Abstract
Background: Dengue virus (DENV) is the most widespread mosquito-borne virus, which can cause dengue fever with mild symptoms, or progress to fatal dengue hemorrhagic fever and dengue shock syndrome. As the main target cells of DENV, macrophages are responsible for the innate immune response against the virus. Methods: In this study, we investigated the role of pyroptosis in the pathogenic mechanism of dengue fever by examining the level of pyroptosis in DENV-1-infected macrophages and further screened differentially expressed microRNAs by high-throughput sequencing to predict microRNAs that could affect the pyroptosis of the macrophage. Results: Macrophages infected with DENV-1 were induced with decreased cell viability, decreased release of lactate dehydrogenase and IL-1β, activation of NLRP3 inflammasome and caspase-1, cleavage of GSDMD to produce an N-terminal fragment bound to cell membrane, and finally induced macrophage pyroptosis. MicroRNA expression profiles were obtained by sequencing macrophages from all periods of DENV-1 infection and comparing with the negative control. Sixty-three microRNAs differentially expressed in both the early and later stages of infection were also identified. In particular, miR-223-3p, miR-148a-3p, miR-125a-5p, miR-146a-5p and miR-34a-5p were recognized as small molecules that may be involved in the regulation of inflammation. Conclusions: In summary, this study aimed to understand the pathogenic mechanism of DENV through relevant molecular mechanisms and provide new targets for dengue-specific therapy.
1. Introduction
Dengue virus (DENV) is currently considered to be the most dominant mosquito-borne virus affecting human health, causing more than 100 million confirmed infections in 125 countries each year, with about 10,000 deaths reported annually. The mortality rate related to DENV has been increasing in recent years [1,2]. DENV is mainly transmitted by the bites of Aedes aegypti and Aedes albopictus mosquitoes, and it is generally not transmitted directly from person to person. Clinical manifestations range from asymptomatic infected cases to severe dengue fever, which can be fatal. Dengue fever (DF) is a mild influenza-like syndrome, including fever, nausea, vomiting, rash, pain, lymph node swelling and leukopenia. In severe cases, it can lead to dengue hemorrhagic fever (DHF) or even dengue shock syndrome (DSS), with a mortality rate of up to 20% [3]. As a mosquito-borne virus, the unique transmission route of DENV makes it mainly occur in tropical and subtropical regions where mosquitoes are widely distributed. However, due to global warming, the spread of DENV has intensified, leading the incidence rate of DF to increase year by year. Therefore, more and more populations are exposed to the risk of DENV infection and preventing and controlling the transmission of DENV infection has become an international public safety issue [4,5].
DENV belongs to the flavivirus family and is characterized as an enveloped, single-stranded, positive-sense RNA virus with viral particles ranging from 45 to 55 nm in diameter and a genome length of 10.7 kb. DENV infection causes the body to produce excessive pro-inflammatory cytokines, resulting in increased endothelial cell permeability and impaired endothelial integrity, as well as aggravating symptoms in DF cases [3]. Therefore, ameliorating exacerbated inflammation is an important goal of treatment. Macrophages, as the primary target cells for DENV, are also the key immune cells responsible for releasing pro-inflammatory cytokines, such as interleukin-1β (IL-1β), which is considered to be an essential factor in regulating vascular integrity [6]. Pyroptosis, as a mode of programmed cellular inflammatory death, differs from apoptosis in that it does not preserve the integrity of the cell membrane. It is characterized by the activation of the inflammasome, persistent membrane swelling and rupture, and it then releases IL-1β, which exacerbates the inflammatory response [7]. Previous studies have shown that DENV-2 activates the NOD-like receptor protein 3 (NLRP3) inflammasome via C-type lectin 5A (CLEC5A) in human macrophages, inducing high levels of IL-1β and IL-18 expression, while caspase-4 is involved in the regulation of pyroptosis upstream of caspase-1 [8,9].
MicroRNA (miRNA) is a class of non-coding single-stranded RNA molecules encoded by endogenous genes with a length of about 22 nucleotides, which plays an important role in the regulation of gene expression and is intricately linked to a multitude of life processes, such as growth and development, cellular differentiation, apoptosis, antiviral response and antitumor response [10]. Meanwhile, miRNA can participate in regulating inflammasome activation and pyroptosis. For example, miR-195 was significantly downregulated in SH-SY5Y human neuroblastoma cells infected with enterovirus A71, ultimately demonstrating that miR-195 regulates EV-A71-induced pyroptosis by directly targeting NLRX1 [11]. This provided a new direction for studying DENV-induced macrophage pyroptosis.
Analyzing the miRNA transcriptome to screen for differentially expressed miRNAs and enrichment pathways is a powerful means of revealing the molecular mechanisms of miRNAs in biological processes and disease development. There are two main methods for miRNA transcriptome analysis: high-throughput sequencing [12] and microarray technology [13]. Microarray technology uses pre-designed labeled probes to hybridize with cDNA sequences, while sequencing does not rely on pre-designed probes or known sequences and can cover a wider range of genomes.
Currently, it has been found that miRNAs can regulate DENV replication by targeting the DENV genome or host genes [14,15]. Vascular dysfunction is one of the main causes of severe dengue fever. PPARγ regulates the downregulation of miR-573 after DENV infects vascular endothelial cells and protects endothelial function [16]. In addition, miR-383-5p inhibits DENV replication in liver cells [17]. However, the research on DENV-induced pyroptosis is still limited and the regulatory role of miRNA in it requires further investigation.
In recent years, DENV-1 has been dominant in the local outbreaks of DF in Zhejiang Province, China [18], but the regulatory mechanism of DENV-1-induced macrophage pyroptosis is still unclear. Pyroptosis, as a mode of inflammatory cell death, can lead to excessive production of pro-inflammatory cytokines, possibly leading to vascular leakage and severe DHF/DSS. The aim of this study was to verify that DENV-1 infection induced pyroptosis in macrophages, exacerbating the inflammatory response. Furthermore, miRNA transcriptome sequencing technology was used to screen for differentially expressed miRNAs before and after infection to identify potential targets that regulate macrophage pyroptosis and inflammatory response.
2. Materials and Methods
2.1. Cell Culture
Human monocyte cell line THP-1 cells (MeisenCTCC, Jinhua, China) were cultured in Roswell Park Memorial Institute 1640 medium (RPMI 1640; Gibco, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS; Gibco, New York, NY, USA), 1% L-glutamine (LG; Gibco, USA) and 1% penicillin-streptomycin (PS; Gibco, USA), in a 37 °C, 5% CO2 incubator. THP-1 cells were stimulated using 10 ng/mL of phorbol-12-myristate-13-acetate (PMA; Sigma, Burlington, MA, USA) for 72 h to differentiate THP-1 cells into M0-type macrophages. The PMA-containing culture medium was removed and the cells were washed three times with phosphate-buffered saline (PBS; Gibco, USA), and RPMI 1640 (2% FBS, 1% LG and 1% PS) was added finally for subsequent experiments. C6/36 mosquito cells were preserved by the Zhejiang Provincial Center for Disease Control and Prevention, cultured in RPMI 1640 containing 10% FBS, 1% LG, 1% PS and 1% sodium pyruvate (SP; Gibco, USA) medium at 28 °C with 5% CO2.
2.2. Virus Amplification and Titration
The DENV-1 strain was isolated from a patient with dengue fever in Zhejiang Province, replicated and amplified in C6/36. When C6/36 was almost confluent into a monolayer of cells, 100 μL of serum sample was added and cultured for 7 days, then the cells were repeatedly freeze−thawed three times and centrifuged at 2000 rpm for 10 min to collect the supernatant as the first-generation viral culture, which was stored in portions at –80 °C. The above steps were repeated to obtain the second-generation viral culture, and it was stored at –80 °C separately. Viral RNA was extracted using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) for nucleic acid extraction and detection. The One Step PrimerTM RT-PCR Kit (TaKaRa, Kusatsu, Japan) was combined with DENV-1-specific primer probes to detect viral RNA. The real-time RT-PCR reaction system and program were configured according to the manufacturer’s instructions.
In the meantime, the C6/36 cells were used to determine the titer of viral TCID50. The C6/36 cells were inoculated into 96-well plates (Corning Inc., Corning, NY, USA) and placed in an incubator for 24 h. When the cells grew to a monolayer, a viral strain dilution of RPMI 1640 diluted to 10−1, 10−2, 10−3, 10−4, 10−5, 10−6, 10−7, 10−8 was added to the 96-well plate at 100 μL per well and 100 μL of RPMI 1640 (2% FBS) was supplemented. After 5–7 days of incubation at 28 °C, in a 5% CO2 incubator, the number of wells with lesions was recorded and the TCID50 was calculated using the Reed–Muench method [19].
2.3. Cell Activity
THP-1 cell suspensions were inoculated into 96-well plates at 5 × 104 per well and 100 μL per well, and differentiation was induced by PMA. DENV-1 was added to macrophages at multiplicities of infection (MOIs) of 0.01, 0.1 and 1. After adsorption for 2 h, the virus-containing culture medium was discarded, and the cells were washed with PBS twice. A total of 100 μL of RPMI 1640 (2% FBS) was added to each well, and the cells were incubated at 37 °C in a 5% CO2 incubator. Cells without any treatment were used as the negative control, and 1 mg/mL lipopolysaccharides (LPS; Sigma, USA) and 2 μM Nigericin (Sigma, USA) were treated for 4 h as the positive control. Using a CCK-8 cytotoxicity detection kit (MeilunBio, Dalian, China) at 24 h, 48 h and 72 h after viral infection, the old medium was discarded, and RPMI 1640 (2% FBS) containing 10% CCK-8 was added to each well. After incubation at 37 °C for 2 h, the absorbance value was measured by an enzyme marker at a primary wavelength of OD 450 nm, and a secondary wavelength of OD 600 nm, as the cell viability = (OD experimental group − OD blank)/(OD negative control − OD blank) × 100%.
2.4. Lactate Dehydrogenase (LDH) Release Assay
THP-1 cells were inoculated into 96-well plates, induced to differentiate with PMA and categorized into the infection group (MOIs = 0.01, 0.1, 1), negative control and positive control (LPS and Nigericin treatment) as described previously. An LDH assay kit (Beyotime, Shanghai, China) was used according to the manufacturer’s protocol, and 10% of the original volume of LDH-releasing reagent was added to the maximal enzymatic activity control wells one hour before 24 h, 48 h and 72 h of viral infection, respectively. The incubation was continued for 1 h. The 96-well plate was centrifuged at 400× g for 5 min, and 120 μL of the supernatant was transferred to a new 96-well plate and configured according to the instructions of the LDH working solution. Adding 60 μL of LDH working solution to each well, incubating for 30 min at room temperature and avoiding light then followed, along with measuring the absorbance values with an enzyme marker at a primary wavelength of OD 490 nm and a secondary wavelength of OD 600 nm. The OD value of each group was calculated after subtracting the blank control, according to the following: LDH release rate = (OD experimental group − OD negative control)/(OD maximal enzyme activity − OD negative control) × 100%.
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) to Detect IL-1β
THP-1 cell suspensions were inoculated into six-well plates at 1 × 106 cells per well and 2 mL per well and induced to differentiate with PMA. DENV-1 was infected as described previously and divided into the infected groups (MOIs = 0.01, 0.1, 1), negative control and positive control (LPS and Nigericin treatment). Cell supernatants were collected at the indicated times. The assay was performed using the human IL-1β ELISA kit (Abcam, Cambridge, UK), where 100 μL TMB developer was added according to the manufacturer’s protocol and incubated at room temperature in darkness for 10 min. After incubation, 100 μL of termination solution was added to each well to terminate the reaction. Finally, absorbance values were measured at OD 450 nm, and a standard curve was plotted to calculate the amount of IL-1β in each sample.
2.6. Real-Time Quantitative PCR (RT-qPCR)
THP-1 cells were inoculated into six-well plates, induced to differentiate with PMA, and divided into the infected groups (MOIs = 0.01, 0.1, 1), negative control and positive control (LPS and Nigericin treatment) as described previously. Cellular RNA was extracted at the indicated times using the nucleic acid extraction kit RNeasy Mini Kit (QIAGEN, Germany), and an 8 μL RNA template was reverse transcribed to synthesize cDNA (TaKaRa, Japan). After completing reverse transcription, the obtained cDNA was subjected to RNA concentration detection using NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA) to ensure the quality met the requirement of subsequent experiments. Amplification was performed using TB Green® Premix Ex Taq II (TaKaRa, Japan) on a 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) and pre-denaturation occurred at 95 °C for 30 s, denaturation at 95 °C for 5 s, annealing at 60 °C for 30 s and conducting over 40 cycles. Melting curves were obtained at 95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s. The mRNA and miRNA were used as internal reference genes with GAPDH and U6, respectively. The relative expression levels of the genes were calculated by the 2−ΔΔCt method. The primers (Tsingke Biotechnology, Beijing, China) designed by the laboratory are listed in Table 1.

Table 1.
Primer sequences used in this study.
2.7. Western Blot
THP-1 cells were inoculated into six-well plates, induced to differentiate with PMA, and divided into the infected group (MOI = 1), negative control and positive control (LPS and Nigericin treatment) as described previously. Cellular proteins were extracted at the indicated times using RIPA lysate containing 1% PMSF (Solarbio, Beijing, China), the protein concentration was determined using the BCA Protein Assay Kit (Beyotime, China), and then it was mixed with protein loading buffer (Solarbio, China), denatured in a metal bath at 95 °C for 10 min and stored at −80 °C. The samples were added to an SDS-PAGE gel for electrophoresis, then the protein was transferred on the gel to the PVDF membrane (Millipore, Burlington, MA, USA) with membrane transfer buffer (EpiZyme, Shanghai, China) under an ice bath condition. The PVDF membrane was treated with blocking buffer (EpiZyme, China) for 15 min. The membrane was rinsed with TBST three times, each time for 10 min. After rinsing, the PVDF membrane was incubated with the corresponding primary antibody diluted to 1:1000 at 4 °C overnight. The primary antibody included anti-Pro IL-1β (Cell Signaling Technology, Danvers, MA, USA), anti-cleaved IL-1β (Cell Signaling Technology, USA), anti-NLRP3 (ZEN-BIOSCIENCE, Chengdu, China), anti-GSDMD (ZEN-BIOSCIENCE, China), anti-GSDMD-NT (Abcam, UK), anti-caspase-1 (ZEN-BIOSCIENCE, China) and anti-β-actin (ImmunoWay, Newark, DL, USA). On the next day, the PVDF membrane was rinsed three times with TBST for 10 min each time. The proteins were incubated with 1:10000 diluted horseradish peroxidase-labeled secondary antibody Goat Anti-Mouse IgG/Goat Anti-Rabbit IgG (Abcam, UK) for 1 h at room temperature. After incubation, the PVDF membrane was rinsed three times with TBST for 10 min each time. Protein bands were detected using an enhanced chemiluminescence kit (Biosharp, Hefei, China) and a chemiluminescence analyzer (Bio-Rad, Hercules, CA, USA).
2.8. Small RNA Sequencing and Analysis
THP-1 cells were inoculated into six-well plates, induced to differentiate with PMA, and divided into the infected group (MOI = 1) and negative control as described previously. Cellular RNA was extracted at 24 h, 48 h and 72 h of infection using the RNeasy Mini Kit. The samples were tested for compliance with library requirements, and then a library was constructed using the NEB Next® Multiplex Small RNA Library Prep Set for Illumina® (NEB E7300L). Library quality was assessed on an Agilent 5400 system (Agilent, Santa Clara, CA, USA) and quantified by qPCR (1.5 nM). After purification and size selection, libraries with insert lengths between 18 and 40 bp were ready for sequencing on the Illumina SE50 platform.
Raw data were filtered using customized perl and python scripts to obtain clean reads without low-quality reads and sequencing junctions. Clean reads from each sample were filtered, sRNAs of 18–40 bp were selected and localized to the reference sequence by bowtie [20], and then the distribution of sRNAs in the reference sequence situation were analyzed. The above reads were mapped to the reference sequences, with miRBase20.0 serving as the reference, and modified software mirdeep2 (mirdeep2_0_0_5) [21] and srna-tools-cli (http://srna-tools.cmp.uea.ac.uk/, accessed on 11 May 2023) were used to obtain the potential miRNA and draw secondary structures. At the same time, new miRNAs were predicted by using the signature hairpin structure of the miRNA precursors. Subsequently, the expression levels of the known and new miRNAs in each sample could be counted and normalized by TPM [22], according to the following: normalized expression = mapped reads/total reads × 1,000,000. Two-by-two differential expression analysis was performed using DESeq R package (1.8.3) based on the negative binomial distribution of DESeq2 [23], and p-values were adjusted using the Benjamini and Hochberg method, with a corrected p < 0.05 set as the screening condition for significant differential expression by default. The target genes of miRNAs were predicted using two software programs, miRanda (miRanda-3.3a) and RNAhybrid (RNAhybrid v2.0), and the intersection was taken as the final targeting result. R language was used to perform Gene Ontology (GO) and KEGG enrichment analyses on the candidate target genes of differentially expressed miRNAs through the “clusterProfiler” package (3.8.1).
2.9. Statistical Analysis
All experiments were performed at least 3 times. Data were analyzed using GraphPad Prism 9.0 software and are expressed as the mean ± standard error of mean. One-way ANOVA was used for multi-group comparison and t-test for two-group comparison. A p < 0.05 was considered statistically significant.
3. Results
3.1. DENV-1-Infected Macrophages Cause Cell Death Along with Increased LDH Release
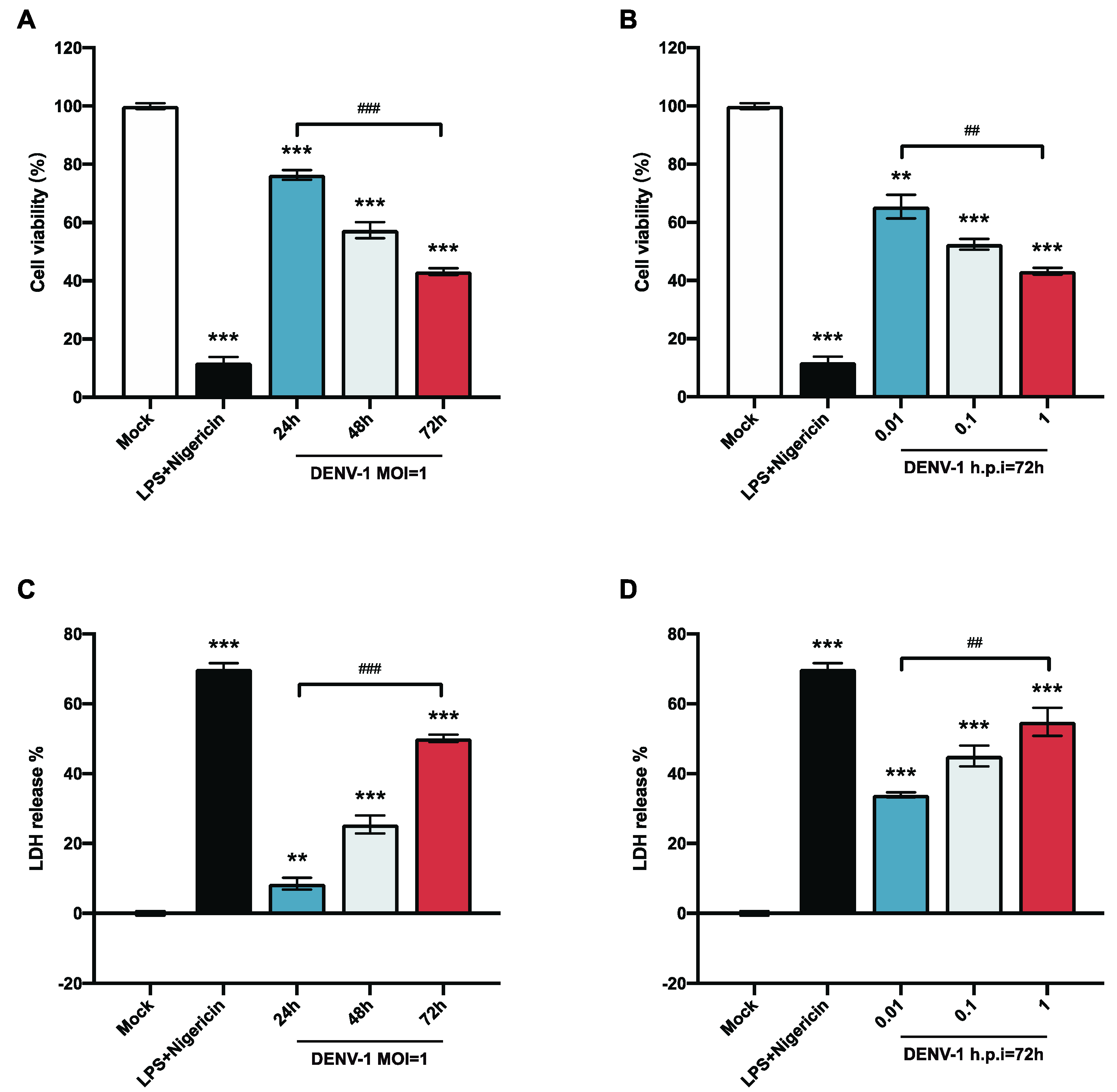
To explore the biological alterations of macrophages after DENV-1 infection, the survival rate of macrophages was detected at different viral loads and infection times. The results showed that the survival rate of macrophages infected with DENV-1 was significantly reduced. The macrophage survival rate of the DENV-1-infected group with MOI = 1 and the positive control group (LPS and Nigericin treatment) were significantly lower than those of the negative control group. The proliferation of living macrophages decreased by approximately 20% after 24 h of infection and worsened with the duration of infection (Figure 1A). In the later stage of infection (h.p.i = 72 h), macrophages were significantly damaged even with a low-dose (MOI = 0.01) infection, and the higher the dose of DENV-1 infection, the lower the survival rate of macrophages (Figure 1B).
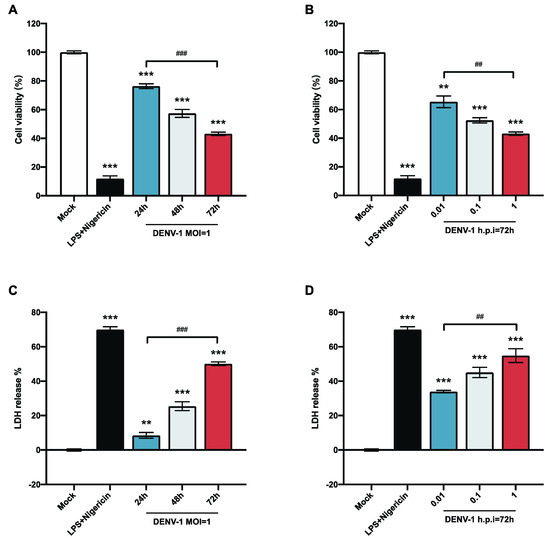
Figure 1.
Results of macrophage cell viability and LDH release. (A,B) Macrophage cell viability was detected using CCK-8; (C,D) LDH release results were detected by collecting cell supernatants in different culture states. ** p < 0.01, *** p < 0.001 compared with the negative control group; ## p < 0.01, ### p < 0.001 compared between the two groups.
LDH is an intracellular plasma enzyme that is released extracellularly when the cell membrane is damaged. To further illustrate the alteration of DENV-1 infection on macrophage membranes, the LDH release rate was measured by collecting the cell supernatant. The results showed that DENV-1 infection of macrophages resulted in a significant increase in LDH release compared with the negative control group, and the LDH release rate was positively correlated with the infection dose and infection time (Figure 1C,D), suggesting that DENV-1 infection leads to the rupture of macrophage membranes and the release of contents. The classical pyroptosis pathway involves perforating the cell membrane through the N-terminal fragment of gasdermin D (GSDMD), so we concluded that DENV-1 infection of macrophages may induce pyroptosis [24,25].
3.2. DENV-1-Infected Macrophages Release Large Amounts of IL-1β and Promote IL-1β mRNA and Its Protein Expression
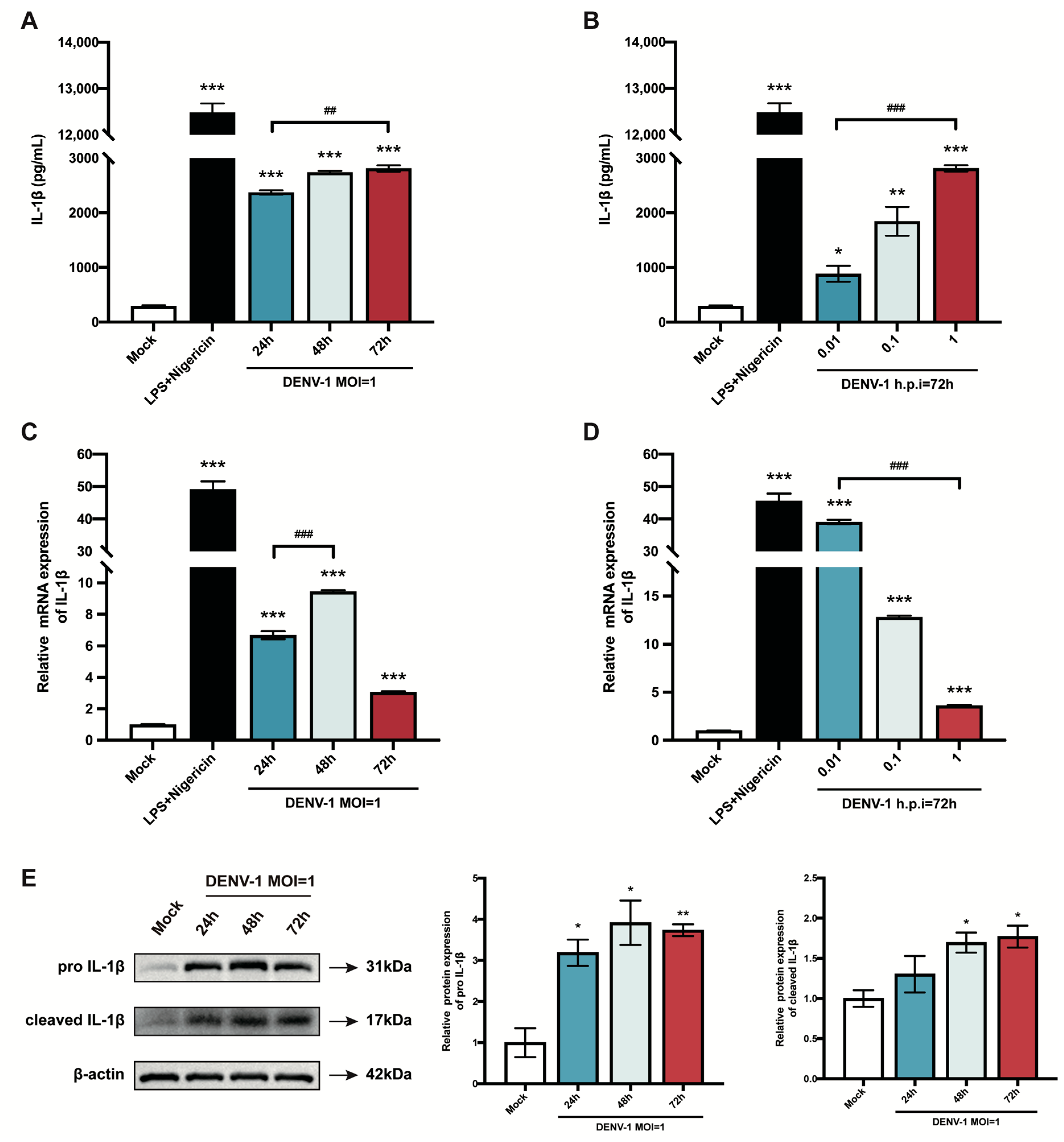
Pyroptosis is a mode of inflammatory programmed cell death, which results in cell death while releasing the pro-inflammatory cytokine IL-1β extracellularly, exacerbating the inflammatory response [26]. Therefore, this study explored the effect of DENV-1 infection on IL-1β. First, the content of IL-1β secreted in the cell supernatant was measured, and the results showed that the release of IL-1β increased significantly after DENV-1 infection compared with the negative control, with the same trend as the positive control, and increased with the increase in infection time and infection dose (Figure 2A,B). Subsequently, the IL-1β mRNA expression level was also examined, which peaked at h.p.i = 48 h in high-dose DENV-1-infected macrophages (Figure 2C). Interestingly, in the later stage of infection, the IL-1β mRNA expression level was negatively correlated with the infected dose, which may have been due to the longer duration of action of the virus at lower doses (Figure 2D). In addition, the Western blot results showed that DENV-1 infection resulted in a significant increase in the expression levels of both IL-1β precursors and lysosomes (Figure 2E), which is a characteristic of pyroptosis, further suggesting that DENV-1 infection induced macrophage pyroptosis.
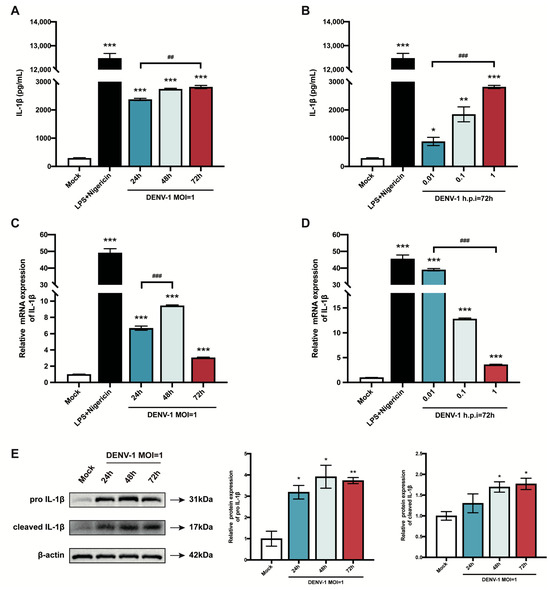
Figure 2.
Results of macrophage IL-1β release and mRNA and protein expression level. (A,B) Supernatants were collected under different culture conditions, and IL-1β content was detected using an ELISA kit; (C,D) Relative quantification of IL-1β mRNA expression level by RT-qPCR. (E) IL-1β protein expression levels were detected using Western blot, and β-actin was used as an internal reference control. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the negative control group; ## p < 0.01, ### p < 0.001 compared between the two groups.
3.3. DENV-1-Infected Macrophages Activate NLRP3 Inflammasome, Induce Caspase-1 and GSDMD Activation, and Promote Pyroptosis
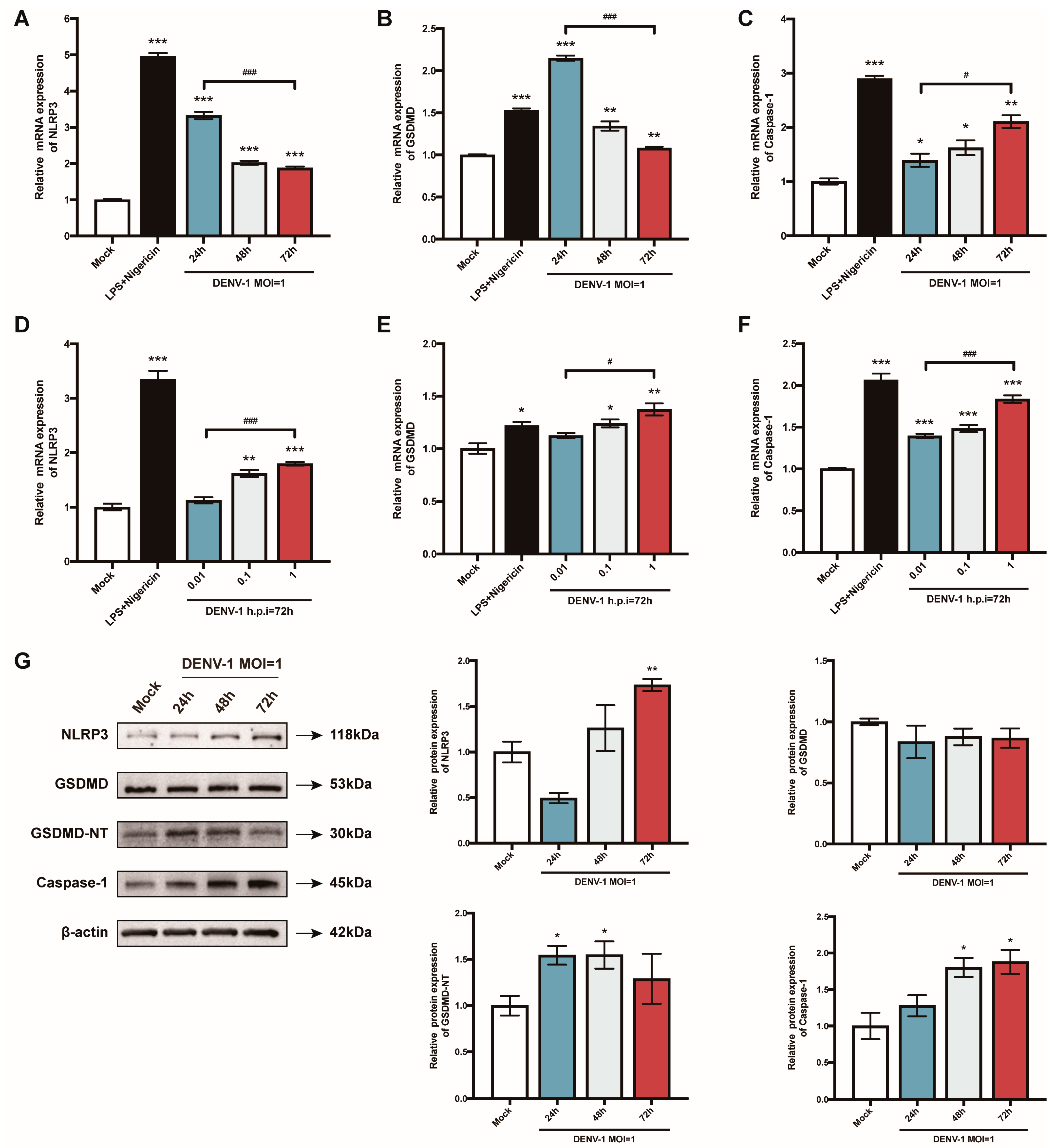
The pyroptosis-related genes were further examined. When macrophages were infected with a high dose of DENV-1 (MOI = 1), NLRP3 and GSDMD mRNA reached a peak value in the early stage of infection (h.p.i = 24 h), while the Caspase-1 mRNA level gradually increased with the infection time (Figure 3A–C). Meanwhile, the expression of all the pyroptosis-related genes showed a dose-dependent pattern (Figure 3D–F). Protein levels were also measured, and the expression of the NLRP3 inflammasome, GSDMD-NT, and caspase-1 were all enhanced after DENV-1 infection, which further verified that DENV-1 induced macrophage pyroptosis (Figure 3G).
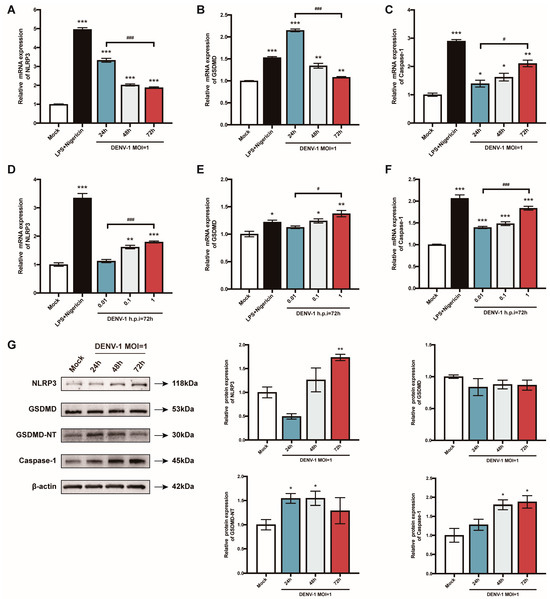
Figure 3.
Results of the detection of macrophage pyroptosis-related gene mRNA and its protein expression level. (A–F) Detection results of the expression levels of pyroptosis-related gene mRNA under different culture conditions; (G) The expression levels of pyroptosis-related proteins in macrophages infected with DENV-1 (MOI = 1) for 24 h, 48 h and 72 h, where β-actin was used as an internal reference control. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the negative control group; # p < 0.05, ### p < 0.001 compared between the two groups.
3.4. DENV-1 Infection of Macrophages Leads to Changes in miRNA Expression Profiles
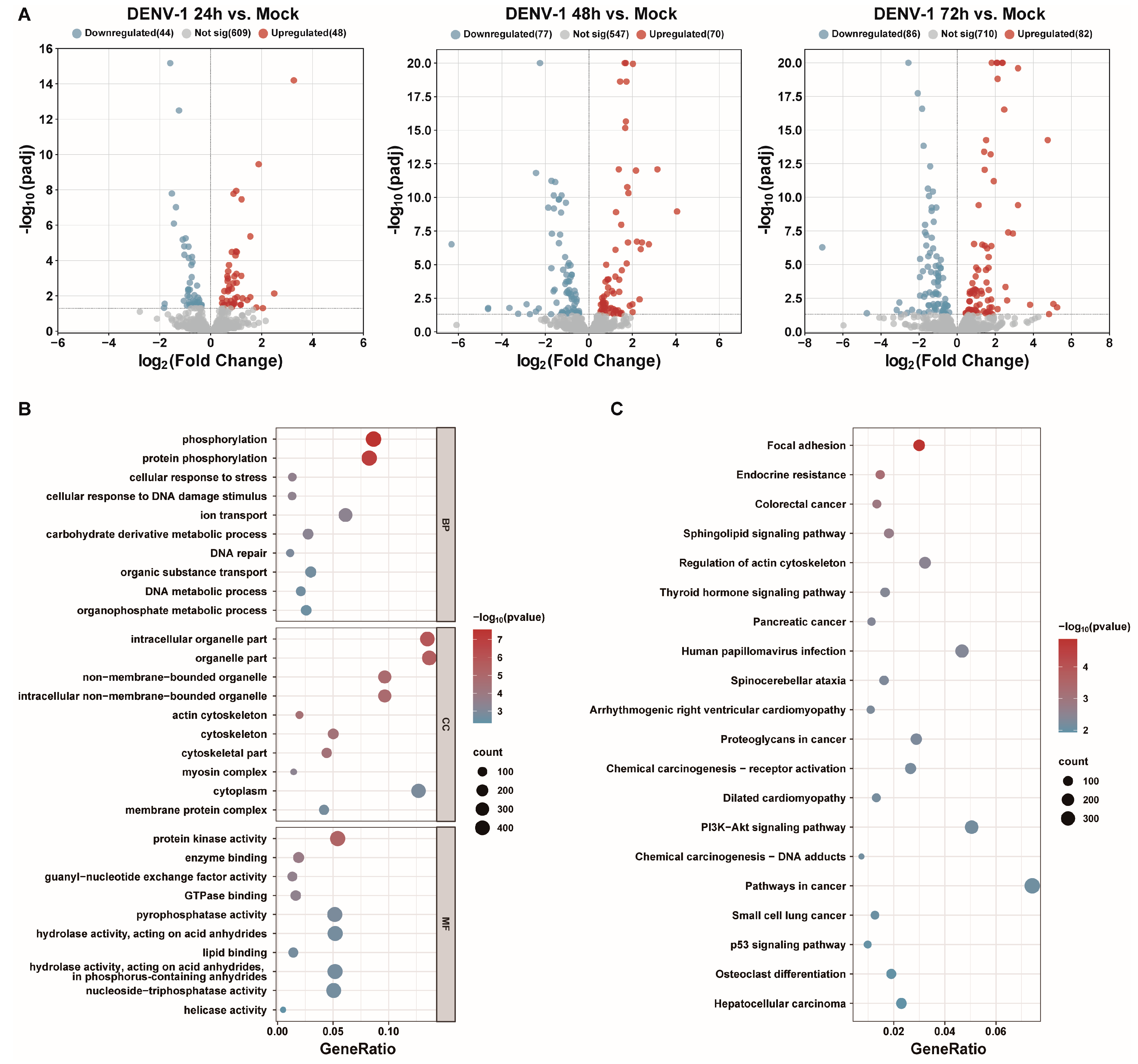
Small RNA transcriptome sequencing was used to obtain the differentially expressed miRNAs at different times after the DENV-1 infection of macrophages. With the increase in DENV-1 infection time, 92, 147 and 168 differentially expressed miRNAs were found at 24 h, 48 h and 72 h, respectively (Figure 4A). Subsequently, the differentially expressed miRNAs screened at an infection time of 72 h were predicted for target genes and subjected to functional enrichment analysis. GO enrichment analysis showed the biological function, pathway and cellular localization of the differentially expressed miRNA-regulated target genes, and the results suggested that the predicted target genes were more related to protein phosphorylation, cellular response to DNA damage stimuli, cytoskeleton, protein kinase activity, etc. (Figure 4B). KEGG enrichment analysis showed that the candidate target genes were mainly involved in actin cytoskeleton regulation, human papillomavirus infection, P13K-AKT signaling pathway, etc. (Figure 4C).
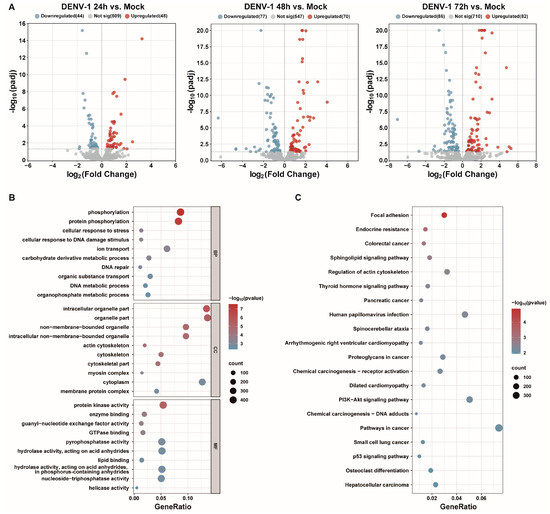
Figure 4.
Transcriptome sequencing results and analysis after DENV-1 infection in macrophages. (A) Differentially expressed miRNAs in the infected group and the negative control group at different infection times, where blue indicates downregulation and red indicates upregulation; (B) GO enrichment analysis of differentially expressed miRNA target genes at h.p.i = 72 h, showing the top 10 entries of difference in each classification; (C) KEGG enrichment analysis of differentially expressed miRNA target genes at h.p.i = 72 h, showing the entries in the top 20 differences.
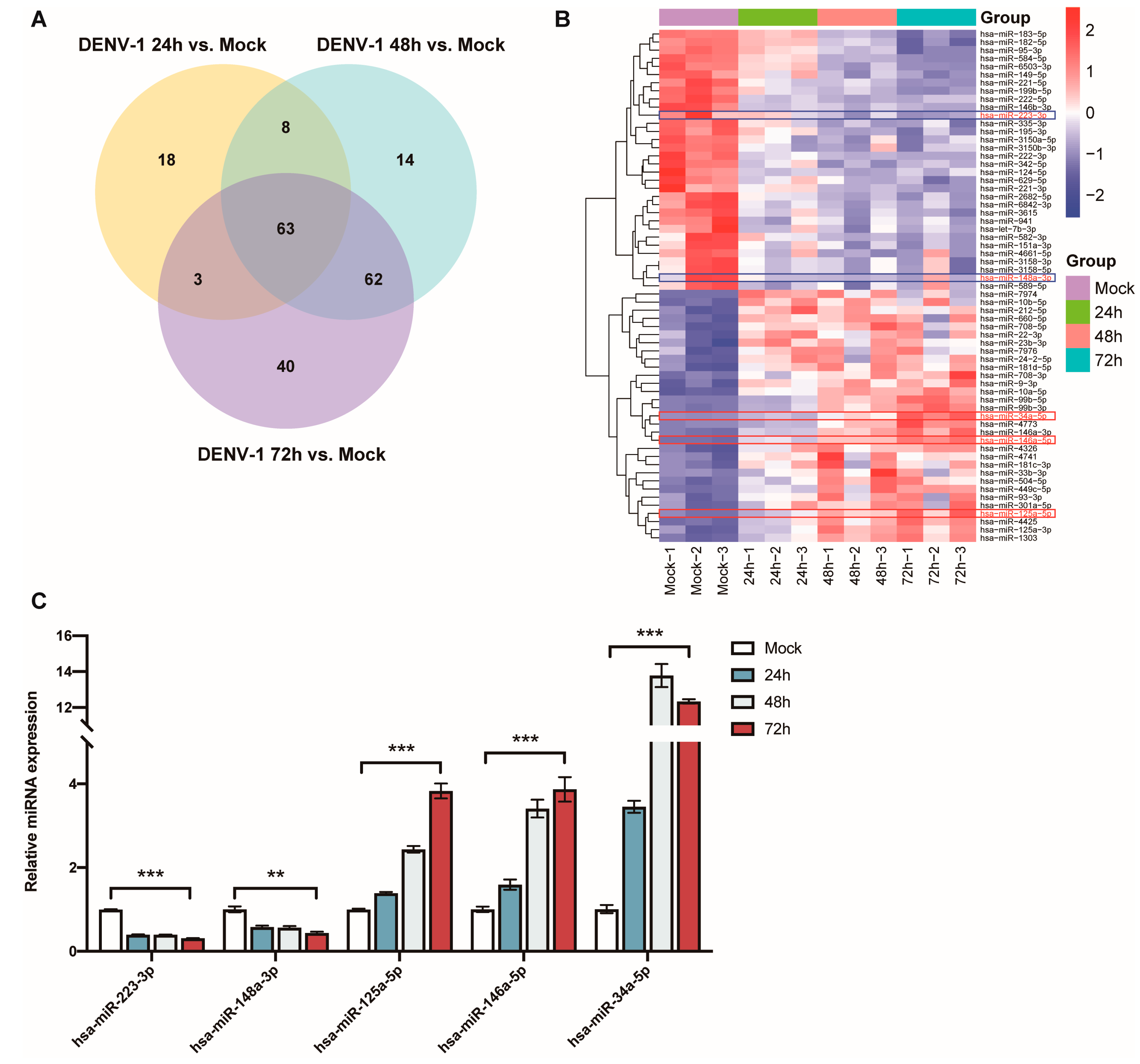
The Venn diagram of the differentially expressed miRNAs at the three different periods shows that a total of 63 miRNAs showed significant differences in their expression levels at 24 h, 48 h and 72 h (Figure 5A). This included the downregulation of miR-223-3p and miR-148a-3p, as well as upregulation of miR-125a-5p, miR-146a-5p and miR-34a-5p, which have been found to be associated with inflammatory responses (Figure 5B).
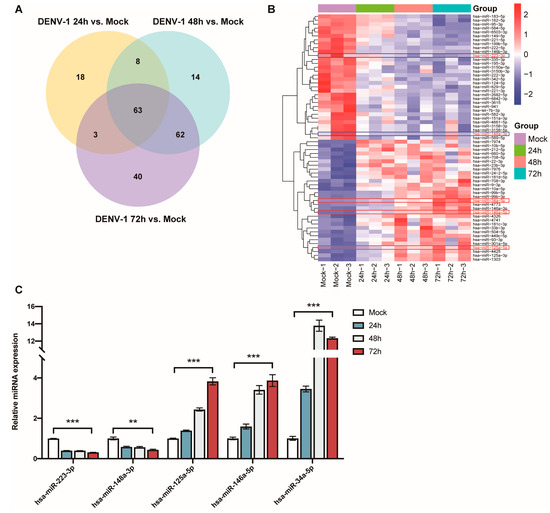
Figure 5.
Analysis and validation of differentially expressed miRNAs after DENV-1 infection of macrophages. (A) Venn diagram of miRNAs differentially expressed at 24 h, 48 h and 72 h of infection; (B) Heatmap of miRNA clustering for miRNAs that were differentially expressed at all infection periods, with the red colored-frame indicating upregulation and blue colored-frame indicating downregulation; (C) Detection of the expression levels of miR-223-3p, miR-148a-3p, miR-125a-5p, miR-146a-5p and miR-34a-5p by RT-qPCR in macrophages at all infection periods. ** p < 0.01, *** p < 0.001 compared with the negative control group.
The expression levels of these miRNAs, which are thought to be associated with inflammation, were detected using RT-qPCR in the DENV-1-infected group (MOI = 1) and in the negative control group, and they were compared and validated with the sequencing results. The results indicated that miR-223-3p and miR-148a-3p were significantly downregulated in the DENV-1 group, with their expression levels negatively correlated with the infection time. Conversely, miR-125a-5p, miR-146a-5p and miR-34a-5p were significantly upregulated in the DENV-1 group, among which miR-125a-5p and miR-146a-5p showed a positive correlation between their expression levels and the infection time. Notably, miR-34a-5p, which exhibited the most differential expression, reached its peak 48 h after infection and maintained a high level thereafter (Figure 5C). In summary, the miRNA qPCR expression trends were consistent with the sequencing results and significantly different from those in the negative control group.
4. Discussion
In this study, we first demonstrated that the DENV-1 infection of macrophages caused a decrease in cell survival rate and membrane disruption, resulting in an increase in LDH release, along with pro-inflammatory cytokines dividing from pro-IL-1β to active IL-1β and massive secretion. Subsequently, we further found that the DENV-1 infection of macrophages activated the NLRP3 inflammasome and caspase-1, cleaved GSDMD to a GSDMD-NT fragment, and combined with cell membrane liposomes to form pores, resulting in the excretion of LDH, IL-1β and other cell contents, causing inflammation and inducing pyroptosis.
Pyroptosis is a pro-inflammatory mode of programmed cell death, which distinguishes itself from apoptosis and autophagy by triggering a series of inflammatory responses [27]. NLRP3, the most extensively studied inflammasome within the NLR family, is associated with a wide range of diseases and functions as a component of its host’s innate immune system. NLRP3 can recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), and it can promote the assembly of an inflammasome complex with the adaptor protein ASC and the effector protease caspase-1, which ultimately lead to pyroptosis [28,29]. Pyroptosis can eliminate viral replication sites within infected cells and mediate innate immune responses in host defense [30]. However, excessive pyroptosis-induced inflammatory responses can result in tissue damage and exacerbate disease progression. For example, Zika virus (ZIKV) infection in mice induces neuronal cell pyroptosis and pathological alterations, leading to brain atrophy, which may be directly associated with microcephaly caused by ZIKV [31]. Furthermore, H7N9 influenza virus-induced alveolar epithelial cell pyroptosis triggers a cytokine storm, thereby increasing the mortality rate of infected mice [32].
Patients with severe DENV infection may present with clinical manifestations such as hemorrhage or even shock, mainly due to the increased permeability of vascular endothelial cells, leading to a large loss of intravascular plasma and albumin. Currently, cytokine storm is considered to be one of the main causes of vascular leakage [33]. Additionally, studies have shown that serum levels of IL-1β in DENV-infected patients are significantly higher than those in uninfected individuals [34]. Further research has revealed that compared to patients with DF, those with DHF produce higher levels of IL-1β [35], suggesting that excessive secretion of IL-1β may influence the clinical progression of the disease. Therefore, in DHF and DSS, we speculate that the large number of pro-inflammatory cytokines released due to pyroptosis may be one of the causes of the increased permeability of vascular endothelial cells and vascular leakage, but the specific mechanism still needs to be further explored.
MiRNAs have the ability to regulate multiple pathways, including inflammatory and innate immune responses [36]. In this study, we found that DENV-1 infection of macrophages caused multiple miRNAs to be differentially expressed, and the changes in miRNAs were further deepened with the extension of infection time. Enrichment analysis revealed that differentially expressed miRNAs predicted target genes highly enriched in protein phosphorylation, stress response and regulation of the cytoskeleton, suggesting that these target genes may be involved in protein activation and a series of antiviral immune responses, which are associated with vascular endothelial cell damage, increased permeability and leakage. In addition, 63 miRNAs with significant differences in the whole process of infection were identified by drawing Venn diagrams of differentially expressed miRNAs at different stages of infection, including miR-223-3p, miR-148a-3p, miR-125a-5p, miR-146a-5p and miR-34a-5p, which have been suggested to be associated with inflammatory responses.
MiR-223-3p is one of the most widely studied inflammatory regulators, with NLRP3 as its direct target, and it is able to negatively regulate inflammasome activation and pyroptosis in inflammatory responses, such as endothelial cell inflammation in syphilis spirochete infections, acute lung injuries and myocarditis [37,38,39]. MiR-148a-3p is also involved in the regulation of inflammation in atherosclerosis and brain injury [40,41]. MiR-125a-5p and miR-146a-5p, which are also reported to be involved in innate immune response and inflammation, have elevated expression in the peripheral blood of patients with rheumatoid arthritis, and they have been considered as biomarkers for detecting rheumatoid arthritis [42,43,44]. In addition, miR-125a-5p was found to promote vascular endothelial cell pyroptosis by inhibiting the DNA demethylase TET2, suggesting that miR-125a-5p is involved in the regulation of vascular function and inflammation [45]. In this study, DENV-1 infection of macrophages caused the most significant upregulation of miR-34a-5p. Previous studies have shown that adipocytes secreting excess miR-34a inhibited macrophage M2 polarization and exacerbated systemic inflammation [46]. Recently, miR-34a-5p was found to induce oxidative stress, inflammation and pyroptosis by negatively regulating the Sirtuin family of proteins (e.g., Sirtuin1, Sirtuin3), whereas inhibition of miR-34a-5p could play a protective role [47,48]. Among these miRNAs, only miR-223-3p and miR-146a have been studied to be involved in regulating DENV infection. miR-223-3p directly targets the microtubule-destabilizing protein stathmin 1 (STMN1) of DENV-2, thereby inhibiting its replication levels [49]. miR-146a promotes the replication of DENV-2 in THP-1 cells by targeting TRAF6 and inhibiting interferon induction. In A549 cells, miR-146a inhibits DENV-2-induced autophagy by targeting TRAF6 [50,51]. However, the relationships between miR-148a-3p, miR-125a-5p and miR-34a-5p with DENV infection have not been confirmed and require further investigation.
Enoxacin is a broad-spectrum antibacterial fluoroquinolone, and it has been proved to be an effective miRNA modulator. Studies have found that enoxacin inhibits the expression of miR-34a-5p in adipocytes, thereby enhancing FGF21 signaling to promote oxidative metabolism and combat obesity [52]. Additionally, based on in silico analysis, enoxacin has been identified as a potential inhibitor of SARS-CoV-2 through the RNAi pathway [53]. Interestingly, enoxacin may also influence the pathogenic mechanisms of DENV by regulating miRNAs.
There were some limitations to this study. Firstly, only DENV-1, which has caused significant human harm and economic losses in Zhejiang Province, was investigated in this study, while the other three serotypes were not studied in depth. Meanwhile, the detection methods could be diversified, such as using electron microscopy to observe cells and detecting other genes and proteins like IL-18 and ASC, which may provide more comprehensive validation of the pyroptotic response. Secondly, the role of miRNAs in regulating DENV-1-induced macrophage pyroptosis has not been verified. Lastly, this study was still confined to cellular experiments in vitro, and the specific molecular mechanisms by which miRNAs affect pyroptosis remain to be further explored.
In summary, our study revealed that DENV-1 induced macrophage pyroptosis and concurrently altered the expression profiles of specific miRNAs, namely miR-223-3p, miR-148a-3p, miR-125a-5p, miR-146a-5p and miR-34a-5p, which are known as small molecules regulating inflammation and pyroptosis. Based on these findings, we hypothesize that these miRNAs may serve as regulators in DENV-1-induced macrophage pyroptosis. Furthermore, verifying the effects of these miRNAs on DENV-1-induced pyroptosis and elucidating its specific mechanism will form the direction of our future research. In conclusion, our study provides a new perspective for exploring the pathogenic mechanism of DENV, suggesting that miRNAs may be a promising therapeutic target for the treatment of DENV-infected patients.
Author Contributions
Conceptualization, H.Y. and Y.Z.; funding acquisition, H.Y. and Y.Z.; methodology, Q.Z., S.Y., X.W., L.S. and H.Y.; project administration, H.Y. and Y.Z.; resources, Z.Y., J.L., X.L., H.M., Y.S. and H.Y.; software, Q.Z., H.Z., Z.Y. and L.F.; writing—original draft, Q.Z.; writing—review and editing, H.Y. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Medical Science and Technology Program of Zhejiang Province (2024KY888, 2024KY907, 2021KY620) and the Medical Science and Technology Program of Hangzhou (B20230010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are provided within the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Paz-Bailey, G.; Adams, L.E.; Deen, J.; Anderson, K.B.; Katzelnick, L.C. Dengue. Lancet 2024, 403, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.M. Challenges and opportunities in controlling mosquito-borne infections. Nature 2018, 559, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Bambrick, H.; Frentiu, F.D.; Devine, G.; Yakob, L.; Williams, G.; Hu, W. Projecting the future of dengue under climate change scenarios: Progress, uncertainties and research needs. PLoS Negl. Trop. Dis. 2020, 14, e0008118. [Google Scholar] [CrossRef]
- Wan, S.W.; Wu-Hsieh, B.A.; Lin, Y.S.; Chen, W.Y.; Huang, Y.; Anderson, R. The monocyte-macrophage-mast cell axis in dengue pathogenesis. J. Biomed. Sci. 2018, 25, 77. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Wu, M.F.; Chen, S.T.; Yang, A.H.; Lin, W.W.; Lin, Y.L.; Chen, N.J.; Tsai, I.S.; Li, L.; Hsieh, S.L. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood 2013, 121, 95–106. [Google Scholar] [CrossRef]
- Cheung, K.T.; Sze, D.M.; Chan, K.H.; Leung, P.H. Involvement of caspase-4 in IL-1β production and pyroptosis in human macrophages during dengue virus infection. Immunobiology 2018, 223, 356–364. [Google Scholar] [CrossRef]
- Trobaugh, D.W.; Klimstra, W.B. MicroRNA Regulation of RNA Virus Replication and Pathogenesis. Trends Mol. Med. 2017, 23, 80–93. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, T.; Chi, Y.; Ge, Y.; Jiao, Y.; Zhu, F.; Cui, L. MicroRNA-195 suppresses enterovirus A71-induced pyroptosis in human neuroblastoma cells through targeting NLRX1. Virus Res. 2021, 292, 198245. [Google Scholar] [CrossRef]
- Pong, L.Y.; Parkkinen, S.; Dhanoa, A.; Gan, H.M.; Wickremesinghe, I.A.C.; Syed Hassan, S. MicroRNA profiling of mouse liver in response to DENV-1 infection by deep sequencing. PeerJ 2019, 7, e6697. [Google Scholar] [CrossRef] [PubMed]
- Rossi, Á.D.; Higa, L.M.; Herlinger, A.L.; Ribeiro-Alves, M.; de Menezes, M.T.; Giannini, A.L.M.; Cardoso, C.C.; Da Poian, A.T.; Tanuri, A.; Aguiar, R.S. Differential Expression of Human MicroRNAs During Dengue Virus Infection in THP-1 Monocytes. Front. Cell. Infect. Microbiol. 2021, 11, 714088. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.R.; Abd-Aziz, N.; Affendi, S.; Poh, C.L. Role of microRNAs in antiviral responses to dengue infection. J. Biomed. Sci. 2020, 27, 4. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lin, T.; Liu, C.; Cheng, C.; Han, X.; Jiang, X. microRNAs, the Link Between Dengue Virus and the Host Genome. Front. Microbiol. 2021, 12, 714409. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Xin, C.W.; Chu, J.J.H. MicroRNA 573 Rescues Endothelial Dysfunction during Dengue Virus Infection under PPARγ Regulation. J. Virol. 2022, 96, e0199621. [Google Scholar] [CrossRef]
- Ahmed, N.; Ahmed, N.; Pezacki, J.P. miR-383 Regulates Hepatic Lipid Homeostasis and Response to Dengue Virus Infection. ACS Infect. Dis. 2022, 8, 928–941. [Google Scholar] [CrossRef]
- Ren, J.; Chen, Z.; Ling, F.; Huang, Y.; Gong, Z.; Liu, Y.; Mao, Z.; Lin, C.; Yan, H.; Shi, X.; et al. Epidemiology of Indigenous Dengue Cases in Zhejiang Province, Southeast China. Front. Public Health 2022, 10, 857911. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Ge, L.; Quan, R.; Chen, J.; Hu, Y.; Sa, R.; Liu, J.; Ran, D.; Fu, Q.; et al. Berbamine, a bioactive alkaloid, suppresses equine herpesvirus type 1 in vitro and in vivo. Front. Vet. Sci. 2023, 10, 1163780. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, J.; Li, Z.; Li, X.; Hu, X.; Huang, Y.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L.; et al. Integrated profiling of microRNAs and mRNAs: microRNAs located on Xq27.3 associate with clear cell renal cell carcinoma. PLoS ONE 2010, 5, e15224. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, M.; Zhang, Y.; Miao, E.A. Detection of pyroptosis by measuring released lactate dehydrogenase activity. Methods Mol. Biol. 2013, 1040, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, Q. Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling. Front. Immunol. 2023, 14, 1128358. [Google Scholar] [CrossRef]
- Robinson, N.; Ganesan, R.; Hegedűs, C.; Kovács, K.; Kufer, T.A.; Virág, L. Programmed necrotic cell death of macrophages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019, 26, 101239. [Google Scholar] [CrossRef]
- Pan, Y.; Cai, W.; Cheng, A.; Wang, M.; Yin, Z.; Jia, R. Flaviviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines. Front. Immunol. 2022, 13, 829433. [Google Scholar] [CrossRef]
- Baroja-Mazo, A.; Martín-Sánchez, F.; Gomez, A.I.; Martínez, C.M.; Amores-Iniesta, J.; Compan, V.; Barberà-Cremades, M.; Yagüe, J.; Ruiz-Ortiz, E.; Antón, J.; et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 2014, 15, 738–748. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- He, Z.; An, S.; Chen, J.; Zhang, S.; Tan, C.; Yu, J.; Ye, H.; Wu, Y.; Yuan, J.; Wu, J.; et al. Neural progenitor cell pyroptosis contributes to Zika virus-induced brain atrophy and represents a therapeutic target. Proc. Natl. Acad. Sci. USA 2020, 117, 23869–23878. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Li, J.; Wang, Y.; Yu, X.; He, X.; Shi, J.; Deng, G.; Zeng, X.; Tian, G.; Li, Y.; et al. H7N9 virus infection triggers lethal cytokine storm by activating gasdermin E-mediated pyroptosis of lung alveolar epithelial cells. Natl. Sci. Rev. 2022, 9, nwab137. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kishimoto, T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 2021, 53, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Lopes, J.F.; Freitas, C.; Valls-de-Souza, R.; Oliveira, M.F.; Bozza, M.T.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, F.A.; et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 2013, 122, 3405–3414. [Google Scholar] [CrossRef]
- Cui, L.; Lee, Y.H.; Thein, T.L.; Fang, J.; Pang, J.; Ooi, E.E.; Leo, Y.S.; Ong, C.N.; Tannenbaum, S.R. Serum Metabolomics Reveals Serotonin as a Predictor of Severe Dengue in the Early Phase of Dengue Fever. PLoS Negl. Trop. Dis. 2016, 10, e0004607. [Google Scholar] [CrossRef]
- Nejad, C.; Stunden, H.J.; Gantier, M.P. A guide to miRNAs in inflammation and innate immune responses. FEBS J. 2018, 285, 3695–3716. [Google Scholar] [CrossRef]
- Long, F.Q.; Kou, C.X.; Li, K.; Wu, J.; Wang, Q.Q. miR-223-3p inhibits rTp17-induced inflammasome activation and pyroptosis by targeting NLRP3. J. Cell. Mol. Med. 2020, 24, 14405–14414. [Google Scholar] [CrossRef]
- Zhang, M.; Lan, H.; Peng, S.; Zhou, W.; Wang, X.; Jiang, M.; Hong, J.; Zhang, Q. miR-223-3p attenuates radiation-induced inflammatory response and inhibits the activation of NLRP3 inflammasome in macrophages. Int. Immunopharmacol. 2023, 122, 110616. [Google Scholar] [CrossRef]
- Pan, L.; Yan, B.; Zhang, J.; Zhao, P.; Jing, Y.; Yu, J.; Hui, J.; Lu, Q. Mesenchymal stem cells-derived extracellular vesicles-shuttled microRNA-223-3p suppress lipopolysaccharide-induced cardiac inflammation, pyroptosis, and dysfunction. Int. Immunopharmacol. 2022, 110, 108910. [Google Scholar] [CrossRef]
- Wang, F.; Ge, J.; Huang, S.; Zhou, C.; Sun, Z.; Song, Y.; Xu, Y.; Ji, Y. KLF5/LINC00346/miR-148a-3p axis regulates inflammation and endothelial cell injury in atherosclerosis. Int. J. Mol. Med. 2021, 48, 152. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, S.; Yin, Y.; Yu, J.; Liu, Y.; Gao, H. Dexmedetomidine suppresses hippocampal astrocyte pyroptosis in cerebral hypoxic-ischemic neonatal rats by upregulating microRNA-148a-3p to inactivate the STAT/JMJD3 axis. Int. Immunopharmacol. 2023, 121, 110440. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, T.S.; Jo, E.K. miR-146 and miR-125 in the regulation of innate immunity and inflammation. BMB Rep. 2016, 49, 311–318. [Google Scholar] [CrossRef]
- Churov, A.V.; Oleinik, E.K.; Knip, M. MicroRNAs in rheumatoid arthritis: Altered expression and diagnostic potential. Autoimmun. Rev. 2015, 14, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhong, S.; He, S.; Weng, J.; Liu, L.; Ye, Y.; Chen, H. Biomarkers (mRNAs and non-coding RNAs) for the diagnosis and prognosis of rheumatoid arthritis. Front. Immunol. 2023, 14, 1087925. [Google Scholar] [CrossRef] [PubMed]
- Zhaolin, Z.; Jiaojiao, C.; Peng, W.; Yami, L.; Tingting, Z.; Jun, T.; Shiyuan, W.; Jinyan, X.; Dangheng, W.; Zhisheng, J.; et al. OxLDL induces vascular endothelial cell pyroptosis through miR-125a-5p/TET2 pathway. J. Cell. Physiol. 2019, 234, 7475–7491. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hui, X.; Hoo, R.L.C.; Ye, D.; Chan, C.Y.C.; Feng, T.; Wang, Y.; Lam, K.S.L.; Xu, A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J. Clin. Investig. 2019, 129, 834–849. [Google Scholar] [CrossRef]
- Hao, R.; Ge, J.; Li, F.; Jiang, Y.; Sun-Waterhouse, D.; Li, D. miR-34a-5p/Sirt1 axis: A novel pathway for puerarin-mediated hepatoprotection against benzo(a)pyrene. Free Radic. Biol. Med. 2022, 186, 53–65. [Google Scholar] [CrossRef]
- Zhong, Z.; Gao, Y.; Zhou, J.; Wang, F.; Zhang, P.; Hu, S.; Wu, H.; Lou, H.; Chi, J.; Lin, H.; et al. Inhibiting miR-34a-5p regulates doxorubicin-induced autophagy disorder and alleviates myocardial pyroptosis by targeting Sirt3-AMPK pathway. Biomed. Pharmacother. 2023, 168, 115654. [Google Scholar] [CrossRef]
- Wu, N.; Gao, N.; Fan, D.; Wei, J.; Zhang, J.; An, J. miR-223 inhibits dengue virus replication by negatively regulating the microtubule-destabilizing protein STMN1 in EAhy926 cells. Microbes Infect. 2014, 16, 911–922. [Google Scholar] [CrossRef]
- Wu, S.; He, L.; Li, Y.; Wang, T.; Feng, L.; Jiang, L.; Zhang, P.; Huang, X. miR-146a facilitates replication of dengue virus by dampening interferon induction by targeting TRAF6. J. Infect. 2013, 67, 329–341. [Google Scholar] [CrossRef]
- Pu, J.; Wu, S.; Xie, H.; Li, Y.; Yang, Z.; Wu, X.; Huang, X. miR-146a Inhibits dengue-virus-induced autophagy by targeting TRAF6. Arch. Virol. 2017, 162, 3645–3659. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.L.; de Lima, T.I.; de Souza, G.P.; Corrêa, R.O.; Ferrucci, D.L.; Rodrigues, B.; Lopes-Ramos, C.; Nilsson, D.; Knittel, T.L.; Castro, P.R.; et al. Enoxacin induces oxidative metabolism and mitigates obesity by regulating adipose tissue miRNA expression. Sci. Adv. 2020, 6, eabc6250. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Moradi, S. In silico analysis suggests the RNAi-enhancing antibiotic enoxacin as a potential inhibitor of SARS-CoV-2 infection. Sci. Rep. 2021, 11, 10271. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).