Anti-Tetanus Vaccination Is Associated with Reduced Occurrence and Slower Progression of Parkinson’s Disease—A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design
2.3. Cohort Definition
2.4. Diphtheria-Tetanus Toxoid Vaccinations
2.5. Data Preparation
2.6. Statistical Analyses
2.7. Machine Learning Model for Disease Severity Tracking
3. Results
3.1. The Study Cohort
| PD Patients N = 1446 | Control N = 7230 | p | Odds Ratio | ||
|---|---|---|---|---|---|
| Gender | Female | 655 (45.3%) | 3275 (45.3%) | 1.000 | 1 |
| Male | 791 (54.7%) | 3955 (54.7%) | 1.000 | 1 | |
| Age (years) | 65.7 ± 7.2 | 65.6 ± 7.3 | 0.537 | ||
| Age category | 45–49 | 47 (3.25%) | 243 (3.36%) | 0.873 | 0.97 |
| 50–59 | 247 (17.08%) | 1249 (17.28%) | 0.879 | 0.99 | |
| 60–69 | 591 (40.87%) | 3081 (42.61%) | 0.232 | 0.93 | |
| 70- | 561 (38.80%) | 2657 (36.74%) | 0.136 | 1.09 | |
| Weight (kg) | 77.1 ± 15.8 | 78.1 ± 15.7 | 0.026 | ||
| Missing | 29 (2.01%) | 109 (1.51%) | |||
| Height (cm) | 165 ± 12 | 165 ± 11 | 0.264 | ||
| Missing | 53 (3.67%) | 182 (2.52%) | |||
| BMI (kg/m2) | 28.3 ± 5.1 | 28.6 ± 5.1 | 0.035 | ||
| Missing | 55 (3.80%) | 188 (2.60%) | |||
| BMI category | <18.5 Underweight | 17 (1.22%) | 55 (0.78%) | 0.110 | 1.57 |
| 18.5–24.9 Normal | 330 (23.72%) | 1618 (22.98%) | 0.554 | 1.04 | |
| 25–29.9 Overweight | 581 (41.77%) | 2951 (41.91%) | 0.929 | 0.99 | |
| ≥30 Obese | 463 (33.29%) | 2418 (34.34%) | 0.458 | 0.95 | |
| Missing | 55 (3.80%) | 188 (2.60%) | 0.014 | 1.48 | |
| BP systolic (mmHg) | 134 ± 25 | 133 ± 21 | 0.246 | ||
| Missing | 4 (0.28%) | 41 (0.57%) | |||
| BP diastolic (mmHg) | 78.2 ± 9.6 | 78.0 ± 9.5 | 0.491 | ||
| Missing | 5 (0.35%) | 45 (0.62%) | |||
| Smoking status | Non-smoker | 1155 (79.88%) | 5478 (75.77%) | 0.001 | 1.27 |
| Past smoker | 43 (2.97%) | 275 (3.80%) | 0.145 | 0.78 | |
| Smoker | 132 (9.13%) | 1051 (14.54%) | <0.001 | 0.59 | |
| Missing | 116 (8.02%) | 426 (5.89%) | 0.003 | 1.39 | |
| Socio-economic status (1–20) | 9.72 ± 3.40 | 9.73 ± 3.40 | 0.948 | ||
| Missing | 74 (5.12%) | 370 (5.12%) | |||
| eGFR MDRD (mL/min/1.73 m2) | 79.4 ± 20.2 | 80.6 ± 24.5 | 0.089 | ||
| Missing | 132 (9.13%) | 844 (11.67%) | |||
| Glucose (mg/dL) | 111 ± 32 | 109 ± 33 | 0.107 | ||
| Missing | 127 (8.78%) | 807 (11.16%) | |||
| Hemoglobin A1c (%) | 6.22 ± 1.10 | 6.22 ± 1.16 | 0.945 | ||
| Missing | 418 (28.9%) | 2490 (34.4%) | |||
| Record of prior tetanus–diphtheria toxoid vaccination | 22 (1.52%) | 216 (2.99%) | 0.001 | 0.50 | |
3.2. Vaccination Is Associated with Decreased Risk of PD Occurrence
3.3. PD Risk in Vaccinated Patients Is Associated with Time Elapsed Since Vaccination

3.4. Vaccinated PD Patients Have Slower Rates of Disease Progression

3.5. Disease Progression Is Associated with Time Elapsed Since Vaccination
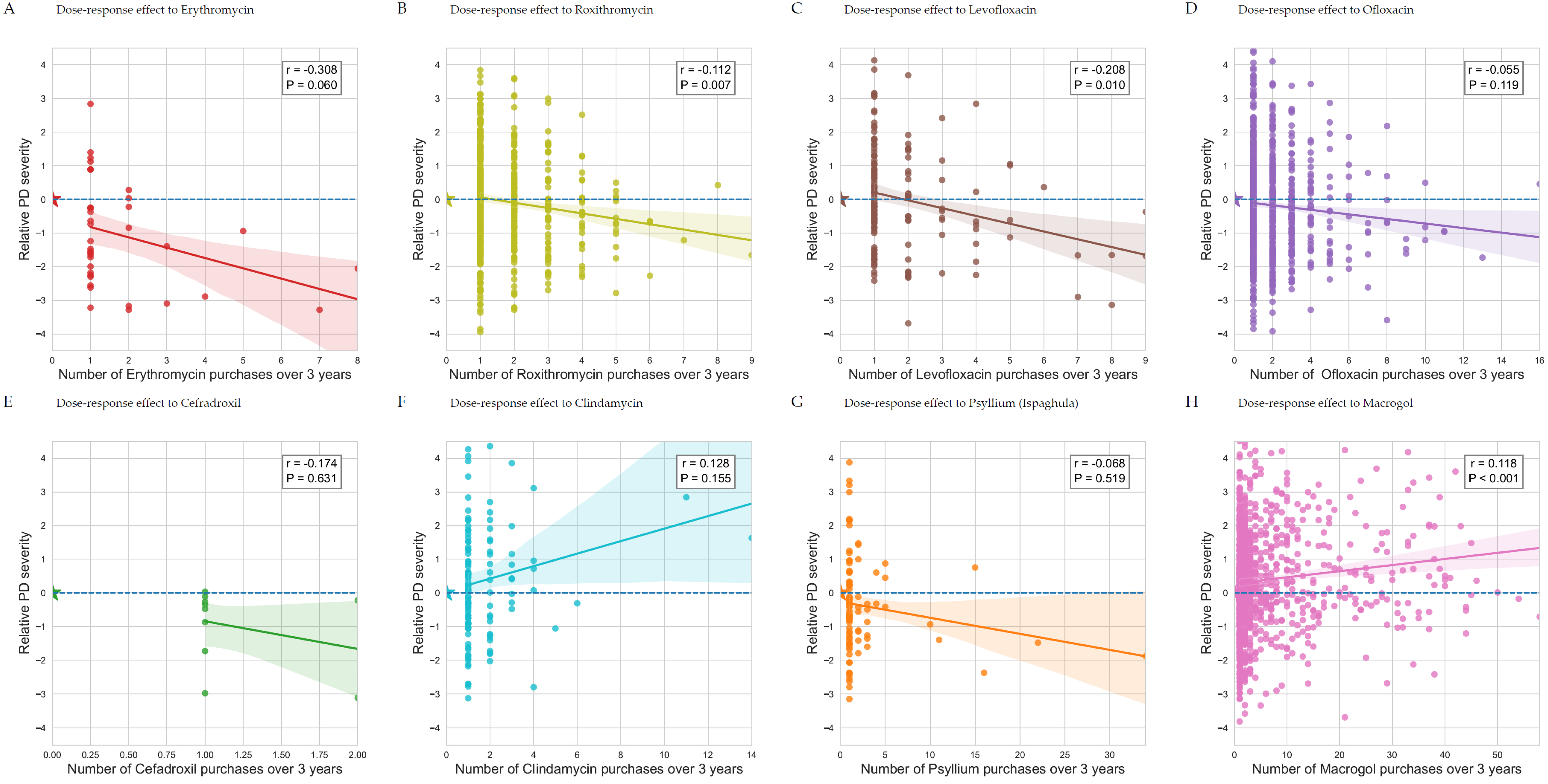
3.6. Disease Severity Is Affected by Antimicrobial Treatments
| ATC Code | Patient-Years | Coefficient | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|---|
| Age | −0.00 | [−0.01 to 0.00] | 0.157 | ||
| Smoker | 0.18 | [0.05 to 0.32] | 0.006 | ||
| J01MA01 | Ofloxacin | 981/8793 | −0.45 | [−0.55 to −0.34] | <0.0001 |
| A06AD15 | Macrogol | 999/8793 | 0.32 | [0.22 to 0.43] | <0.0001 |
| J01FA01 | Erythromycin | 66/8793 | −0.73 | [−1.11 to −0.35] | 0.0002 |
| A06AC01 | Ispaghula (psyllium) | 184/8793 | −0.42 | [−0.65 to −0.19] | 0.0003 |
| J07AM51 | Tetanus toxoid vaccine | 26/8793 | −1.11 | [−1.72 to −0.51] | 0.0003 |
| J01CE08 | Benzathine benzylpenicillin | 46/8793 | −0.71 | [−1.16 to −0.25] | 0.0023 |
| J01DB05 | Cefadroxil | 21/8793 | −1.03 | [−1.70 to −0.36] | 0.0028 |
| J01FF01 | Clindamycin | 160/8793 | 0.39 | [0.15 to 0.64] | 0.0017 |
| J01FA06 | Roxithromycin | 1159/8793 | −0.14 | [−0.24 to −0.04] | 0.0055 |
| J01MA12 | Levofloxacin | 269/8793 | −0.23 | [−0.42 to −0.04] | 0.0193 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.Y.J.; Collado-Mateo, D.; et al. Global, Regional, and National Burden of Parkinson’s Disease, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Funayama, M.; Nishioka, K.; Li, Y.; Hattori, N. Molecular Genetics of Parkinson’s Disease: Contributions and Global Trends. J. Hum. Genet. 2023, 68, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R.; Bouvet, P. Clostridial Toxins. Future Microbiol. 2009, 4, 1021–1064. [Google Scholar] [CrossRef] [PubMed]
- Eisel, U.; Jarausch, W.; Goretzki, K.; Henschen, A.; Engels, J.; Weller, U.; Hudel, M.; Habermann, E.; Niemann, H. Tetanus Toxin: Primary Structure, Expression in E. coli, and Homology with Botulinum Toxins. EMBO J. 1986, 5, 2495–2502. [Google Scholar] [CrossRef]
- Brüggemann, H.; Bäumer, S.; Fricke, W.F.; Wiezer, A.; Liesegang, H.; Decker, I.; Herzberg, C.; Martínez-Arias, R.; Merkl, R.; Henne, A.; et al. The Genome Sequence of Clostridium tetani, the Causative Agent of Tetanus Disease. Proc. Natl. Acad. Sci. USA 2003, 100, 1316–1321. [Google Scholar] [CrossRef]
- Pirazzini, M.; Montecucco, C.; Rossetto, O. Toxicology and Pharmacology of Botulinum and Tetanus Neurotoxins: An Update. Arch. Toxicol. 2022, 96, 1521–1539. [Google Scholar] [CrossRef]
- Hassel, B. Tetanus: Pathophysiology, Treatment, and the Possibility of Using Botulinum Toxin against Tetanus-Induced Rigidity and Spasms. Toxins 2013, 5, 73–83. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Diphtheria, Tetanus, and Pertussis Vaccine Recommendations. Available online: https://www.cdc.gov/vaccines/vpd/dtap-tdap-td/hcp/recommendations.html (accessed on 30 April 2024).
- Israeli Ministry of Health Tetanus Vaccine. Available online: https://www.gov.il/en/pages/tetanus-vaccine (accessed on 30 April 2024).
- McQuillan, G.M.; Kruszon-Moran, D.; Deforest, A.; Chu, S.Y.; Wharton, M. Serologic Immunity to Diphtheria and Tetanus in the United States. Ann. Intern. Med. 2002, 136, 660. [Google Scholar] [CrossRef]
- Gergen, P.J.; McQuillan, G.M.; Kiely, M.; Ezzati-Rice, T.M.; Sutter, R.W.; Virella, G. A Population-Based Serologic Survey of Immunity to Tetanus in the United States. N. Engl. J. Med. 1995, 332, 761–767. [Google Scholar] [CrossRef]
- Li, Z.; Liang, H.; Hu, Y.; Lu, L.; Zheng, C.; Fan, Y.; Wu, B.; Zou, T.; Luo, X.; Zhang, X.; et al. Gut Bacterial Profiles in Parkinson’s Disease: A Systematic Review. CNS Neurosci. Ther. 2023, 29, 140–157. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic Bacterial Composition in Parkinson’s Disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Van Hao, N.; Huyen, N.N.M.; Ny, N.T.H.; Trang, V.T.N.; Hoang, N.V.M.; Thuy, D.B.; Nguyen, N.T.; Lieu, P.T.; Duong, H.T.H.; Thuy, T.T.D.; et al. The Role of the Gastrointestinal Tract in Toxigenic Clostridium Tetani Infection: A Case-Control Study. Am. J. Trop. Med. Hyg. 2021, 105, 494–497. [Google Scholar] [CrossRef]
- Israel, A.; Merzon, E.; Schäffer, A.A.; Shenhar, Y.; Green, I.; Golan-Cohen, A.; Ruppin, E.; Magen, E.; Vinker, S. Elapsed Time since BNT162b2 Vaccine and Risk of SARS-CoV-2 Infection: Test Negative Design Study. BMJ 2021, 375, e067873. [Google Scholar] [CrossRef] [PubMed]
- Mappin-Kasirer, B.; Pan, H.; Lewington, S.; Kizza, J.; Gray, R.; Clarke, R.; Peto, R. Tobacco Smoking and the Risk of Parkinson Disease. Neurology 2020, 94, e2132–e2138. [Google Scholar] [CrossRef]
- Kandinov, B.; Giladi, N.; Korczyn, A.D. Smoking and Tea Consumption Delay Onset of Parkinson’s Disease. Park. Relat. Disord. 2009, 15, 41–46. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay Following BNT162b2 MRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2021, 10, 64. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism. Neurology 1967, 17, 427. [Google Scholar] [CrossRef]
- Ramaker, C.; Marinus, J.; Stiggelbout, A.M.; van Hilten, B.J. Systematic Evaluation of Rating Scales for Impairment and Disability in Parkinson’s Disease. Mov. Disord. 2002, 17, 867–876. [Google Scholar] [CrossRef]
- Israel, A.; Schäffer, A.A.; Cicurel, A.; Cheng, K.; Sinha, S.; Schiff, E.; Feldhamer, I.; Tal, A.; Lavie, G.; Ruppin, E. Identification of Drugs Associated with Reduced Severity of COVID-19—A Case-Control Study in a Large Population. Elife 2021, 10, e68165. [Google Scholar] [CrossRef]
- Smith, J.W.G. Penicillin in Prevention of Tetanus. Br. Med. J. 1964, 2, 1293. [Google Scholar] [CrossRef]
- Duffy, C.R.; Huang, Y.; Andrikopoulou, M.; Stern-Ascher, C.N.; Wright, J.D.; Goffman, D.; D’Alton, M.E.; Friedman, A.M. Clindamycin, Gentamicin, and Risk of Clostridium Difficile Infection and Acute Kidney Injury During Delivery Hospitalizations. Obstet. Gynecol. 2020, 135, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Tomkovich, S.; Taylor, A.; King, J.; Colovas, J.; Bishop, L.; McBride, K.; Royzenblat, S.; Lesniak, N.A.; Bergin, I.L.; Schloss, P.D. An Osmotic Laxative Renders Mice Susceptible to Prolonged Clostridioides Difficile Colonization and Hinders Clearance. mSphere 2021, 6, e0062921. [Google Scholar] [CrossRef] [PubMed]
- Tungland, B.C.; Meyer, D. Nondigestible Oligo- and Polysaccharides (Dietary Fiber): Their Physiology and Role in Human Health and Food. Compr. Rev. Food Sci. Food Saf. 2002, 1, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases, 14th ed.; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021.
- Xu, J.; Wang, L.; Chen, X.; Le, W. New Understanding on the Pathophysiology and Treatment of Constipation in Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 917499. [Google Scholar] [CrossRef]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s Disease: Possible Routes by Which Vulnerable Neuronal Types May Be Subject to Neuroinvasion by an Unknown Pathogen. J. Neural Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s Disease. Ann. N. Y. Acad. Sci. 2009, 1170, 615–622. [Google Scholar] [CrossRef]
- Reichmann, H. View Point: Etiology in Parkinson’s Disease. Dual Hit or Spreading Intoxication. J. Neurol. Sci. 2011, 310, 9–11. [Google Scholar] [CrossRef]
- Hensel, B.; Seib, U.C.; Wellhöner, H.H. Vagal Ascent and Distribution of 125I-Tetanus Toxin after Injection into the Anterior Wall of the Stomach. Naunyn Schmiedebergs Arch. Pharmacol. 1973, 276, 395–402. [Google Scholar] [CrossRef]
- Ovespian, S.V.; Bodeker, M.; O’Leary, V.B.; Lawrence, G.W.; Oliver Dolly, J. Internalization and Retrograde Axonal Trafficking of Tetanus Toxin in Motor Neurons and Trans-Synaptic Propagation at Central Synapses Exceed Those of Its C-Terminal-Binding Fragments. Brain Struct. Funct. 2015, 220, 1825–1838. [Google Scholar] [CrossRef]
- Keller, A.; Sweeney, S.T.; Zars, T.; O’Kane, C.J.; Heisenberg, M. Targeted Expression of Tetanus Neurotoxin Interferes with Behavioral Responses to Sensory Input in Drosophila. J. Neurobiol. 2002, 50, 221–233. [Google Scholar] [CrossRef]
- Blandini, F.; Nappi, G.; Tassorelli, C.; Martignoni, E. Functional Changes of the Basal Ganglia Circuitry in Parkinson’s Disease. Prog. Neurobiol. 2000, 62, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Bagetta, G.; Nisticò, G. Tetanus Toxin as a Neurobiological Tool to Study Mechanisms of Neuronal Cell Death in the Mammalian Brain. Pharmacol. Ther. 1994, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, P.; Fang, L.; Ge, S.; Huang, F.; Polverini, P.J.; Heng, W.; Zheng, L.; Hu, Q.; Yan, F.; et al. Active Smoking Induces Aberrations in Digestive Tract Microbiota of Rats. Front. Cell. Infect. Microbiol. 2021, 11, 737204. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-J.; Yang, S.-T.; Sha, Y.; Ge, Y.-Y.; Wang, J.-M. Hyperbaric Oxygen Treatment for Parkinson’s Disease with Severe Depression and Anxiety. Medicine 2018, 97, e0029. [Google Scholar] [CrossRef]
- Gorell, J.M.; Johnson, C.C.; Rybicki, B.A.; Peterson, E.L.; Richardson, R.J. The Risk of Parkinson’s Disease with Exposure to Pesticides, Farming, Well Water, and Rural Living. Neurology 1998, 50, 1346–1350. [Google Scholar] [CrossRef]
- Fan, B.; Jabeen, R.; Bo, B.; Guo, C.; Han, M.; Zhang, H.; Cen, J.; Ji, X.; Wei, J. What and How Can Physical Activity Prevention Function on Parkinson’s Disease? Oxidative Med. Cell. Longev. 2020, 2020, 4293071. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Israel, A.; Magen, E.; Ruppin, E.; Merzon, E.; Vinker, S.; Giladi, N. Anti-Tetanus Vaccination Is Associated with Reduced Occurrence and Slower Progression of Parkinson’s Disease—A Retrospective Study. Biomedicines 2024, 12, 2687. https://doi.org/10.3390/biomedicines12122687
Israel A, Magen E, Ruppin E, Merzon E, Vinker S, Giladi N. Anti-Tetanus Vaccination Is Associated with Reduced Occurrence and Slower Progression of Parkinson’s Disease—A Retrospective Study. Biomedicines. 2024; 12(12):2687. https://doi.org/10.3390/biomedicines12122687
Chicago/Turabian StyleIsrael, Ariel, Eli Magen, Eytan Ruppin, Eugene Merzon, Shlomo Vinker, and Nir Giladi. 2024. "Anti-Tetanus Vaccination Is Associated with Reduced Occurrence and Slower Progression of Parkinson’s Disease—A Retrospective Study" Biomedicines 12, no. 12: 2687. https://doi.org/10.3390/biomedicines12122687
APA StyleIsrael, A., Magen, E., Ruppin, E., Merzon, E., Vinker, S., & Giladi, N. (2024). Anti-Tetanus Vaccination Is Associated with Reduced Occurrence and Slower Progression of Parkinson’s Disease—A Retrospective Study. Biomedicines, 12(12), 2687. https://doi.org/10.3390/biomedicines12122687







