The Interplay of Molecular Factors and Morphology in Human Placental Development and Implantation
Abstract
:1. Introduction
2. The Organogenesis of the Placenta
- Leukemia Inhibitory Factor (LIF): A member of the IL-6 cytokine family, LIF is essential for endometrial receptivity. It promotes the differentiation of endometrial cells and facilitates the adhesion of the blastocyst to the uterine lining. The study by Alzaidi Z. et al. showed that reduced LIF expression is associated with implantation failure and infertility [17].
- Interleukin-11 (IL-11): Also part of the IL-6 cytokine family, IL-11 is essential for decidualization—the transformation of endometrial stromal cells into specialized decidual cells that support embryo implantation. Deficiencies in IL-11 signaling can lead to impaired decidualization and subsequent implantation failures [17].
- Tumor Necrosis Factor-alpha (TNF-α): This cytokine has a dual role in implantation. At physiological levels, TNF-α contributes to tissue remodeling and immune regulation necessary for implantation. However, elevated levels of TNF-α are associated with inflammatory conditions that can disrupt implantation and are linked to pregnancy complications [18].
2.1. Early Placental Development
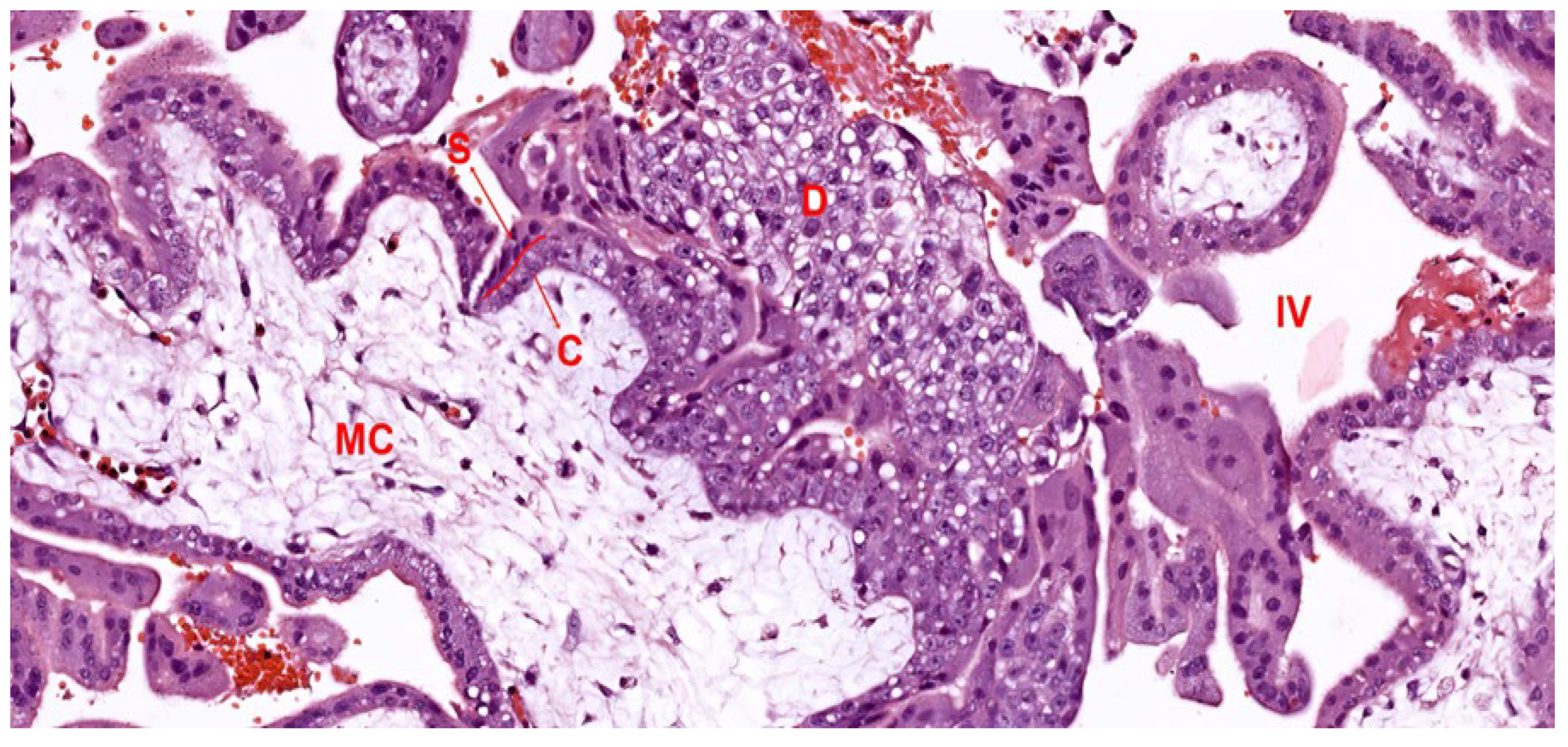
2.2. Development of the Villous Tree
2.2.1. Mesenchymal Villi
2.2.2. Immature Intermediate Villi
2.2.3. Stem Villi
2.2.4. Mature Intermediate Villi
2.2.5. Terminal Villi
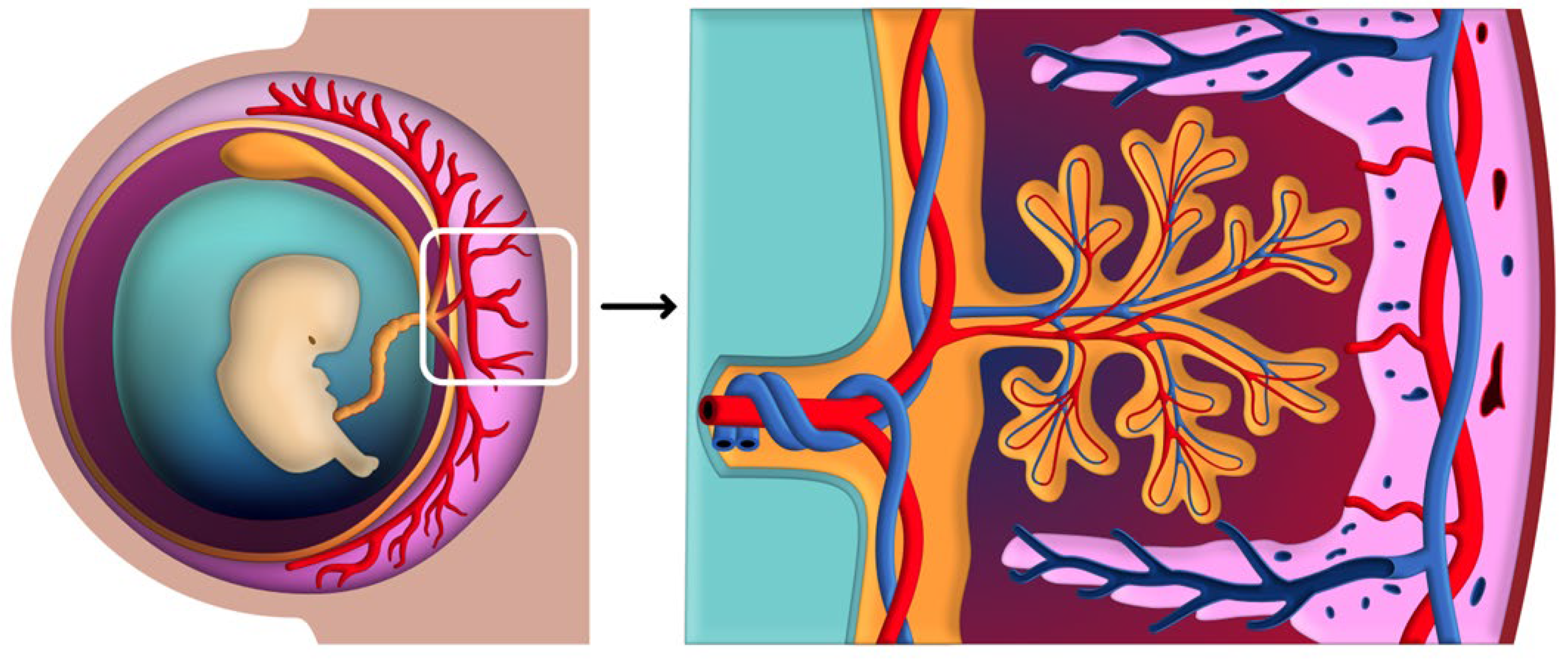
3. Placental-Uterine Interface and Circulatory Systems
3.1. Structural Components of the Placental-Uterine Interface
3.1.1. Decidua
- Decidua Basalis: This is the portion of the endometrium located directly beneath the implanted embryo and is in direct contact with the chorionic villi. The decidua basalis undergoes extensive remodeling, supporting the attachment and invasion of trophoblast cells from the placenta. It is rich in blood vessels and maternal immune cells, which play a role in both nutrient supply and immunological adaptation, helping prevent fetal rejection.
- Decidua Parietalis: This region lines the remaining uterine cavity, not directly adjacent to the implantation site. It undergoes mild changes during pregnancy but does not directly interact with the chorionic villi. However, the decidua parietalis contributes to the structural integrity of the uterine wall and, in later stages, fuses with the decidua capsularis as the amniotic sac expands to fill the uterine cavity.
- Decidua Capsularis: This portion initially covers the embryo and separates it from the uterine cavity. As pregnancy progresses and the fetus grows, the decidua capsularis stretches and thins. By around the second trimester, it typically fuses with the decidua parietalis, leading to the obliteration of the uterine cavity. This fusion provides additional support to the expanding gestational sac and maintains the structural cohesion of the placental environment [62].
3.1.2. Trophoblast Invasion and Decidual Reaction
- Cytotrophoblasts: These are characterized by their single nucleus, comprise the inner trophoblast layer, and exhibit prolific growth. During placental maturation, these cells differentiate further into villous cytotrophoblasts—supporting the villous structure—and extravillous cytotrophoblasts, which actively penetrate maternal tissues to remodel spiral arteries and secure the placental position. This invasion is crucial as it allows the cytotrophoblasts to replace the endothelial lining of maternal blood vessels, creating a low-resistance pathway that facilitates increased blood flow to the placenta [65].
- Syncytiotrophoblasts: Formed by the fusion of cytotrophoblast cells, the syncytiotrophoblast layer represents the outermost barrier of the placenta, in direct contact with maternal blood. This multinucleated layer plays an essential role in nutrient and gas exchange, hormone production, and immunological protection. By secreting hCG, the syncytiotrophoblast sustains the corpus luteum in the early stages of pregnancy, ensuring continued progesterone production until the placenta can take over hormone synthesis. Furthermore, the syncytiotrophoblasts express specific proteins that help evade maternal immune detection, supporting the immune tolerance necessary for a successful pregnancy [66].
3.2. Molecular Regulation of Placental Development
3.3. Establishment and Regulation of Uteroplacental Circulation
3.3.1. Invasion and Remodeling of Spiral Arteries
3.3.2. Intervillous Space and Maternal-Fetal Blood Flow
3.3.3. Hormonal and Biochemical Factors Influencing Blood Flow
3.4. Immunological Aspects of the Placental-Uterine Interface
3.5. Pathophysiological Implications and Clinical Relevance
3.5.1. Complications Arising from Defective Trophoblast Invasion
3.5.2. Impact on Long-Term Health
4. Morphology and Functional Layers of the Placenta
4.1. Chorionic Plate
4.2. Basal Plate
4.3. Intervillous Space
4.4. Clinical Relevance of Placental Morphology
4.4.1. Accessory Lobes: Succenturiate and Bilobed Placenta
4.4.2. Circumvallate and Circummarginate Placenta
4.4.3. Placental Shape and Umbilical Cord Insertion Variations
4.4.4. Implications of Morphological Variations for Long-Term Health
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Ser. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef]
- Moser, G.; Weiss, G.; Sundl, M.; Gauster, M.; Siwetz, M.; Lang-Olip, I.; Huppertz, B. Extravillous trophoblasts invade more than uterine arteries: Evidence for the invasion of uterine veins. Histochem. Cell Biol. 2017, 147, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Colucci, F. Uterine NK cells: Active regulators at the maternal-fetal interface. J. Clin. Investig. 2014, 124, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Moriarty, K.L.; Lee, Y.; Crombleholme, T.M. Placental Gene Therapy for Fetal Growth Restriction and Preeclampsia: Preclinical Studies and Prospects for Clinical Application. J. Clin. Med. 2024, 13, 5647. [Google Scholar] [CrossRef]
- Salavati, N.; Smies, M.; Ganzevoort, W.; Charles, A.K.; Erwich, J.J.; Plösch, T.; Gordijn, S.J. The Possible Role of Placental Morphometry in the Detection of Fetal Growth Restriction. Front. Physiol. 2019, 9, 1884. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, J.A.; McNamara, H.; Platt, R.W.; Benjamin, A.; Kramer, M.S. Placental weight for gestational age and adverse perinatal outcomes. Obstet. Gynecol. 2012, 119, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.; Parker, M.; Cerda, S.; Pearson, C.; Fu, L.; Gillman, M.W.; Zuckerman, B.; Wang, X. Placental weight mediates the effects of prenatal factors on fetal growth: The extent differs by preterm status. Obesity 2013, 21, 609–620. [Google Scholar] [CrossRef]
- Chen, D.B.; Zheng, J. Regulation of placental angiogenesis. Microcirculation 2014, 21, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]
- Myatt, L. Placental adaptive responses and fetal programming. J. Physiol. 2006, 572, 25–30. [Google Scholar] [CrossRef]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. The human placenta: New perspectives on its formation and function during early pregnancy. Proc. Biol. Sci. 2023, 290, 20230191. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, Z.; Lin, M.; Zhu, L.; Xu, L.; Wang, X. Deciphering the Epigenetic Landscape: Placental Development and Its Role in Pregnancy Outcomes. Stem Cell Rev. Rep. 2024, 20, 996–1014. [Google Scholar] [CrossRef]
- Red-Horse, K.; Zhou, Y.; Genbacev, O.; Prakobphol, A.; Foulk, R.; McMaster, M.; Fisher, S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investig. 2004, 114, 744–754. [Google Scholar] [CrossRef]
- Khan, Y.S.; Ackerman, K.M. Embryology, Week 1. In StatPearls [Internet]; [Updated 17 April 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554562/ (accessed on 8 November 2024).
- Alzaidi, Z.; Yildiz, Ş.M.; Saatçi, Ç.; Akalin, H.Ü.; Muderris, I.I.; Aynekin, B.; Şahin, I.O.; Dündar, M. The Effect of Cytokine Leukemia-Inhibitory Factor (LIF) and Interleukin-11 (IL-11) Gene Expression on Primary Infertility Related to Polycystic Ovary Syndrome, Tubal Factor, and Unexplained Infertility in Turkish Women. Egypt. J. Med. Hum. Genet. 2021, 22, 85. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, J.S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.D.; Kimber, S.J. Trophoblast-uterine interactions at implantation. Reprod. Biol. Endocrinol. 2004, 2, 48. [Google Scholar] [CrossRef]
- Gauster, M.; Moser, G.; Wernitznig, S.; Kupper, N.; Huppertz, B. Early human trophoblast development: From morphology to function. Cell. Mol. Life Sci. 2022, 79, 345. [Google Scholar] [CrossRef] [PubMed]
- Ruane, P.T.; Garner, T.; Parsons, L.; Babbington, P.A.; Wangsaputra, I.; Kimber, S.J.; Stevens, A.; Westwood, M.; Brison, D.R.; Aplin, J.D. Trophectoderm differentiation to invasive syncytiotrophoblast is promoted by endometrial epithelial cells during human embryo implantation. Hum. Reprod. 2022, 37, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Dias Da Silva, I.; Wuidar, V.; Zielonka, M.; Pequeux, C. Unraveling the Dynamics of Estrogen and Progesterone Signaling in the Endometrium: An Overview. Cells 2024, 13, 1236. [Google Scholar] [CrossRef]
- Deryabin, P.I.; Borodkina, A.V. The Role of the Endometrium in Implantation: A Modern View. Int. J. Mol. Sci. 2024, 25, 9746. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K. A historical review of blastocyst implantation research. Biol. Reprod. 2018, 99, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Varma, S.; Duttaroy, A.K. Modulation of fetoplacental growth, development and reproductive function by endocrine disrupters. Front. Endocrinol. 2023, 14, 1215353. [Google Scholar] [CrossRef]
- Stute, P.; Neulen, J.; Wildt, L. The Impact of Micronized Progesterone on the Endometrium: A Systematic Review. Climacteric 2016, 19, 316–328. [Google Scholar] [CrossRef]
- Sherwood, O.D. Relaxin’s Physiological Roles and Other Diverse Actions. Endocr. Rev. 2004, 25, 205–234. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Bogdan, A.; Balassa, T.; Csabai, T.; Szekeres-Bartho, J. The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin. Immunopathol. 2016, 38, 635–649. [Google Scholar] [CrossRef]
- Burton, G.J.; Watson, A.L.; Hempstock, J.; Skepper, J.N.; Jauniaux, E. Uterine Glands Provide Histiotrophic Nutrition for the Human Fetus during the First Trimester of Pregnancy. J. Clin. Endocrinol. Metab. 2002, 87, 2954–2959. [Google Scholar] [CrossRef]
- Kapila, V.; Chaudhry, K. Physiology, Placenta. In StatPearls [Internet]; [Updated 24 July 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538332/ (accessed on 8 November 2024).
- Scott, R.L.; Vu, H.T.H.; Jain, A.; Iqbal, K.; Tuteja, G.; Soares, M.J. Conservation at the uterine-placental interface. Proc. Natl. Acad. Sci. USA 2022, 119, e2210633119. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The Biology of VEGF and Its Receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Red-Horse, K.; Rivera, J.; Schanz, A.; Zhou, Y.; Winn, V.; Kapidzic, M.; Fisher, S.J. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J. Clin. Investig. 2006, 116, 2643–2652. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer and Other Diseases. Nat. Rev. Cancer 2005, 5, 273–285. [Google Scholar] [CrossRef]
- Nevo, O.; Lee, D.K.; Caniggia, I. Attenuation of VEGFR-2 Expression by sFlt-1 and Low Oxygen in Human Placenta. PLoS ONE 2013, 8, e81176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, S. Vascular Biology of the Placenta. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 4, Cell Types of the Placenta. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53245/ (accessed on 8 November 2024).
- Kojima, J.; Ono, M.; Kuji, N.; Nishi, H. Human Chorionic Villous Differentiation and Placental Development. Int. J. Mol. Sci. 2022, 23, 8003. [Google Scholar] [CrossRef]
- Turowski, G.; Vogel, M. Re-view and view on maturation disorders in the placenta. APMIS 2018, 126, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Bedzhov, I. Mechanisms of formation and functions of the early embryonic cavities. Semin. Cell Dev. Biol. 2022, 131, 110–116. [Google Scholar] [CrossRef]
- Peñailillo, R.; Velásquez, V.; Acuña-Gallardo, S.; García, F.; Sánchez, M.; Nardocci, G.; Illanes, S.E.; Monteiro, L.J. FOXM1 Participates in Trophoblast Migration and Early Trophoblast Invasion: Potential Role in Blastocyst Implantation. Int. J. Mol. Sci. 2024, 25, 1678. [Google Scholar] [CrossRef]
- Goldie, L.C.; Nix, M.K.; Hirschi, K.K. Embryonic vasculogenesis and hematopoietic specification. Organogenesis 2008, 4, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Basta, M.; Lipsett, B.J. Anatomy, Abdomen and Pelvis: Umbilical Cord. In StatPearls [Internet]; [Updated 24 July 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557389/ (accessed on 8 November 2024).
- Pechriggl, E.; Blumer, M.; Tubbs, R.S.; Olewnik, Ł.; Konschake, M.; Fortélny, R.; Stofferin, H.; Honis, H.R.; Quinones, S.; Maranillo, E.; et al. Embryology of the Abdominal Wall and Associated Malformations—A Review. Front. Surg. 2022, 9, 891896. [Google Scholar] [CrossRef] [PubMed]
- Demir, R.; Seval, Y.; Huppertz, B. Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem. 2007, 109, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kreis, N.-N.; Ritter, A.; Louwen, F.; Yuan, J. A Message from the Human Placenta: Structural and Immunomodulatory Defense against SARS-CoV-2. Cells 2020, 9, 1777. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Liao, H.; Lin, W.; Li, Z.; Ma, X.; Xu, Q.; Yu, F. The Role of TGF-β during Pregnancy and Pregnancy Complications. Int. J. Mol. Sci. 2023, 24, 16882. [Google Scholar] [CrossRef] [PubMed]
- Soncin, F.; Natale, D.; Parast, M.M. Signaling pathways in mouse and human trophoblast differentiation: A comparative review. Cell. Mol. Life Sci. 2015, 72, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, B.; Wang, Y.; Tan, S.; Xu, Q.; Wang, Z.; Zhou, K.; Liu, H.; Ren, Z.; Jiang, Z. Ang-1 and VEGF: Central regulators of angiogenesis. Mol. Cell. Biochem. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, T.M.; Charnock-Jones, D.S.; Kaufmann, P. Aspects of Human Fetoplacental Vasculogenesis and Angiogenesis. Placenta 2004, 25, 103–113. [Google Scholar] [CrossRef]
- Ietta, F.; Wu, Y.; Winter, J.; Xu, J.; Wang, J.; Post, M.; Caniggia, I. Dynamic HIF1A regulation during human placental development. Biol. Reprod. 2006, 75, 112–121. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L. Review: The placenta and developmental programming: Balancing fetal nutrient demands with maternal resource allocation. Placenta 2012, 33, S23–S27. [Google Scholar] [CrossRef]
- Mayhew, T.M. Taking tissue samples from the placenta: An illustration of principles and strategies. Placenta 2008, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B.; Peeters, L.L. Vascular biology in implantation and placentation. Angiogenesis 2005, 8, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; McMaster, M.; Woo, K.; Janatpour, M.; Perry, J.; Karpanen, T.; Alitalo, K.; Damsky, C.; Fisher, S.J. Vascular endothelial growth factor ligand and receptor regulation of trophoblast invasion. Am. J. Pathol. 2002, 160, 1405–1423. [Google Scholar] [CrossRef]
- Cross, J.C.; Baczyk, D.; Dobric, N.; Hemberger, M.; Hughes, M.; Simmons, D.G.; Yamamoto, H.; Kingdom, J.C. Genes, Development, and Evolution of the Placenta. Placenta 2003, 24, 123–130. [Google Scholar] [CrossRef]
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Pathology of the Human Placenta; Springer: New York, NY, USA, 2012; pp. 105–142. [Google Scholar] [CrossRef]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and Function of the Normal Human Placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.; Perez-Garcia, V.; Hemberger, M. Regulation of Placental Development and Its Impact on Fetal Growth-New Insights From Mouse Models. Front. Endocrinol. 2018, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Regnault, T.R.H.; Barker, P.L.; Botting, K.J.; McMillen, I.C.; McMillan, C.M.; Roberts, C.T.; Morrison, J.L. Placental Adaptations in Growth Restriction. Nutrients 2015, 7, 360–389. [Google Scholar] [CrossRef] [PubMed]
- Herrick, E.J.; Bordoni, B. Embryology, Placenta. In StatPearls [Internet]; [Updated 1 May 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551634/ (accessed on 8 November 2024).
- Ochoa-Bernal, M.A.; Fazleabas, A.T. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int. J. Mol. Sci. 2020, 21, 1973. [Google Scholar] [CrossRef]
- Beck, A.P.; Erdelyi, I.; Zeiss, C.J. Endometrial decidualization and deciduosis in aged rhesus macaques (Macaca mulatta). Comp. Med. 2014, 64, 148–156. [Google Scholar]
- Huppertz, B. Traditional and New Routes of Trophoblast Invasion and Their Implications for Pregnancy Diseases. Int. J. Mol. Sci. 2020, 21, 289. [Google Scholar] [CrossRef]
- Bačenková, D.; Trebuňová, M.; Čížková, D.; Hudák, R.; Dosedla, E.; Findrik-Balogová, A.; Živčák, J. In Vitro Model of Human Trophoblast in Early Placentation. Biomedicines 2022, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- McConkey, C.A.; Delorme-Axford, E.; Nickerson, C.A.; Kim, K.S.; Sadovsky, Y.; Boyle, J.P.; Coyne, C.B. A three-dimensional culture system recapitulates placental syncytiotrophoblast development and microbial resistance. Sci. Adv. 2016, 2, e1501462. [Google Scholar] [CrossRef]
- Abbas, Y.; Turco, M.Y.; Burton, G.J.; Moffett, A. Investigation of human trophoblast invasion in vitro. Hum. Reprod. Update 2020, 26, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Wu, H.; Sferruzzi-Perri, A.N.; Wang, Y.L.; Shao, X. Endocytosis at the maternal-fetal interface: Balancing nutrient transport and pathogen defense. Front. Immunol. 2024, 15, 1415794. [Google Scholar] [CrossRef]
- Basak, T.; Ain, R. Molecular regulation of trophoblast stem cell self-renewal and giant cell differentiation by the Hippo components YAP and LATS1. Stem Cell Res. Ther. 2022, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, Y.; Bian, Q.; Zhang, N.; Wang, M.; Wang, J.; Li, X.; Lai, L.; Zhao, Z.; Yu, H. Molecular mechanisms of syncytin-1 in tumors and placental development related diseases. Discov. Oncol. 2023, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Nien, J.K.; Espinoza, J.; Todem, D.; Fu, W.; Chung, H.; Kusanovic, J.P.; Gotsch, F.; Erez, O.; Mazaki-Tovi, S.; et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small-for-gestational-age neonate. J. Matern.-Fetal Neonatal Med. 2008, 21, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wong, R.J.; Stevenson, D.K. The Impact of Hypoxia in Early Pregnancy on Placental Cells. Int. J. Mol. Sci. 2021, 22, 9675. [Google Scholar] [CrossRef]
- Gopinathan, G.; Diekwisch, T.G.H. Epigenetics and Early Development. J. Dev. Biol. 2022, 10, 26. [Google Scholar] [CrossRef]
- Papúchová, H.; Meissner, T.B.; Li, Q.; Strominger, J.L.; Tilburgs, T. The Dual Role of HLA-C in Tolerance and Immunity at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2730. [Google Scholar] [CrossRef] [PubMed]
- Degner, K.; Magness, R.R.; Shah, D.M. Establishment of the Human Uteroplacental Circulation: A Historical Perspective. Reprod. Sci. 2017, 24, 753–761. [Google Scholar] [CrossRef]
- Lyall, F.; Bulmer, J.N.; Duffie, E.; Cousins, F.; Theriault, A.; Robson, S.C. Human trophoblast invasion and spiral artery transformation: The role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am. J. Pathol. 2001, 158, 1713–1721. [Google Scholar] [CrossRef]
- Lawless, L.; Qin, Y.; Xie, L.; Zhang, K. Trophoblast Differentiation: Mechanisms and Implications for Pregnancy Complications. Nutrients 2023, 15, 3564. [Google Scholar] [CrossRef]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C. Rheological and physiological consequences of conversion of the ma-ternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Kim, S.H.; Cha, D.H.; Park, H.J. Defective Uteroplacental Vascular Remodeling in Preeclampsia: Key Molecular Factors Leading to Long Term Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 11202. [Google Scholar] [CrossRef]
- Arumugasaamy, N.; Rock, K.D.; Kuo, C.Y.; Bale, T.L.; Fisher, J.P. Microphysiological systems of the placental barrier. Adv. Drug Deliv. Rev. 2020, 161–162, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Betz, D.; Fane, K. Human Chorionic Gonadotropin. In StatPearls [Internet]; [Updated 14 August 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532950/ (accessed on 8 November 2024).
- Chen, K.; Pittman, R.N.; Popel, A.S. Nitric oxide in the vasculature: Where does it come from and where does it go? A quan-titative perspective. Antioxid. Redox Signal. 2008, 10, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Ramanlal, R.; Gupta, V. Physiology, Vasodilation. In StatPearls [Internet]; [Updated 23 January 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557562/ (accessed on 8 November 2024).
- Figarska, A.; Witkowska-Piłaszewicz, O. Immunological Response during Pregnancy in Humans and Mares. Agriculture 2022, 12, 431. [Google Scholar] [CrossRef]
- Tersigni, C.; Meli, F.; Neri, C.; Iacoangeli, A.; Franco, R.; Lanzone, A.; Scambia, G.; Di Simone, N. Role of Human Leukocyte Antigens at the Feto-Maternal Interface in Normal and Pathological Pregnancy: An Update. Int. J. Mol. Sci. 2020, 21, 4756. [Google Scholar] [CrossRef]
- Tilburgs, T.; Crespo, Â.C.; van der Zwan, A.; Rybalov, B.; Raj, T.; Stranger, B.; Gardner, L.; Moffett, A.; Strominger, J.L. Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 7219–7224. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef]
- Huang, N.; Chi, H.; Qiao, J. Role of Regulatory T Cells in Regulating Fetal-Maternal Immune Tolerance in Healthy Pregnancies and Reproductive Diseases. Front. Immunol. 2020, 11, 1023. [Google Scholar] [CrossRef] [PubMed]
- Gusella, A.; Martignoni, G.; Giacometti, C. Behind the Curtain of Abnormal Placentation in Pre-Eclampsia: From Molecular Mechanisms to Histological Hallmarks. Int. J. Mol. Sci. 2024, 25, 7886. [Google Scholar] [CrossRef] [PubMed]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth re-striction: Relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torres, J.; Espino-y-Sosa, S.; Martinez-Portilla, R.; Borboa-Olivares, H.; Estrada-Gutierrez, G.; Acevedo-Gallegos, S.; Ruiz-Ramirez, E.; Velasco-Espin, M.; Cerda-Flores, P.; Ramirez-Gonzalez, A.; et al. A Narrative Review on the Pathophysiology of Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7569. [Google Scholar] [CrossRef] [PubMed]
- Turbeville, H.R.; Sasser, J.M. Preeclampsia beyond pregnancy: Long-term consequences for mother and child. Am. J. Physiol. Ren. Physiol. 2020, 318, F1315–F1326. [Google Scholar] [CrossRef]
- Liu, X.; Wang, G.; Huang, H.; Lv, X.; Si, Y.; Bai, L.; Wang, G.; Li, Q.; Yang, W. Exploring maternal-fetal interface with in vitro placental and trophoblastic models. Front. Cell Dev. Biol. 2023, 11, 1279227. [Google Scholar] [CrossRef] [PubMed]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knöfler, M. Regulation of Placental Extravillous Trophoblasts by the Maternal Uterine Environment. Front. Immunol. 2018, 9, 2597. [Google Scholar] [CrossRef] [PubMed]
- Knöfler, M.; Simmons, D.G.; Lash, G.E.; Harris, L.K.; Armant, D.R. Regulation of trophoblast invasion—A workshop report. Placenta 2008, 29, S26–S28. [Google Scholar] [CrossRef] [PubMed]
- Kupper, N.; Pritz, E.; Siwetz, M.; Guettler, J.; Huppertz, B. Placental Villous Explant Culture 2.0: Flow Culture Allows Studies Closer to the In Vivo Situation. Int. J. Mol. Sci. 2021, 22, 7464. [Google Scholar] [CrossRef]
- Bushway, M.E.; Gerber, S.A.; Fenton, B.M.; Miller, R.K.; Lord, E.M.; Murphy, S.P. Morphological and phenotypic analyses of the human placenta using whole mount immunofluorescence. Biol. Reprod. 2014, 90, 110. [Google Scholar] [CrossRef]
- 6Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.; et al. The Pivotal Role of the Placenta in Normal and Pathological Pregnancies: A Focus on Preeclampsia, Fetal Growth Restriction, and Maternal Chronic Venous Disease. Cells 2022, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Goidescu, I.G.; Nemeti, G.; Preda, A.; Staicu, A.; Goidescu, C.M.; Surcel, M.; Rotar, I.C.; Cruciat, G.; Muresan, D. Succenturiate Placental Lobe Abruption. Int. J. Women’s Health 2024, 16, 1041–1047. [Google Scholar] [CrossRef]
- Oyelese, Y.; Javinani, A.; Shamshirsaz, A.A. Vasa Previa. Obstet. Gynecol. 2023, 142, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Aoki, S.; Sakamaki, K.; Kurasawa, K.; Okuda, M.; Takahashi, T.; Hirahara, F. Circumvallate placenta: Associated clinical manifestations and complications-a retrospective study. Obstet. Gynecol. Int. 2014, 986230. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, K.M.; Hildebrand, J.P. Placenta Abnormalities. In StatPearls [Internet]; [Updated 17 October 2022]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459355/ (accessed on 8 November 2024).
- Brouillet, S.; Dufour, A.; Prot, F.; Feige, J.J.; Equy, V.; Alfaidy, N.; Gillois, P.; Hoffmann, P. Influence of the umbilical cord insertion site on the optimal individual birth weight achievement. BioMed Res. Int. 2014, 341251. [Google Scholar] [CrossRef]
- Siargkas, A.; Tsakiridis, I.; Pachi, C.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Impact of marginal cord insertion on perinatal outcomes: A systematic review and meta-analysis. Am. J. Obs. Gynecol. MFM 2023, 5, 100876. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhong, Q.; Mei, L.; Gao, L.; Lan, X.; Xiong, J.; Luo, S.; Wang, L. Associations between Velamentous or Marginal Cord Insertion and Risk of Adverse Perinatal Outcomes in Twin Pregnancies: A Retrospective Cohort Study. BMC Pregnancy Childbirth 2023, 23, 648. [Google Scholar] [CrossRef] [PubMed]
- Salafia, C.M.; Yampolsky, M.; Misra, D.P.; Shlakhter, O.; Haas, D.; Eucker, B.; Thorp, J. Placental surface shape, function, and effects of maternal and fetal vascular pathology. Placenta 2010, 31, 958–962. [Google Scholar] [CrossRef]
- Winje, B.A.; Roald, B.; Kristensen, N.P.; Frøen, J.F. Placental pathology in pregnancies with maternally perceived decreased fetal movement--a population-based nested case-cohort study. PLoS ONE 2012, 7, e39259. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Igarashi, M. Clinical significance of pregnancies with succenturiate lobes of placenta. Arch. Gynecol. Obstet. 2008, 277, 299–301. [Google Scholar] [CrossRef]
- Than, N.G.; Hahn, S.; Rossi, S.W.; Szekeres-Bartho, J. Editorial: Fetal-Maternal Immune Interactions in Pregnancy. Front. Immunol. 2019, 10, 2729. [Google Scholar] [CrossRef]
- Soares, M.J.; Varberg, K.M.; Iqbal, K. Hemochorial placentation: Development, function, and adaptations. Biol. Reprod. 2018, 99, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Derisoud, E.; Jiang, H.; Zhao, A.; Chavatte-Palmer, P.; Deng, Q. Revealing the molecular landscape of human placenta: A systematic review and meta-analysis of single-cell RNA sequencing studies. Hum. Reprod. Update 2024, 30, 410–441. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Highlighting the trajectory from intrauterine growth restriction to future obesity. Front. Endocrinol. 2022, 13, 1041718. [Google Scholar] [CrossRef] [PubMed]
- Wojczakowski, W.; Kimber-Trojnar, Ż.; Dziwisz, F.; Słodzińska, M.; Słodziński, H.; Leszczyńska-Gorzelak, B. Preeclampsia and Cardiovascular Risk for Offspring. J. Clin. Med. 2021, 10, 3154. [Google Scholar] [CrossRef]
- Fox, K.A.; Lee, W. Prenatal Diagnosis and Evaluation of Abnormal Placentation. Clin. Obstet. Gynecol. 2017, 60, 596–607. [Google Scholar] [CrossRef] [PubMed]



| Stage | Timing | Key Processes | Structures Formed | Hormonal Activity |
|---|---|---|---|---|
| Pre-implantation | Days 1–5 post-fertilization | Fertilization in the fallopian tube; the zygote divides forming a morula and then a blastocyst; endometrial preparation through hormonal changes (progesterone and estrogen) [21]. | Blastocyst (trophoblast and inner cell mass); endometrial decidual cells rich in glycogen. | Increased progesterone and estrogen prepare the endometrium for implantation, promoting decidualization [23]. |
| Implantation Proper | Days 6–7 post-fertilization | Blastocyst attachment to endometrium; trophoblast differentiation into syncytiotrophoblast and cytotrophoblast; formation of maternal-fetal interface with trophoblastic lacunae [22]. | Cytotrophoblast and syncytiotrophoblast layers; trophoblastic lacunae. | Continued progesterone activity supports implantation; syncytiotrophoblasts release human chorionic gonadotropin (hCG) to maintain corpus luteum function [24]. |
| Post-implantation | Days 8–12 post-fertilization | Complete embedding of blastocyst within endometrium; formation of early placental structures (primary villi); establishment of uteroplacental circulation; decidua differentiation [25]. | Primary villi; decidua basalis, capsularis, parietalis; early uteroplacental circulation. | hCG levels increase, maintaining the corpus luteum and sustaining progesterone and estrogen production for endometrial support [26]. |
| Villous Type | Timing | When Maximum | % Volume at Term | Size | Characteristic Features | Primary Function |
|---|---|---|---|---|---|---|
| Mesenchymal Villi [56] | 5 weeks–term | 0 to 8 weeks | <1% | 120–250 μm (<8 weeks), 60–100 μm (>8 weeks) | Primitive stroma, thick trophoblastic cover, few fetal vessels | Proliferation and growth precursor for other villous types |
| Immature Intermediate Villi [50] | 8 weeks–term, peaks 14–20 weeks | 14 to 20 weeks | 5–10% | 100–200 μm, up to 400 μm | Reticular stroma with fluid-filled channels, visible Hofbauer cells, limited vascularization | Growth centers for villous tree development and branching |
| Stem Villi [57] | 8 weeks–term | Term | 20–25% | 150–300 μm | Fibrotic stroma, large vessels with media and adventitia, primary structural support | Provides structural support as the ‘trunk’ of a villous tree, minimal exchange |
| Mature Intermediate Villi [58] | Third trimester | Third trimester | 25% | 80–150 μm | Loose, unoriented connective tissue fibers, capillary-rich with vascular lumens < 50% | Significant role in exchange, structural support for terminal villi formation |
| Terminal Villi [3] | Third trimester | Term | 40–50% | 60 μm | High capillary density (>50% vascular lumen), thin trophoblastic cover, main exchange site | Primary site of feto-maternal exchange due to high capillary volume and efficiency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vornic, I.; Buciu, V.; Furau, C.G.; Zara, F.; Novacescu, D.; Barb, A.C.; Cumpanas, A.A.; Latcu, S.C.; Sas, I.; Serban, D.; et al. The Interplay of Molecular Factors and Morphology in Human Placental Development and Implantation. Biomedicines 2024, 12, 2908. https://doi.org/10.3390/biomedicines12122908
Vornic I, Buciu V, Furau CG, Zara F, Novacescu D, Barb AC, Cumpanas AA, Latcu SC, Sas I, Serban D, et al. The Interplay of Molecular Factors and Morphology in Human Placental Development and Implantation. Biomedicines. 2024; 12(12):2908. https://doi.org/10.3390/biomedicines12122908
Chicago/Turabian StyleVornic, Ioana, Victor Buciu, Cristian George Furau, Flavia Zara, Dorin Novacescu, Alina Cristina Barb, Alin Adrian Cumpanas, Silviu Constantin Latcu, Ioan Sas, Denis Serban, and et al. 2024. "The Interplay of Molecular Factors and Morphology in Human Placental Development and Implantation" Biomedicines 12, no. 12: 2908. https://doi.org/10.3390/biomedicines12122908
APA StyleVornic, I., Buciu, V., Furau, C. G., Zara, F., Novacescu, D., Barb, A. C., Cumpanas, A. A., Latcu, S. C., Sas, I., Serban, D., Cut, T. G., & Dumitru, C. S. (2024). The Interplay of Molecular Factors and Morphology in Human Placental Development and Implantation. Biomedicines, 12(12), 2908. https://doi.org/10.3390/biomedicines12122908










