The Role of Sphingolipid Metabolism in Pregnancy-Associated Breast Cancer After Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Study Population
2.3. RNA Isolation, Reverse Transcription, and Real-Time PCR
2.4. Mass Spectrometry of Sphingolipids
2.5. Statistical Analysis
3. Results
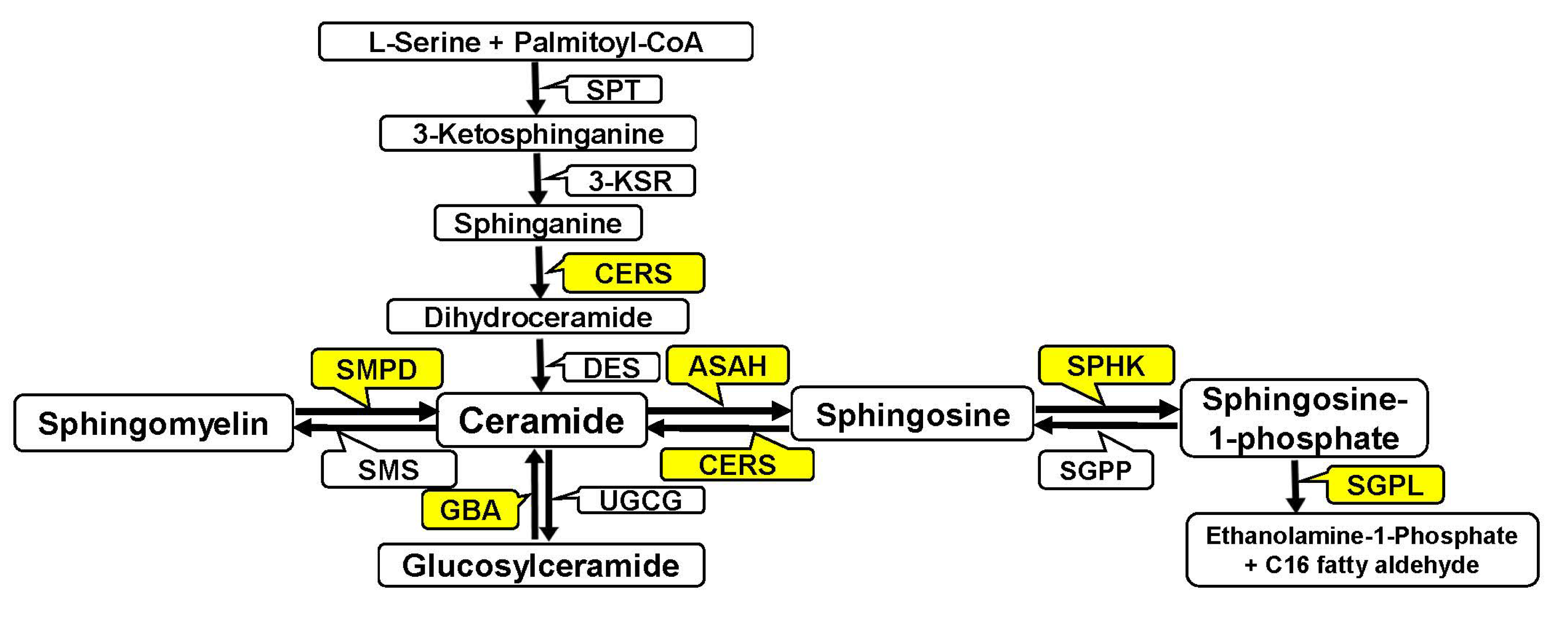
3.1. Analysis of Gene Expression of Enzymes of Ceramide and Sphingoid Base Metabolism (Sphingosine and S1P) in Pregnancy-Associated Breast Cancer After Treatment
3.1.1. Analysis of Sphingomyelinase Gene Expression in Pregnancy-Associated Breast Cancer After Treatment
3.1.2. Analysis of Ceramide Synthase Gene Expression in Pregnancy-Associated Breast Cancer After Treatment
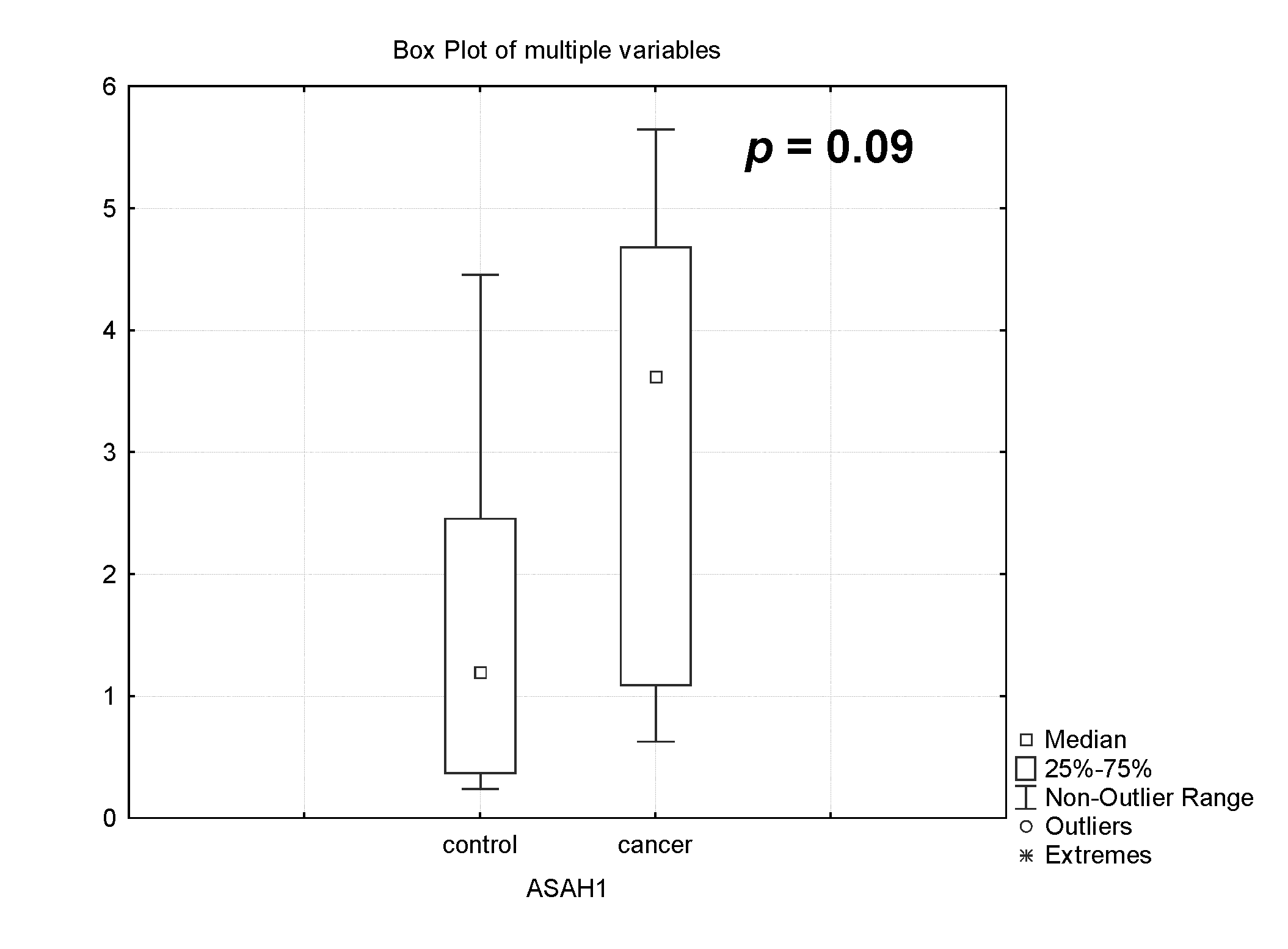
3.1.3. Analysis of Acid Ceramidase (ASAH1) Gene Expression in Pregnancy-Associated Breast Cancer After Treatment
3.2. Analysis of Ceramide Species Level in the Placenta of Healthy Pregnant Women and Women with Pregnancy-Associated Breast Cancer After Chemotherapy
3.3. Changes in the Expression of Genes Controlling S1P Metabolism and Level of Sphingosine-1-Phosphate in the Placenta of Healthy Pregnant Women and Women with Pregnancy-Associated Breast Cancer After Chemotherapy
3.3.1. Analysis of Sphingosine and Sphingosine-1-Phosphate Level in the Placenta of Healthy Pregnant Women and Women with Pregnancy-Associated Breast Cancer After Chemotherapy
3.3.2. Analysis of Sphingosine-1-Phosphate Receptors (S1PR1, S1PR2 and S1PR3) Gene Expression in Pregnancy-Associated Breast Cancer After Treatment
3.4. The Effect of PABC and Therapy on the Newborn and Mother
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASAH1 | acid ceramidase |
| GBA | glucosylceramidase |
| CDase | ceramidase |
| CER | ceramide |
| CER + DTX | CER in combination with docetaxel |
| C1P | ceramide-1-phosphate |
| CERS 1–6 | ceramide synthases |
| DTX | docetaxel |
| FTY720 | fingolimod |
| GCS | glucosylceramide synthase |
| MCF-7 | human breast carcinoma cell |
| MRM | multiple reaction monitoring |
| PABC | pregnancy-associated breast cancer |
| S1P | sphingosine-1-phosphate |
| SPH | sphingosine |
| SGPL1 | sphingosine-1-phosphate lyase 1 |
| SMPD1 | acidic sphingomyelinase |
| SMPD3 | neutral sphingomyelinase |
| SMS | sphingomyelin synthase |
| SPHK1 | sphingosine kinase1 |
References
- Alkafaas, S.S.; Elsalahaty, M.I.; Ismail, D.F.; Radwan, M.A.; Elkafas, S.S.; Loutfy, S.A.; Elshazli, R.M.; Baazaoui, N.; Ahmed, A.E.; Hafez, W.; et al. The Emerging Roles of Sphingosine 1-Phosphate and SphK1 in Cancer Resistance: A Promising Therapeutic Target. Cancer Cell Int. 2024, 24, 89. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Z.; Wang, X.-R.; Wang, J.; Xie, C.; Wang, X.-X.; Pan, H.-D.; Meng, W.-Y.; Liang, T.-L.; Li, J.-X.; Yan, P.-Y.; et al. The Key Role of Sphingolipid Metabolism in Cancer: New Therapeutic Targets, Diagnostic and Prognostic Values, and Anti-Tumor Immunotherapy Resistance. Front. Oncol. 2022, 12, 941643. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, T.; Giuliano, A.E.; Cabot, M.C. Ceramide Glycosylation Potentiates Cellular Multidrug Resistance. FASEB J. 2001, 15, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, Y.; Wang, S.; Cao, B.; Li, C.; Li, G. Sphingolipid Metabolism and Signaling in Lung Cancer: A Potential Therapeutic Target. J. Oncol. 2022, 2022, 9099612. [Google Scholar] [CrossRef]
- Noda, S.; Yoshimura, S.; Sawada, M.; Naganawa, T.; Iwama, T.; Nakashima, S.; Sakai, N. Role of Ceramide during Cisplatin-Induced Apoptosis in C6 Glioma Cells. J. Neuro-Oncol. 2001, 52, 11–21. [Google Scholar] [CrossRef]
- Pherez-Farah, A.; López-Sánchez, R.D.C.; Villela-Martínez, L.M.; Ortiz-López, R.; Beltrán, B.E.; Hernández-Hernández, J.A. Sphingolipids and Lymphomas: A Double-Edged Sword. Cancers 2022, 14, 2051. [Google Scholar] [CrossRef]
- Piazzesi, A.; Afsar, S.Y.; Van Echten-Deckert, G. Sphingolipid Metabolism in the Development and Progression of Cancer: One Cancer’s Help Is Another’s Hindrance. Mol. Oncol. 2021, 15, 3256–3279. [Google Scholar] [CrossRef]
- Pitman, M.; Oehler, M.K.; Pitson, S.M. Sphingolipids as Multifaceted Mediators in Ovarian Cancer. Cell. Signal. 2021, 81, 109949. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, L.; Mao, F.; Chen, J. Sphingolipids in Prostate Cancer Prognosis: Integrating Single-Cell and Bulk Sequencing. Aging 2024, 16, 8031. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of Bioactive Lipid Signalling: Lessons from Sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Clarke, C.J. Neutral Sphingomyelinases in Cancer: Friend or Foe? In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 140, pp. 97–119. ISBN 978-0-12-814223-3. [Google Scholar]
- Datta, A.; Loo, S.Y.; Huang, B.; Wong, L.; Tan, S.S.L.; Tan, T.Z.; Lee, S.-C.; Thiery, J.P.; Lim, Y.C.; Yong, W.P.; et al. SPHK1 Regulates Proliferation and Survival Responses in Triple-Negative Breast Cancer. Oncotarget 2014, 5, 5920–5933. [Google Scholar] [CrossRef] [PubMed]
- Hertervig, E.; Nilsson, A.; Nyberg, L.; Duan, R.D. Alkaline Sphingomyelinase Activity Is Decreased in Human Colorectal Carcinoma. Cancer 1997, 3, 448–453. [Google Scholar] [CrossRef]
- Jacobi, J.; García-Barros, M.; Rao, S.; Rotolo, J.A.; Thompson, C.; Mizrachi, A.; Feldman, R.; Manova, K.; Bielawska, A.; Bielawska, J.; et al. Targeting Acid Sphingomyelinase with Anti-Angiogenic Chemotherapy. Cell. Signal. 2017, 29, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Miyoshi, Y. Targeting Sphingosine-1-Phosphate Signaling in Breast Cancer. Available online: https://www.mdpi.com/1422-0067/25/6/3354 (accessed on 4 September 2024).
- Chen, H.; Haddadi, N.; Zhu, X.; Hatoum, D.; Chen, S.; Nassif, N.T.; Lin, Y.; McGowan, E.M. Expression Profile of Sphingosine Kinase 1 Isoforms in Human Cancer Tissues and Cells: Importance and Clinical Relevance of the Neglected 1b-Isoform. J. Oncol. 2022, 2022, 2250407. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, F.; Chen, K.; Tian, M.; Zhao, D. Sphingosine Kinase 1 as an Anticancer Therapeutic Target. Drug Des. Dev. Ther. 2015, 9, 3239–3245. [Google Scholar] [CrossRef]
- Fakhr, Y.; Brindley, D.N.; Hemmings, D.G. Physiological and Pathological Functions of Sphingolipids in Pregnancy. Cell. Signal. 2021, 85, 110041. [Google Scholar] [CrossRef]
- Huang, Q.; Hao, S.; Yao, X.; You, J.; Li, X.; Lai, D.; Han, C.; Schilling, J.; Hwa, K.Y.; Thyparambil, S.; et al. High-Throughput Quantitation of Serological Ceramides/Dihydroceramides by LC/MS/MS: Pregnancy Baseline Biomarkers and Potential Metabolic Messengers. J. Pharm. Biomed. Anal. 2021, 192, 113639. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Olson, D.M.; Bennekom, M.V.; Brindley, D.N.; Hemmings, D.G. Increased Expression of Enzymes for Sphingosine 1-Phosphate Turnover and Signaling in Human Decidua During Late Pregnancy1. Biol. Reprod. 2010, 82, 628–635. [Google Scholar] [CrossRef]
- Apicella, C.; Ruano, C.S.M.; Thilaganathan, B.; Khalil, A.; Giorgione, V.; Gascoin, G.; Marcellin, L.; Gaspar, C.; Jacques, S.; Murdoch, C.E.; et al. Pan-Genomic Regulation of Gene Expression in Normal and Pathological Human Placentas. Cells 2023, 12, 578. [Google Scholar] [CrossRef]
- Suryawanshi, H.; Max, K.; Bogardus, K.A.; Sopeyin, A.; Chang, M.S.; Morozov, P.; Castano, P.M.; Tuschl, T.; Williams, Z. Dynamic Genome-Wide Gene Expression and Immune Cell Composition in the Developing Human Placenta. J. Reprod. Immunol. 2022, 151, 103624. [Google Scholar] [CrossRef]
- Saben, J.; Zhong, Y.; McKelvey, S.; Dajani, N.K.; Andres, A.; Badger, T.M.; Gomez-Acevedo, H.; Shankar, K. A Comprehensive Analysis of the Human Placenta Transcriptome. Placenta 2014, 35, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, A.M.; Nabekura, T.; Kaddoumi, A.; Bammler, T.K.; Govindarajan, R.; Hebert, M.F.; Unadkat, J.D. Profiling Gene Expression in Human Placentae of Different Gestational Ages: An OPRU Network and UW SCOR Study. Reprod. Sci. 2008, 15, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Alexander, H. Lead Genetic Studies in Dictyostelium Discoideum and Translational Studies in Human Cells Demonstrate That Sphingolipids Are Key Regulators of Sensitivity to Cisplatin and Other Anticancer Drugs. Semin. Cell Dev. Biol. 2011, 22, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Larrauri, A.; Das Adhikari, U.; Aramburu-Nuñez, M.; Custodia, A.; Ouro, A. Ceramide Metabolism Enzymes—Therapeutic Targets against Cancer. Medicina 2021, 57, 729. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Xu, X.; Lim, Y.-W.; Iau, P.; Sukri, N.; Lim, S.-E.; Yap, H.L.; Yeo, W.-L.; Tan, P.; Tan, S.-H.; et al. Chemotherapy-Induced Tumor Gene Expression Changes in Human Breast Cancers. Pharmacogenetics Genom. 2009, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Stegner, A.L.; Alexander, H.; Alexander, S. Overexpression of Sphingosine-1-Phosphate Lyase or Inhibition of Sphingosine Kinase in Dictyostelium Discoideum Results in a Selective Increase in Sensitivity to Platinum-Based Chemotherapy Drugs. Eukaryot. Cell 2004, 3, 795–805. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prinetti, A.; Millimaggi, D.; D’Ascenzo, S.; Clarkson, M.; Bettiga, A.; Chigorno, V.; Sonnino, S.; Pavan, A.; Dolo, V. Lack of Ceramide Generation and Altered Sphingolipid Composition Are Associated with Drug Resistance in Human Ovarian Carcinoma Cells. Biochem. J. 2006, 395, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Sattar, R.S.A.; Sumi, M.P.; Kumar, A.; Sharma, A.K.; Ahmad, E.; Ali, A.; Mahajan, B.; Saluja, S.S. S1P Signaling, Its Interactions and Cross-Talks with Other Partners and Therapeutic Importance in Colorectal Cancer. Cell. Signal. 2021, 86, 110080. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, V.; Priceman, S.J.; Li, W.; Cherryholmes, G.; Lee, H.; Makovski-Silverstein, A.; Borriello, L.; DeClerck, Y.A.; Yu, H. Sphingosine-1-Phosphate Receptor-1 Promotes Environment-Mediated and Acquired Chemoresistance. Mol. Cancer Ther. 2017, 16, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Spiegel, S. Sphingosine-1-Phosphate Signaling: A Novel Target for Simultaneous Adjuvant Treatment of Triple Negative Breast Cancer and Chemotherapy-Induced Neuropathic Pain. Adv. Biol. Regul. 2020, 75, 100670. [Google Scholar] [CrossRef] [PubMed]
- Das, M. GM1 for Taxane-Induced Neuropathy in Breast Cancer. Lancet Oncol. 2019, 20, e348. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.-X.; Li, M.; Liu, Y.-J.; Yang, S.-M.; Zhang, N. Synergistic Enhancement of Cancer Therapy Using a Combination of Ceramide and Docetaxel. Int. J. Mol. Sci. 2014, 15, 4201–4220. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.L.; Olzomer, E.M.; Lolies, N.; Hoehn, K.L.; Wegner, M.-S. Update on Glycosphingolipids Abundance in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 4477. [Google Scholar] [CrossRef] [PubMed]
- Saddoughi, S.A.; Gencer, S.; Peterson, Y.K.; Ward, K.E.; Mukhopadhyay, A.; Oaks, J.; Bielawski, J.; Szulc, Z.M.; Thomas, R.J.; Selvam, S.P.; et al. Sphingosine Analogue Drug FTY720 Targets I2PP2A/SET and Mediates Lung Tumour Suppression via Activation of PP2A-RIPK1-dependent Necroptosis. EMBO Mol. Med. 2013, 5, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Takahara, S.; Horie, S.; Muto, S.; Otsuki, Y.; Katsuoka, Y. Induction of Apoptosis in Human Bladder Cancer Cells In Vitro and In Vivo Caused by FTY720 Treatment. J. Urol. 2003, 169, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Takahara, S.; Ichimaru, N.; Wang, J.D.; Itoh, Y.; Otsuki, Y.; Morimoto, J.; Fukui, R.; Hoshiga, M.; Ishihara, T.; et al. Marked Prevention of Tumor Growth and Metastasis by a Novel Immunosuppressive Agent, FTY720, in Mouse Breast Cancer Models. Cancer Res. 2002, 62, 1410–1419. [Google Scholar]
- Hait, N.C.; Avni, D.; Yamada, A.; Nagahashi, M.; Aoyagi, T.; Aoki, H.; Dumur, C.I.; Zelenko, Z.; Gallagher, E.J.; Leroith, D.; et al. The Phosphorylated Prodrug FTY720 Is a Histone Deacetylase Inhibitor That Reactivates ERα Expression and Enhances Hormonal Therapy for Breast Cancer. Oncogenesis 2015, 4, e156. [Google Scholar] [CrossRef]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of Histone Acetylation in the Nucleus by Sphingosine-1-Phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef]
- Lim, K.G.; Tonelli, F.; Li, Z.; Lu, X.; Bittman, R.; Pyne, S.; Pyne, N.J. FTY720 Analogues as Sphingosine Kinase 1 Inhibitors: Enzyme Inhibition Kinetics, Allosterism, Proteasomal Degradation, and Actin Rearrangement in MCF-7 Breast Cancer Cells. J. Biol. Chem. 2011, 286, 18633–18640. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Molino, S.; Tate, E.; McKillop, W.; Medin, J. Sphingolipid Pathway Enzymes Modulate Cell Fate and Immune Responses. Immunotherapy 2017, 9, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; El Buri, A.; Adams, D.R.; Pyne, S. Sphingosine 1-Phosphate and Cancer. Adv. Biol. Regul. 2018, 68, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; McNaughton, M.; Boomkamp, S.; MacRitchie, N.; Evangelisti, C.; Martelli, A.M.; Jiang, H.-R.; Ubhi, S.; Pyne, S. Role of Sphingosine 1-Phosphate Receptors, Sphingosine Kinases and Sphingosine in Cancer and Inflammation. Adv. Biol. Regul. 2016, 60, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Pettus, B.J.; Chalfant, C.E.; Hannun, Y.A. Ceramide in Apoptosis: An Overview and Current Perspectives. Biochim. Biophys. Acta 2002, 1585, 114–125. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Many Ceramides. J. Biol. Chem. 2011, 286, 27855–27862. [Google Scholar] [CrossRef]
- Pyne, N.J.; Tonelli, F.; Lim, K.G.; Long, J.S.; Edwards, J.; Pyne, S. Sphingosine 1-Phosphate Signalling in Cancer. Biochem. Soc. Trans. 2012, 40, 94–100. [Google Scholar] [CrossRef]
- Healthcare in Russia. Statistical Book; Rosstat: Moscow, Russia, 2023. [Google Scholar]
- Margioula-Siarkou, G.; Margioula-Siarkou, C.; Petousis, S.; Vavoulidis, E.; Margaritis, K.; Almperis, A.; Haitoglou, C.; Mavromatidis, G.; Dinas, K. Breast Carcinogenesis during Pregnancy: Molecular Mechanisms, Maternal and Fetal Adverse Outcomes. Biology 2023, 12, 408. [Google Scholar] [CrossRef]
- Ohuma, E.O.; Moller, A.-B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, Regional, and Global Estimates of Preterm Birth in 2020, with Trends from 2010: A Systematic Analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef]
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, Regional, and National Causes of under-5 Mortality in 2000–19: An Updated Systematic Analysis with Implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef]







| Parameter | Data |
|---|---|
| Age, median -Minimum–maximum | 37 years old 31–43 years old |
| Stage of the disease: -II -III | -5 out of 7 (71.4%) -2 out of 7 (28.6%) |
| Luminal A Luminal B HER2-negative Luminal B HER2-positive Triple negative | -1 out of 7 (14%) -3 out of 7 (43%) -1 out of 7 (14%) -2 out of 7 (29%) |
| Chemotherapy: -Taxanes -Chloroethylamines, anthracyclines -Taxanes, chloroethylamines, anthracyclines -Taxanes, chloroethylamines | -1 out of 7 (14.3%) -4 out of 7 (57.1%) -1 out of 7 (14.3%) -1 out of 7 (14.3%) |
| Gene, Number in NCBI | Sequence | Length, b.p. |
|---|---|---|
| SPHK1 NM_001142602.2 | F: GAGCAGGTCACCAATGAAG R: ATCAGCAATGAAGCCCCAG | 150 |
| SGPL1 NM_003901.4 | F: TTCCATTCCCCATCTCAGG R: CACACACACACACACACAC | 240 |
| ASAH1 NM_001363743.2 | F: AGTCAATAGCTTGTCTTCGTC R: GTGTTTACTGTCCCGTTACTC | 265 |
| SMPD1 NM_001365135.2 | F: AGTCAATAGCTTGTCTTCGTC R: GTGTTTACTGTCCCGTTACTC | 265 |
| GBA1 NM_001171812.2 | F: GCCACAGCATCATCACGAAC R: TAGCACGACCACAACAGCAG | 293 |
| SMPD3 NM_018667.4 | F: CCTTCATACCCACCACCTAC R: CAGAAGAGAAAGCCGAGAAAC | 145 |
| CERS2 NM_022075.5 | F: CACCCCATCCTCAATAACAAC R: CCTCTCACTTTCTCCTTTTTCC | 148 |
| CERS1 NM_001387444.1 | F: CCCCAAGCCTACTCCAAAAC R: AACTACTCCTCACCACCCAC | 216 |
| CERS4 NM_024552.3 | F: AGACCAGGAGGCAAGTGAAG R: CGAAGGAGGACAGGTAGAAGAG | 225 |
| CERS6 NM_203463.3 | F: AGGACAGGAGTGGACAAAG R: AGGGGAAAAGCGAGATAGAG | 154 |
| CERS3 NM_001378789.1 | F: GAAGAGGAAGAGGAAGAGGAAG R: TGGTGAGAAAGAGGGAAGGG | 226 |
| CERS5 NM_147190.5 | F: GCCCTTCCCATATCTACTCTTC R: GCACAAACGCACATCAAC | 179 |
| S1PR1 NM_001400.5 | F: AATTCAGCCGCAGCAAATC R: AACTCTACCCACCAACACCC | 279 |
| S1PR2 NM_004230.4 | F: TGTATGGCAGCGACAAGAG R: ACAGGATGATGGAGAAGATGG | 192 |
| S1PR3 NM_005226.4 | F: CCCACTCTTCATCCTCTTCC R: GCTGCTATTGTTGCTGCTG | 268 |
| Sphingolipid | MRM Transition | Fragmentor Voltage, V | Collision Energy, V | Dwell Time, ms | Capillary Voltage, kV |
|---|---|---|---|---|---|
C16 Cer (d18:1/16:0) C22 Cer (d18:1/22:0) C24 Cer (d18:1/24:0) C24:1 Cer (d18:1/24:1(15Z)) Sph S1P | 538.0→264.2 520.0→264.2 622.5→264.2 604.5→264.2 650.5→264.2 632.5→264.2 649.0→264.2 631.0→264.2 305.5→264.2 380.5→362.5 | 100 150 100 150 100 100 100 100 150 100 | 35 20 35 35 35 35 35 35 5 15 | 45 45 45 45 55 55 | 4000 4000 4000 4000 3000 3000 |
| Number of Patient | Pathological Condition | Number of Pathological Conditions in the Newborn |
|---|---|---|
| BC1 BC2 BC3 BC4 BC5 BC6 BC7 C1 C2 C3 C4 C5 C6 C7 C8 | Physiological birth tumor, Rash on face Toxic erythema, rhinitis, otitis, jaundice Interatrial communication, thermoregulation disorder, asymmetry of the sizes of the lateral ventricles of the brain, birth tumor Ossification disorder of the skull bones Serious condition, respiratory distress syndrome, central nervous system depression, patent ductus arteriosus, interatrial communication, thermoregulation disorder, anemia No Jaundice, anemia, physiological birth tumor No No No Physiological birth tumor No Physiological birth tumor No No | 2 4 4 1 7 0 3 0 0 0 1 0 1 0 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blokhin, V.; Zavarykina, T.; Kotsuba, V.; Kapralova, M.; Gutner, U.; Shupik, M.; Kozyrko, E.; Luzina, E.; Lomskova, P.; Bajgazieva, D.; et al. The Role of Sphingolipid Metabolism in Pregnancy-Associated Breast Cancer After Chemotherapy. Biomedicines 2024, 12, 2843. https://doi.org/10.3390/biomedicines12122843
Blokhin V, Zavarykina T, Kotsuba V, Kapralova M, Gutner U, Shupik M, Kozyrko E, Luzina E, Lomskova P, Bajgazieva D, et al. The Role of Sphingolipid Metabolism in Pregnancy-Associated Breast Cancer After Chemotherapy. Biomedicines. 2024; 12(12):2843. https://doi.org/10.3390/biomedicines12122843
Chicago/Turabian StyleBlokhin, Victor, Tatiana Zavarykina, Vasily Kotsuba, Maria Kapralova, Uliana Gutner, Maria Shupik, Elena Kozyrko, Evgenia Luzina, Polina Lomskova, Darya Bajgazieva, and et al. 2024. "The Role of Sphingolipid Metabolism in Pregnancy-Associated Breast Cancer After Chemotherapy" Biomedicines 12, no. 12: 2843. https://doi.org/10.3390/biomedicines12122843
APA StyleBlokhin, V., Zavarykina, T., Kotsuba, V., Kapralova, M., Gutner, U., Shupik, M., Kozyrko, E., Luzina, E., Lomskova, P., Bajgazieva, D., Khokhlova, S., & Alessenko, A. (2024). The Role of Sphingolipid Metabolism in Pregnancy-Associated Breast Cancer After Chemotherapy. Biomedicines, 12(12), 2843. https://doi.org/10.3390/biomedicines12122843









