 |
| Right hindlimb | 3.5699 | 6 | 36 | 0.594991 | 55.50016 | 1.00 × 10−16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ALL, A.H.; Wong, K.-L.; Al-Nashash, H.A. Characterization of Contusive Spinal Cord Injury by Monitoring Motor-Evoked Potential. Biomedicines 2024, 12, 2548. https://doi.org/10.3390/biomedicines12112548
ALL AH, Wong K-L, Al-Nashash HA. Characterization of Contusive Spinal Cord Injury by Monitoring Motor-Evoked Potential. Biomedicines. 2024; 12(11):2548. https://doi.org/10.3390/biomedicines12112548
Chicago/Turabian StyleALL, Angelo H., Ka-Leung Wong, and Hasan A. Al-Nashash. 2024. "Characterization of Contusive Spinal Cord Injury by Monitoring Motor-Evoked Potential" Biomedicines 12, no. 11: 2548. https://doi.org/10.3390/biomedicines12112548
APA StyleALL, A. H., Wong, K.-L., & Al-Nashash, H. A. (2024). Characterization of Contusive Spinal Cord Injury by Monitoring Motor-Evoked Potential. Biomedicines, 12(11), 2548. https://doi.org/10.3390/biomedicines12112548







