Effect of SARS-CoV-2 Infection on Selected Parameters of the Apelinergic System in Repeat Blood Donors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Samples Collection
- -
- The donor had to have a confirmed (positive result of RT-PCR test) SARS-CoV-2 infection in the past;
- -
- The donor had to be healthy in each study period, i.e., not be a carrier and have no symptoms of COVID-19 or any other disease;
- -
- The donor had to meet the basic donation criteria regarding their age, weight, blood pressure, heart rate, and hemoglobin concentration (described in the regulation of the Minister of Health [22]).
2.2. Apelinergic System Parameters Determination
2.3. Data Analysis
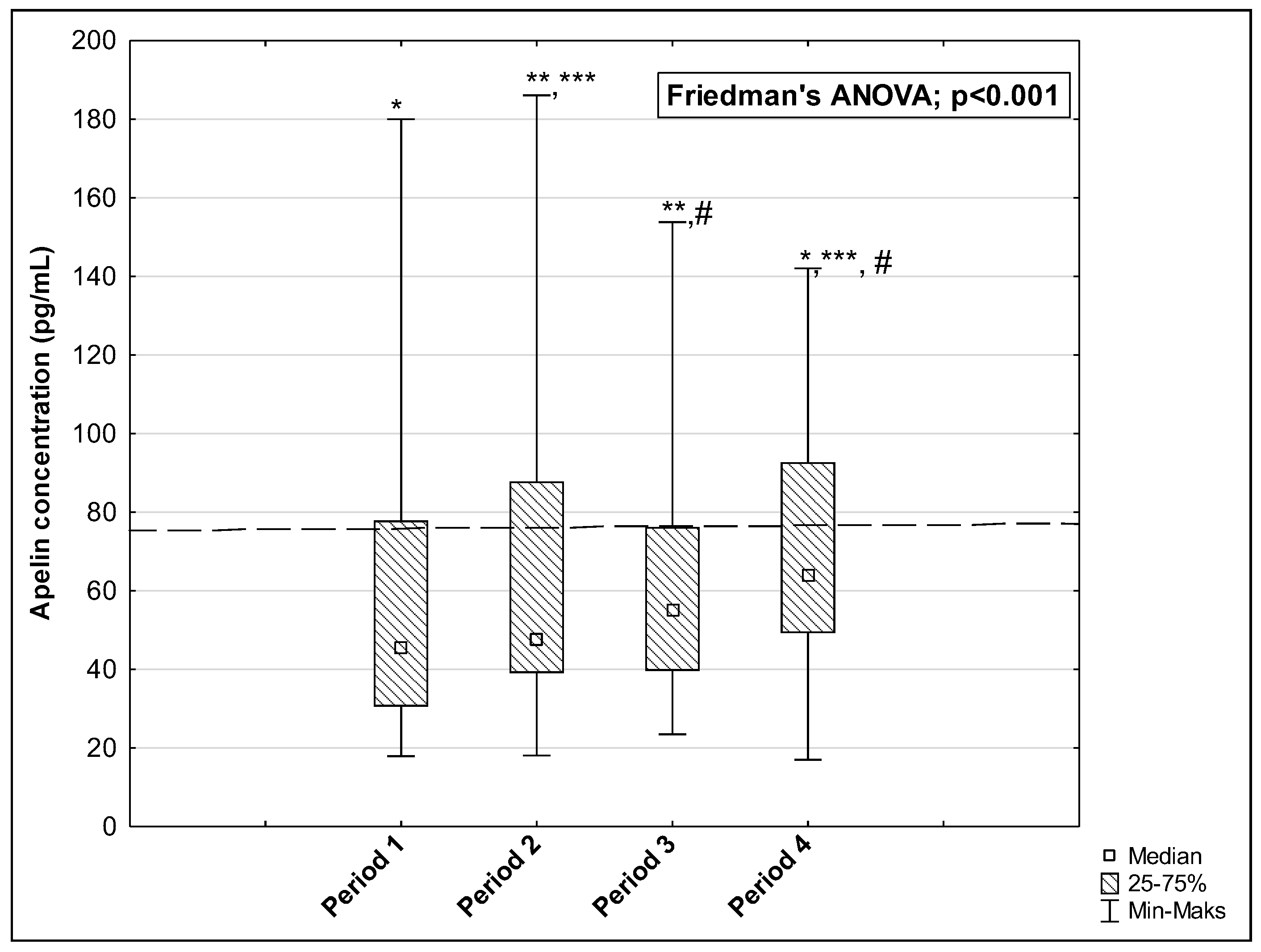
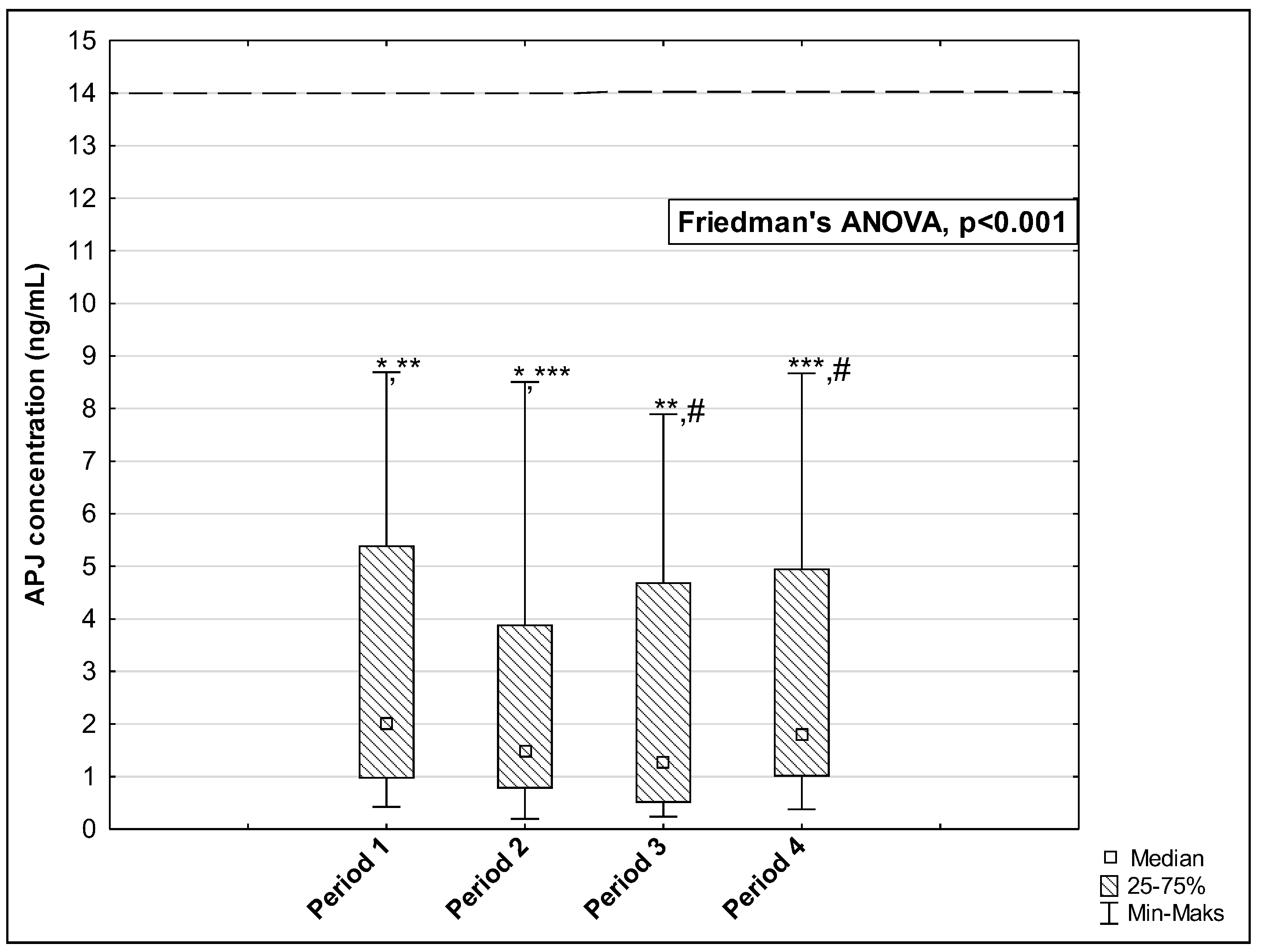
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wahid, M.; Jawed, A.; Mandal, R.K.; Areeshi, M.Y.; El-Shall, N.A.; Mohapatra, R.K.; Tuli, H.S.; Dhama, K.; Pellicano, R.; Fagoonee, S.; et al. Role of Available COVID-19 Vaccines in Reducing Deaths and Perspective for next Generation Vaccines and Therapies to Counter Emerging Viral Variants: An Update. Minerva Med. 2023, 114, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 Vaccines for Their Characteristics, Efficacy and Effectiveness against SARS-CoV-2 and Variants of Concern: A Narrative Review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Postlethwait, J.H.; Massaquoi, M.S.; Farnsworth, D.R.; Yan, Y.L.; Guillemin, K.; Miller, A.C. The SARS-CoV-2 Receptor and Other Key Components of the Renin-Angiotensin-Aldosterone System Related to COVID-19 Are Expressed in Enterocytes in Larval Zebrafish. Biol. Open 2021, 10, bio058172. [Google Scholar] [CrossRef] [PubMed]
- Jami, G.; Ataee, M.; Esmaeili, V.; Chamani, S.; Rezaei, A.; Naghizadeh, A. Characterization of the Angiotensin-Converting Enzyme 2 (ACE2), the Main Receptor for the SARS-CoV-2 Virus. Am. J. Clin. Exp. Immunol. 2023, 12, 24–44. [Google Scholar] [PubMed]
- Mehrabadi, M.E.; Hemmati, R.; Tashakor, A.; Homaei, A.; Yousefzadeh, M.; Hemati, K.; Hosseinkhani, S. Induced Dysregulation of ACE2 by SARS-CoV-2 Plays a Key Role in COVID-19 Severity. Biomed. Pharmacother. 2021, 137, 111363. [Google Scholar] [CrossRef]
- Maza, M.d.C.; Úbeda, M.; Delgado, P.; Horndler, L.; Llamas, M.A.; van Santen, H.M.; Alarcón, B.; Abia, D.; García-Bermejo, L.; Serrano-Villar, S.; et al. ACE2 Serum Levels as Predictor of Infectability and Outcome in COVID-19. Front. Immunol. 2022, 13, 836516. [Google Scholar] [CrossRef]
- Yang, P.; Kuc, R.E.; Brame, A.L.; Dyson, A.; Singer, M.; Glen, R.C.; Cheriyan, J.; Wilkinson, I.B.; Davenport, A.P.; Maguire, J.J. [Pyr1]Apelin-13(1-12) Is a Biologically Active ACE2 Metabolite of the Endogenous Cardiovascular Peptide [Pyr1]Apelin-13. Front. Neurosci. 2017, 11, 246158. [Google Scholar] [CrossRef]
- Park, J.; Park, M.Y.; Kim, Y.; Jun, Y.; Lee, U.; Oh, C.M. Apelin as a New Therapeutic Target for COVID-19 Treatment. QJM Int. J. Med. 2023, 116, 197–204. [Google Scholar] [CrossRef]
- Saeedi Saravi, S.S.; Beer, J.H. Apelin-Potential Therapy for COVID-19? J. Mol. Cell. Cardiol. 2020, 145, 84–87. [Google Scholar] [CrossRef]
- Wyderka, R.; Diakowska, D.; Łoboz-Rudnicka, M.; Mercik, J.; Borger, M.; Osuch, Ł.; Brzezińska, B.; Leśków, A.; Krzystek-Korpacka, M.; Jaroch, J. Influence of the Apelinergic System on Conduction Disorders in Patients after Myocardial Infarction. J. Clin. Med. 2023, 12, 7603. [Google Scholar] [CrossRef]
- Shin, K.; Kenward, C.; Rainey, J.K. Apelinergic System Structure and Function. Compr. Physiol. 2017, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Chapman, F.A.; Maguire, J.J.; Newby, D.E.; Davenport, A.P.; Dhaun, N. Targeting the Apelin System for the Treatment of Cardiovascular Diseases. Cardiovasc. Res. 2023, 119, 2683–2696. [Google Scholar] [CrossRef] [PubMed]
- İşbil, E.N.; Şahintürk, S.; Demirel, S. Vascular Functional Effects of the Apelinergic System. J. Lit. Pharm. Sci. 2021, 10, 12–20. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Zhang, Z.; Cao, Y.; Liu, X.; Miao, R. Abnormal Apelin-ACE2 and SGLT2 Signaling Contribute to Adverse Cardiorenal Injury in Patients with COVID-19. Int. J. Cardiol. 2020, 336, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kenoosh, H.A.; Kenoosh, A.; Awad, M.M. Comparative Study of Apelin and Glucose Levels in COVID-19 Patients. Int. J. Health Sci. 2022, 6, 6428–6438. [Google Scholar] [CrossRef]
- Rostamzadeh, F.; Najafipour, H.; Yazdani, R.; Nakhaei, S.; Alinaghi Langari, A. Changes in Serum Levels of Apelin and Nitric Oxide in Hospitalized Patients with COVID-19: Association with Hypertension, Diabetes, Obesity, and Severity of Disease. Eur. J. Med. Res. 2022, 27, 243. [Google Scholar] [CrossRef]
- Gadhi, M.H.; Saleh, E.S. Measurement of the Serum Level of Elabela for the Early Detection of Acute Kidney Injury in Hospitalized Iraqi COVID-19 Patients. J. Fac. Med. Baghdad 2022, 64, 163–169. [Google Scholar] [CrossRef]
- Van Den Hurk, K.; Zalpuri, S.; Prinsze, F.J.; Merz, E.M.; De Kort, W.L.A.M. Associations of Health Status with Subsequent Blood Donor Behavior—An Alternative Perspective on the Healthy Donor Effect from Donor InSight. PLoS ONE 2017, 12, e0186662. [Google Scholar] [CrossRef]
- Su, S.; Sun, Y.; Gu, X.; Wu, W.; Su, X.; Ma, T.; Song, A.; Xie, X.; Wang, L.; Cheng, Q.; et al. Exploration of the Healthy Donor Effect Among 0.6 Million Blood Donors in China: Longitudinal Study. JMIR Public Health Surveill. 2024, 10, e48617. [Google Scholar] [CrossRef]
- Brodersen, T.; Rostgaard, K.; Lau, C.J.; Juel, K.; Erikstrup, C.; Nielsen, K.R.; Ostrowski, S.R.; Titlestad, K.; Sækmose, S.G.; Pedersen, O.B.V.; et al. The Healthy Donor Effect and Survey Participation, Becoming a Donor and Donor Career. Transfusion 2023, 63, 143–155. [Google Scholar] [CrossRef]
- Stanek, M.; Leśków, A.; Szymczyk-Nużka, M.; Wojciechowska-Chorębała, A.; Diakowska, D. Evaluation of Serological and Hematological Parameters in Donors of SARS-CoV-2 Convalescent Plasma of Respect to Time Periods of Donation. Acta Haematol. Pol. 2024, 55, 157–167. [Google Scholar] [CrossRef]
- Rozporządzenie Ministra Zdrowia w Sprawie Warunków Pobierania Krwi Od Kandydatów Na Dawców Krwi i Dawców Krwi; Dziennik Ustaw Rzeczypospolitej Polskiej poz. 1741: Polska. 2017. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170001741 (accessed on 19 August 2024).
- Song, J.; Tang, J.; Zhang, Z.; Liu, Y.; Zhong, J. Targeting the Elabela/Apelin-Apelin Receptor Axis as a Novel Therapeutic Approach for Hypertension. Chin. Med. J. 2022, 135, 1019. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, D.; Wang, M.; Wang, Q.; Kouznetsova, J.; Yang, R.; Qian, K.; Wu, W.; Shuldiner, A.; Sztalryd, C.; et al. Elabela-Apelin Receptor Signaling Pathway Is Functional in Mammalian Systems. Sci. Rep. 2015, 5, 8170. [Google Scholar] [CrossRef] [PubMed]
- Marzoog, B.A. Recent Advances in Molecular Biology of Metabolic Syndrome Pathophysiology: Endothelial Dysfunction as a Potential Therapeutic Target. J. Diabetes Metab. Disord. 2022, 21, 1903–1911. [Google Scholar] [CrossRef]
- He, X.; Liu, C.; Peng, J.; Li, Z.; Li, F.; Wang, J.; Hu, A.; Peng, M.; Huang, K.; Fan, D.; et al. COVID-19 Induces New-Onset Insulin Resistance and Lipid Metabolic Dysregulation via Regulation of Secreted Metabolic Factors. Signal Transduct. Target. Ther. 2021, 6, 427. [Google Scholar] [CrossRef]
- Berber, N.K.; Geçkil, A.A.; Altan, N.Ö.; Kıran, T.R.; Otlu, Ö.; Erdem, M.; İn, E. Efficacy of Serum Apelin and Galectin-3 as Potential Predictors of Mortality in Severe COVID-19 Patients. J. Med. Virol. 2023, 95, e28494. [Google Scholar] [CrossRef]
- Reindl-Schwaighofer, R.; Hödlmoser, S.; Eskandary, F.; Poglitsch, M.; Bonderman, D.; Strassl, R.; Aberle, J.H.; Oberbauer, R.; Zoufaly, A.; Hecking, M. ACE2 Elevation in Severe COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 1191–1196. [Google Scholar] [CrossRef]
- Mcgrail, J.; Martín-Banderas, L.; Durán-Lobato, M. Cannabinoids as Emergent Therapy Against COVID-19. Cannabis Cannabinoid Res. 2022, 7, 582. [Google Scholar] [CrossRef]
- Mehta, N.; Kalra, A.; Nowacki, A.S.; Anjewierden, S.; Han, Z.; Bhat, P.; Carmona-Rubio, A.E.; Jacob, M.; Procop, G.W.; Harrington, S.; et al. Association of Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1020–1026. [Google Scholar] [CrossRef]
- Yang, P.; Read, C.; Kuc, R.E.; Buonincontri, G.; Southwood, M.; Torella, R.; Upton, P.D.; Crosby, A.; Sawiak, S.J.; Carpenter, T.A.; et al. Elabela/Toddler Is an Endogenous Agonist of the Apelin APJ Receptor in the Adult Cardiovascular System, and Exogenous Administration of the Peptide Compensates for the Downregulation of Its Expression in Pulmonary Arterial Hypertension. Circulation 2017, 135, 1160–1173. [Google Scholar] [CrossRef]
- Mermutluoglu, C.; Tekin, R. Evaluation of Elabela, Visfatin, and Chemerin Levels as Inflammation Biomarkers in COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 11180–11184. [Google Scholar] [PubMed]



| Control Group n = 30 | Study Group n = 30 | p-Value | |
|---|---|---|---|
| Gender: | 0.717 | ||
| Man | 25 (83.3%) | 26 (86.7%) | |
| Woman | 5 (16.7%) | 4 (13.3%) | |
| Age in years; | 0.226 | ||
| Median [IQR] | 37 [34–42] | 44 [32–47] | |
| Titer of anti-SARS-CoV-2 antibodies: | - | - | |
| Low (<500) | 10 (33.3%) | ||
| High (>500) | 20 (66.6%) |
| Control Group (n = 30) | Period 1 | Period 4 | p-Value (Control vs. Period 1; Mann–Whitney Test) | p-Value (Control vs. Period 4; Mann–Whitney Test) | p-Value (Period 1 vs. Period 4, Post Hoc Test) | |
|---|---|---|---|---|---|---|
| AP (pg/mL) | 75.50 [59.50–126.16] | 45.37 [30.60–77.91] | 63.82 [49.30–92.70] | 0.005 * | 0.191 | 0.004 * |
| ELA (pg/mL) | 1398.12 [923.75–1943.75] | 1071.50 [920.00–1182.00] | 1255.50 [1150.00–1365.00] | 0.039 * | 0.445 | <0.001 * |
| APJ (ng/mL) | 13.95 [5.43–20.05] | 2.01 [0.96–5.39] | 1.82 [1.00–4.96] | <0.001 * | <0.001 * | 0.100 |
| Age (Years) | Sex | Titer of Anti-SARS-CoV-2 Antibodies | AP | ELA | APJ | |
|---|---|---|---|---|---|---|
| Age (years) | 1 | |||||
| Sex | R = −0.164 p = 0.385 | 1 | ||||
| Titer of anti-SARS-CoV-2 antibodies | R = 0.102 p = 0.590 | R = −0.346 p = 0.060 | 1 | |||
| AP | R = 0.283 p = 0.128 | R = −0.022 p = 0.905 | R = 0.057 p = 0.764 | 1 | ||
| ELA | R = −0.012 p = 0.945 | R = 0.181 p = 0.337 | R = 0.187 p = 0.319 | R = 0.129 p = 0.495 | 1 | |
| APJ | R = 0.123 p = 0.515 | R = 0.101 p = 0.591 | R = −0.38 p = 0.464 | R = 0.566 p = 0.001 * | R = 0.285 p = 0.126 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanek, M.; Leśków, A.; Diakowska, D. Effect of SARS-CoV-2 Infection on Selected Parameters of the Apelinergic System in Repeat Blood Donors. Biomedicines 2024, 12, 2583. https://doi.org/10.3390/biomedicines12112583
Stanek M, Leśków A, Diakowska D. Effect of SARS-CoV-2 Infection on Selected Parameters of the Apelinergic System in Repeat Blood Donors. Biomedicines. 2024; 12(11):2583. https://doi.org/10.3390/biomedicines12112583
Chicago/Turabian StyleStanek, Marta, Anna Leśków, and Dorota Diakowska. 2024. "Effect of SARS-CoV-2 Infection on Selected Parameters of the Apelinergic System in Repeat Blood Donors" Biomedicines 12, no. 11: 2583. https://doi.org/10.3390/biomedicines12112583
APA StyleStanek, M., Leśków, A., & Diakowska, D. (2024). Effect of SARS-CoV-2 Infection on Selected Parameters of the Apelinergic System in Repeat Blood Donors. Biomedicines, 12(11), 2583. https://doi.org/10.3390/biomedicines12112583





