Murine vs. Human Osteoblast Responses to Coagulation and Inflammatory Factors: Reconsidering the Use of Animal Models in Hemophilia A Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Growth Conditions
2.2. Coagulation Factors and Cytokines
2.3. Experimental Setup
2.4. Cell Viability
2.5. Alizarin Red S Staining and Photometric Quantification
2.6. Statistical Analysis
3. Results
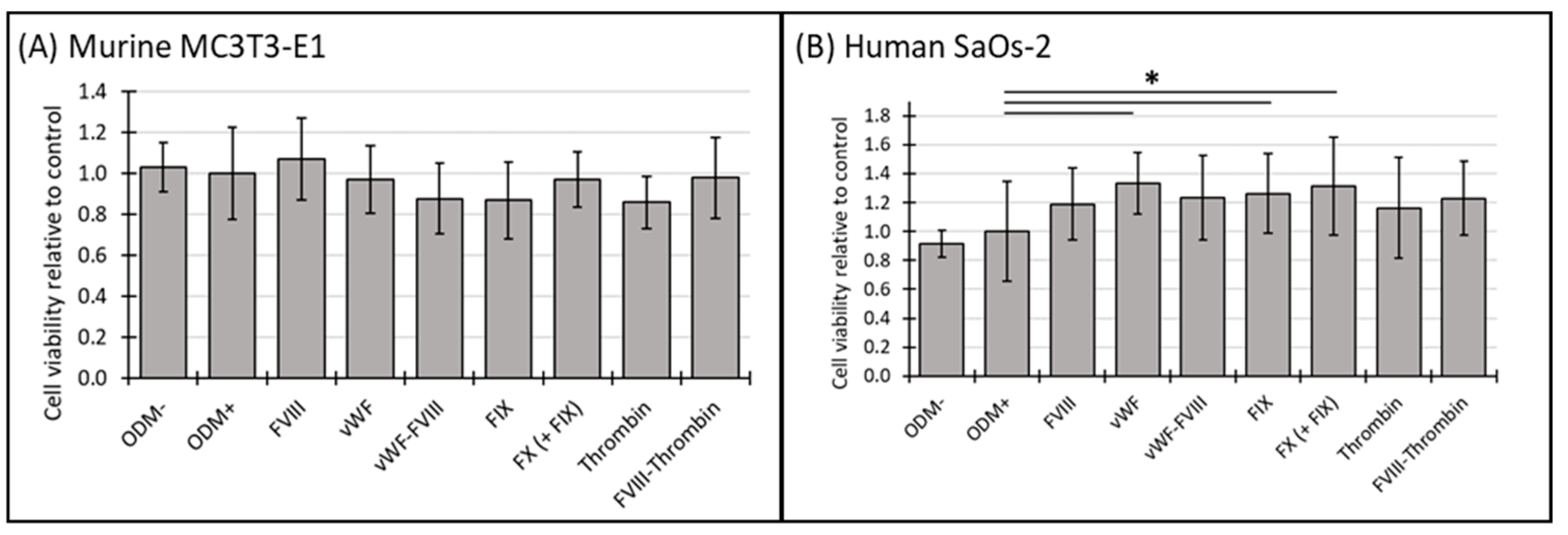
3.1. Cell Viability of Murine MC3T3-E1 and Human SaOs-2 Osteoblasts After Stimulation with Different Coagulation Factors
3.2. Mineralization of Murine MC3T3-E1 and Human SaOs-2 Osteoblasts After Stimulation with Different Coagulation Factors
3.3. Mineralization Relative to Cell Viability of Murine MC3T3-E1 and Human SaOs-2 Osteoblasts After Stimulation with Different Coagulation Factors
3.4. Cell Viability of Murine MC3T3-E1 and Human SaOs-2 Osteoblasts After Stimulation with Cytokines
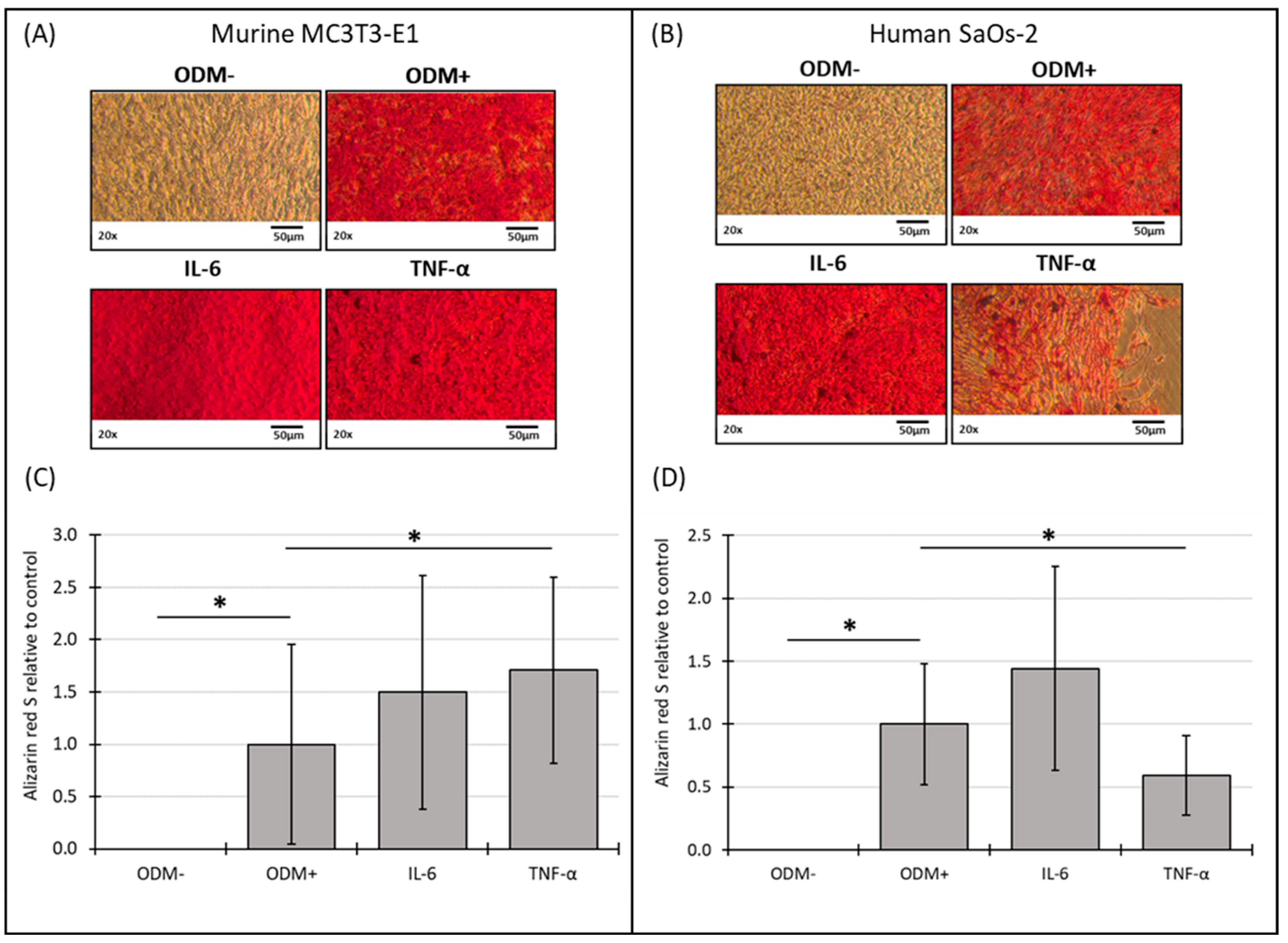
3.5. Mineralization of Murine MC3T3-E1 and Human SaOs-2 Osteoblasts After Stimulation with Cytokines
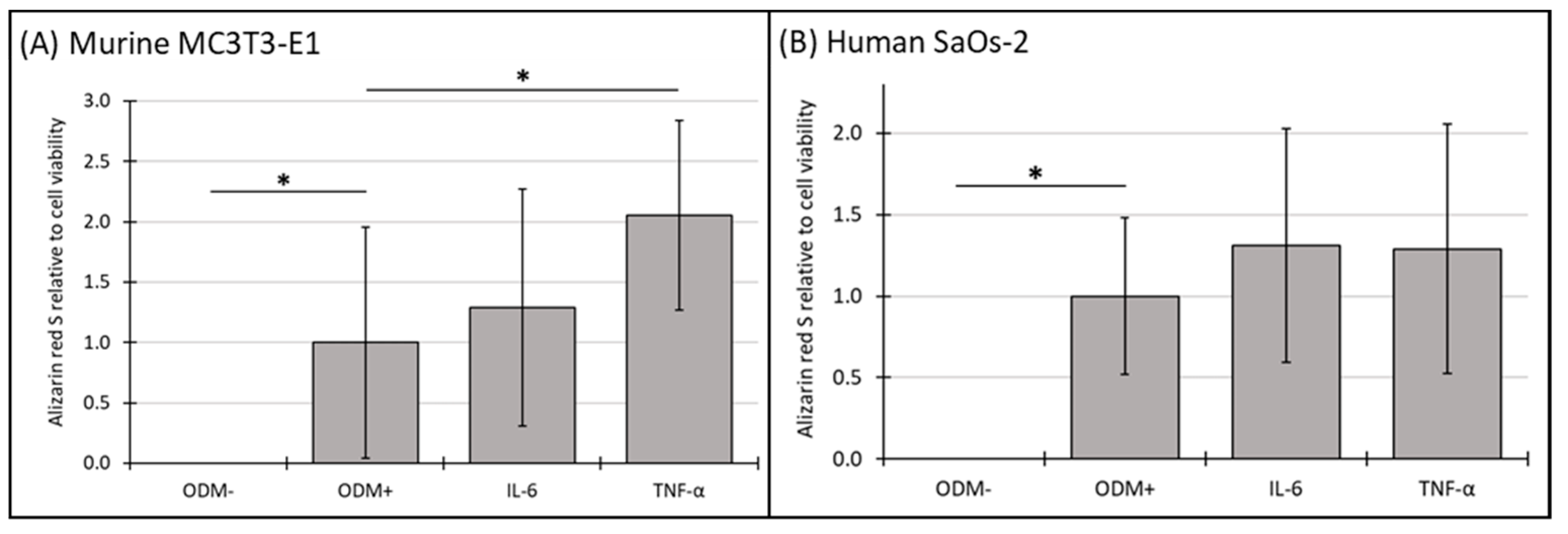
3.6. Mineralization Relative to Cell Viability of Murine MC3T3-E1 and Human SaOs-2 Osteoblasts After Stimulation with Cytokines
4. Discussion
4.1. Coagulation Factors and Species-Specific Responses
4.2. Coagulation Factors and Mineralization
4.3. Cytokines and Osteoblast Function
4.4. Implications for Hemophilia A Research
4.5. Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebetsberger, J.; Schirmer, M.; Wurzer, W.J.; Streif, W. Low Bone Mineral Density in Hemophiliacs. Front. Med. 2022, 9, 794456. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, G.; Damiano, M.L.; Tom, A.; Worman, C.; Schultz, W.; Recht, M.; Stopeck, A.T. Prevalence and risk factors associated with decreased bone mineral density in patients with haemophilia. Haemophilia 2009, 15, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Samuelson Bannow, B.; Recht, M.; Négrier, C.; Hermans, C.; Berntorp, E.; Eichler, H.; Mancuso, M.E.; Klamroth, R.; O’Hara, J.; Santagostino, E.; et al. Factor VIII: Long-established role in haemophilia A and emerging evidence beyond haemostasis. Blood Rev. 2019, 35, 43–50. [Google Scholar] [CrossRef]
- Daubie, V.; De Decker, R.; Nicaise, C.; Pochet, R. Osteosarcoma cell-calcium signaling through tissue factor-factor VIIa complex and factor Xa. FEBS Lett. 2007, 581, 2611–2615. [Google Scholar] [CrossRef]
- Pagel, C.N.; Sivagurunathan, S.; Loh, L.H.; Tudor, E.M.; Pike, R.N.; Mackie, E.J. Functional responses of bone cells to thrombin. Biol. Chem. 2006, 387, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Ichikawa, J.; Wako, M.; Ohba, T.; Saito, M.; Sato, H.; Koyama, K.; Hagino, T.; Schoenecker, J.G.; Ando, T.; et al. Thrombin induced by the extrinsic pathway and PAR-1 regulated inflammation at the site of fracture repair. Bone 2016, 83, 23–34. [Google Scholar] [CrossRef]
- Tatakis, D.N.; Dolce, C.; Dziak, R. Thrombin’s effects on osteoblastic cells. I. Cytosolic calcium and phosphoinositides. Biochem. Biophys. Res. Commun. 1989, 164, 119–127. [Google Scholar] [CrossRef]
- Pagel, C.N.; De Niese, M.R.; Abraham, L.A.; Chinni, C.; Song, S.J.; Pike, R.N.; Mackie, E.J. Inhibition of osteoblast apoptosis by thrombin. Bone 2003, 33, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Lv, Y.; Yan, Q.; Guo, W. Cytokines: The links between bone and the immune system. Injury 2024, 55, 111203. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Nauman, P.; Mandat, T.; Paradowska-Gorycka, A.; Romanowska-Próchnicka, K.; Szukiewicz, D.; Kotela, A.; Kubaszewski, Ł.; Kotela, I.; et al. Cytokines in the pathogenesis of hemophilic arthropathy. Cytokine Growth Factor Rev. 2018, 39, 71–91. [Google Scholar] [CrossRef]
- Lau, A.G.; Sun, J.; Hannah, W.B.; Livingston, E.W.; Heymann, D.; Bateman, T.A.; Monahan, P.E. Joint bleeding in factor VIII deficient mice causes an acute loss of trabecular bone and calcification of joint soft tissues which is prevented with aggressive factor replacement. Haemophilia 2014, 20, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Liel, M.S.; Klein, R.; Recht, M.; Greenberg, D.L.; Taylor, J. Reduced Bone Mineral Density in Factor VIII Deficient Mice and the Role of Inflammatory Cytokines. Blood 2011, 118, 21. [Google Scholar] [CrossRef]
- Liel, M.S.; Greenberg, D.L.; Recht, M.; Vanek, C.; Klein, R.F.; Taylor, J.A. Decreased bone density and bone strength in a mouse model of severe factor VIII deficiency. Br. J. Haematol. 2012, 158, 140–143. [Google Scholar] [CrossRef]
- Recht, M.; Liel, M.S.; Turner, R.T.; Klein, R.F.; Taylor, J.A. The bone disease associated with factor VIII deficiency in mice is secondary to increased bone resorption. Haemophilia 2013, 19, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Taves, S.; Sun, J.; Livingston, E.W.; Chen, X.; Amiaud, J.; Brion, R.; Hannah, W.B.; Bateman, T.A.; Heymann, D.; Monahan, P.E. Hemophilia A and B mice, but not VWF(-/-)mice, display bone defects in congenital development and remodeling after injury. Sci. Rep. 2019, 9, 14428. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Roser-Page, S.; Vikulina, T.; Weiss, D.; Hao, L.; Baldwin, W.H.; Yu, K.; Del Mazo Arbona, N.; McGee-Lawrence, M.E.; Meeks, S.L.; et al. Reduced bone formation in males and increased bone resorption in females drive bone loss in hemophilia A mice. Blood Adv. 2019, 3, 288–300. [Google Scholar] [CrossRef]
- Rydell-Törmänen, K.; Johnson, J.R. The Applicability of Mouse Models to the Study of Human Disease. Methods Mol. Biol. 2019, 1940, 3–22. [Google Scholar] [CrossRef]
- Jilka, R.L. The relevance of mouse models for investigating age-related bone loss in humans. J. Gerontology. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1209–1217. [Google Scholar] [CrossRef]
- Proudfoot, D. Calcium Signaling and Tissue Calcification. Cold Spring Harb. Perspect. Biol. 2019, 11, a035303. [Google Scholar] [CrossRef]
- Blair, H.C.; Schlesinger, P.H.; Huang, C.L.; Zaidi, M. Calcium signalling and calcium transport in bone disease. Sub-Cell. Biochem. 2007, 45, 539–562. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Bootman, M.D.; Taylor, C.W.; Mackie, E.J.; Stone, S.R. Characterization of the receptor responsible for thrombin-induced intracellular calcium responses in osteoblast-like cells. J. Biol. Chem. 1993, 268, 21432–21437. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.M.; Monroe, D.M.; Gailani, D. Mouse models of hemostasis. Platelets 2020, 31, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Borensztajn, K.; Spek, C.A. Targeting coagulation factor receptors–protease-activated receptors in idiopathic pulmonary fibrosis. J. Thromb. Haemost. 2017, 15, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Hogan, K.A.; Weiler, H.; Lord, S.T. Mouse models in coagulation. Thromb. Haemost. 2002, 87, 563–574. [Google Scholar] [CrossRef]
- Yen, C.T.; Fan, M.N.; Yang, Y.L.; Chou, S.C.; Yu, I.S.; Lin, S.W. Current animal models of hemophilia: The state of the art. Thromb J. 2016, 14 (Suppl. S1), 22. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Bernar, A.; Gebetsberger, J.V.; Bauer, M.; Streif, W.; Schirmer, M. Optimization of the Alizarin Red S Assay by Enhancing Mineralization of Osteoblasts. Int. J. Mol. Sci. 2022, 24, 723. [Google Scholar] [CrossRef]
- Emeis, J.J.; Jirouskova, M.; Muchitsch, E.M.; Shet, A.S.; Smyth, S.S.; Johnson, G.J. A guide to murine coagulation factor structure, function, assays, and genetic alterations. J. Thromb. Haemost. JTH 2007, 5, 670–679. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Yao, Q.; He, L.; Bao, C.; Yan, X.; Ao, J. The role of TNF-α in osteoporosis, bone repair and inflammatory bone diseases: A review. Tissue Cell 2024, 89, 102422. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. TNF-α and IL-6: The Link between Immune and Bone System. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed]
- Ueda, O.; Tateishi, H.; Higuchi, Y.; Fujii, E.; Kato, A.; Kawase, Y.; Wada, N.A.; Tachibe, T.; Kakefuda, M.; Goto, C.; et al. Novel genetically-humanized mouse model established to evaluate efficacy of therapeutic agents to human interleukin-6 receptor. Sci. Rep. 2013, 3, 1196. [Google Scholar] [CrossRef]
- Qing, H.; Desrouleaux, R.; Israni-Winger, K.; Mineur, Y.S.; Fogelman, N.; Zhang, C.; Rashed, S.; Palm, N.W.; Sinha, R.; Picciotto, M.R.; et al. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell 2020, 182, 1660. [Google Scholar] [CrossRef]
- Hammacher, A.; Ward, L.D.; Weinstock, J.; Treutlein, H.; Yasukawa, K.; Simpson, R.J. Structure-function analysis of human IL-6: Identification of two distinct regions that are important for receptor binding. Protein Sci. 1994, 3, 2280–2293. [Google Scholar] [CrossRef]
- Okamoto, K.; Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, a031245. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. Past, present and future of hemophilia: A narrative review. Orphanet J. Rare Dis. 2012, 7, 24. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernar, A.; Bauer, M.; Schirmer, M.; Streif, W.; Gebetsberger, J. Murine vs. Human Osteoblast Responses to Coagulation and Inflammatory Factors: Reconsidering the Use of Animal Models in Hemophilia A Research. Biomedicines 2024, 12, 2666. https://doi.org/10.3390/biomedicines12122666
Bernar A, Bauer M, Schirmer M, Streif W, Gebetsberger J. Murine vs. Human Osteoblast Responses to Coagulation and Inflammatory Factors: Reconsidering the Use of Animal Models in Hemophilia A Research. Biomedicines. 2024; 12(12):2666. https://doi.org/10.3390/biomedicines12122666
Chicago/Turabian StyleBernar, Aline, Monika Bauer, Michael Schirmer, Werner Streif, and Jennifer Gebetsberger. 2024. "Murine vs. Human Osteoblast Responses to Coagulation and Inflammatory Factors: Reconsidering the Use of Animal Models in Hemophilia A Research" Biomedicines 12, no. 12: 2666. https://doi.org/10.3390/biomedicines12122666
APA StyleBernar, A., Bauer, M., Schirmer, M., Streif, W., & Gebetsberger, J. (2024). Murine vs. Human Osteoblast Responses to Coagulation and Inflammatory Factors: Reconsidering the Use of Animal Models in Hemophilia A Research. Biomedicines, 12(12), 2666. https://doi.org/10.3390/biomedicines12122666





