Perioperative LiMAx Test Analysis: Impact of Portal Vein Embolisation, Chemotherapy and Major Liver Resection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Application of the LiMAx Test
2.3. Ethics Approval and Consent to Participate
2.4. Statistical Anaylsis
3. Results
3.1. Patient Data
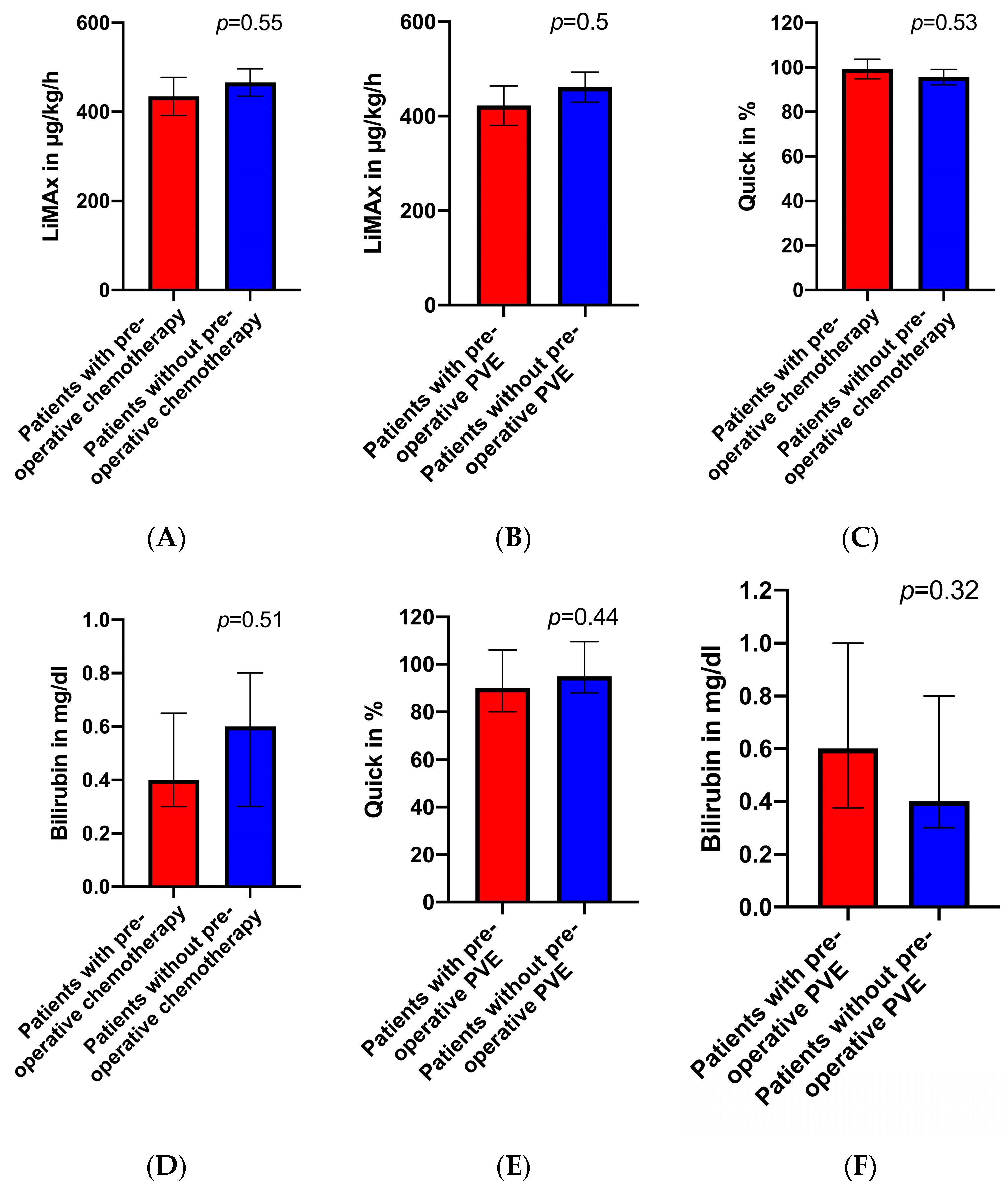
3.2. Preoperative LiMAx Values
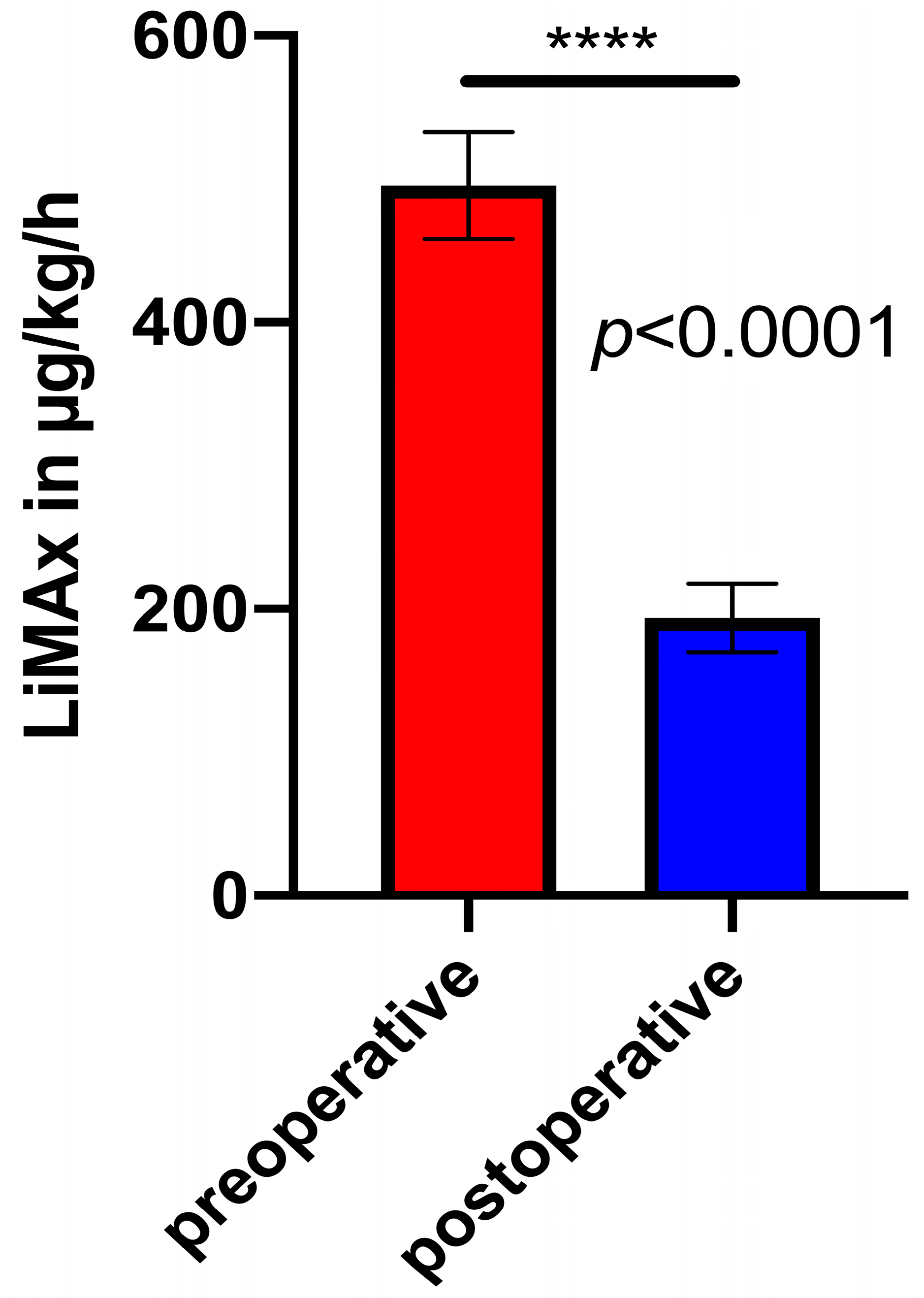
3.3. Pre- and Matched Postoperative LiMAx Values
3.4. Postoperative Complications
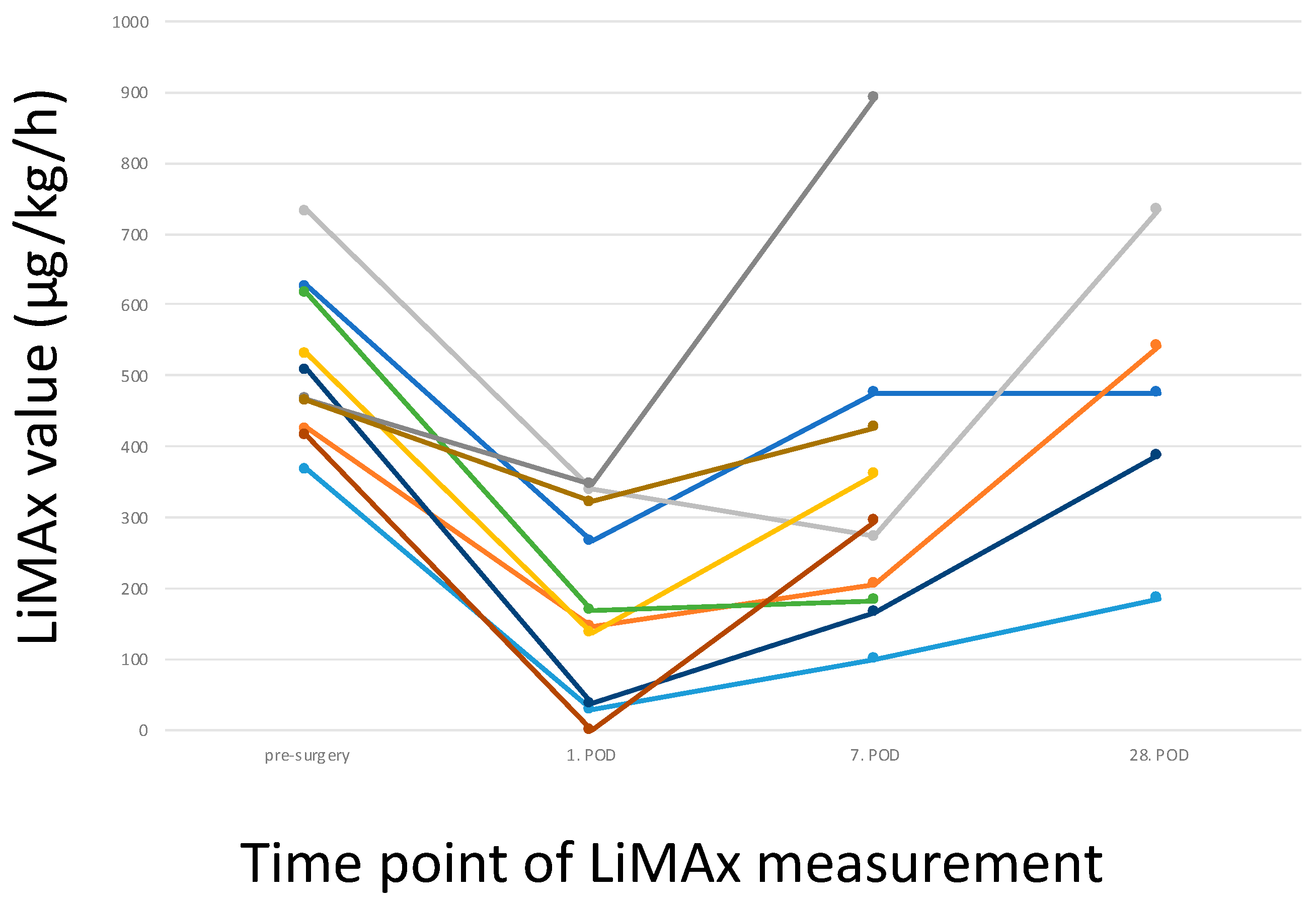
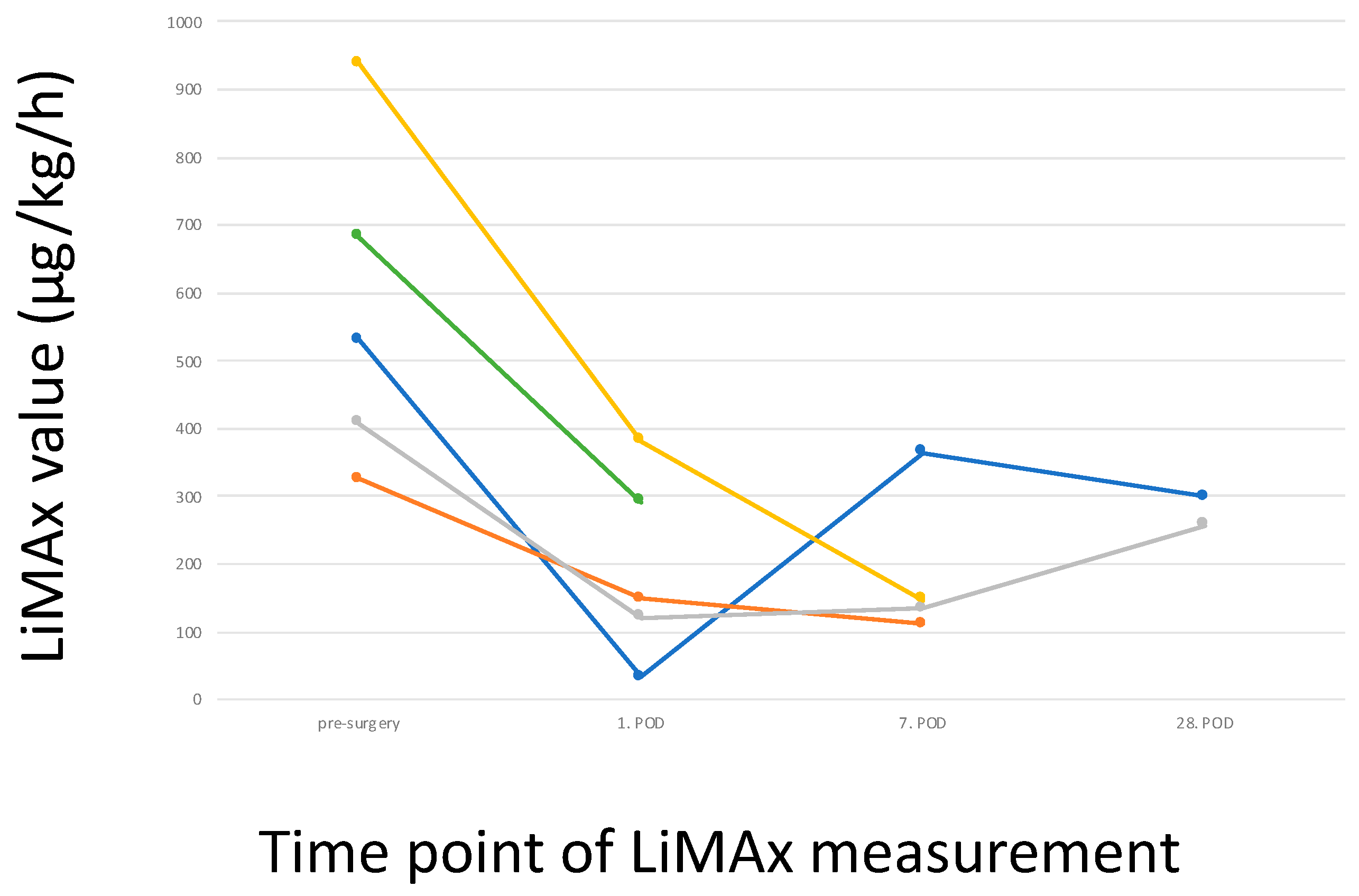
3.5. Postoperative LiMAx Values
4. Discussion
5. Conclusions
Limitations of This Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schneider, P.D. Preoperative assessment of liver function. Surg. Clin. N. Am. 2004, 84, 355–373. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, M.A.; Damink, S.W.M.O.; Dejong, C.H.C.; Lang, H.; Malagó, M.; Jalan, R.; Saner, F.H. Liver failure after partial hepatic resection: Definition, pathophysiology, risk factors and treatment. Liver Int. 2008, 28, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, A.; Ruzzenente, A.; Conci, S.; Valdegamberi, A.; Iacono, C. How much remnant is enough in liver resection? Dig. Surg. 2012, 29, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Jarnagin, W.R.; Gonen, M.; Fong, Y.; DeMatteo, R.P.; Ben-Porat, L.; Little, S.; Corvera, C.; Weber, S.; Blumgart, L.H. Improvement in perioperative outcome after hepatic resection: Analysis of 1803 consecutive cases over the past decade. An. Surg. 2002, 236, 397–406. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Shoup, M.; Gonen, M.; D’Angelica, M.; Jarnagin, W.R.; DeMatteo, R.P.; Schwartz, L.H.; Tuorto, S.; Blumgart, L.H.; Fong, Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J. Gastrointest. Surg. 2003, 7, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, M.; Lock, J.F.; Riecke, B.; Heyne, K.; Martus, P.; Fricke, M.; Lehmann, S.; Niehues, S.M.; Schwabe, M.; Lemke, A.J.; et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann. Surg. 2009, 250, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Jalan, R.; Hayes, P.C. Review article: Quantitative tests of liver function. Aliment. Pharmacol. Ther. 1995, 9, 263–270. [Google Scholar] [CrossRef]
- Truant, S.; Oberlin, O.; Sergent, G.; Lebuffe, G.; Gambiez, L.; Ernst, O.; Pruvot, F.-R. Remnant liver volume to body weight ratio > or =0.5%: A new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J. Am. Coll. Surg. 2007, 204, 22–33. [Google Scholar] [CrossRef]
- Bennink, R.J.; Dinant, S.; Erdogan, D.; Heijnen, B.H.; Straatsburg, I.H.; Van Vliet, A.K.; Van Gulik, T.M. Preoperative assessment of postoperative remnant liver function using hepatobiliary scintigraphy. J. Nucl. Med. 2004, 45, 965–971. [Google Scholar]
- Tomassini, F.; D’Asseler, Y.; Giglio, M.C.; Lecluyse, C.; Lambert, B.; Sainz-Barriga, M.; Van Dorpe, J.; Hoorens, A.; Geboes, K.; Troisi, R.I. Hemodynamic changes in ALPPS influence liver regeneration and function: Results from a prospective study. HPB 2019, 21, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, T.; de Beeck, B.O.; Driessen, A.; Roeyen, G.; Bracke, B.; Hartman, V.; Huyghe, I.; Morrison, S.; Ysebaert, D.; Francque, S. Estimation of the future remnant liver function is a better tool to predict post-hepatectomy liver failure than platelet-based liver scores. Eur. J. Surg. Oncol. 2017, 43, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Olthof, P.B.; Coelen, R.J.S.; Bennink, R.J.; Heger, M.; Lam, M.F.; Besselink, M.G.; Busch, O.R.; van Lienden, K.P.; van Gulik, T.M. (99m)Tc-mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma. HPB 2017, 19, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Rassam, F.; Olthof, P.B.; Bennink, R.J.; van Gulik, T.M. Current Modalities for the Assessment of Future Remnant Liver Function. Visc. Med. 2017, 33, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Rassam, F.; Olthof, P.B.; Richardson, H.; van Gulik, T.M.; Bennink, R.J. Practical guidelines for the use of technetium-99m mebrofenin hepatobiliary scintigraphy in the quantitative assessment of liver function. Nucl. Med. Commun. 2019, 40, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, M.; Lock, J.F.; Malinowski, M.; Niehues, S.M.; Seehofer, D.; Neuhaus, P. The LiMAx test: A new liver function test for predicting postoperative outcome in liver surgery. HPB 2010, 12, 139–146. [Google Scholar] [CrossRef]
- Truant, S.; Boleslawski, E.; Sergent, G.; Leteurtre, E.; Duhamel, A.; Hebbar, M.; Pruvot, F.R. Liver function following extended hepatectomy can be accurately predicted using remnant liver volume to body weight ratio. World J. Surg. 2015, 39, 1193–1201. [Google Scholar] [CrossRef]
- Schnitzbauer, A.A.; Lang, S.A.; Goessmann, H.; Nadalin, S.; Baumgart, J.; Farkas, S.A.; Fichtner-Feigl, S.; Lorf, T.; Goralcyk, A.; Hörbelt, R.; et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012, 255, 405–414. [Google Scholar] [CrossRef]
- Guillaud, A.; Pery, C.; Campillo, B.; Lourdais, A.; Sulpice, L.; Boudjema, K. Incidence and predictive factors of clinically relevant bile leakage in the modern era of liver resections. HPB 2013, 15, 224–229. [Google Scholar] [CrossRef]
- Yamashita, Y.-I.; Hamatsu, T.; Rikimaru, T.; Tanaka, S.; Shirabe, K.; Shimada, M.; Sugimachi, K. Bile leakage after hepatic resection. Ann. Surg. 2001, 233, 45–50. [Google Scholar] [CrossRef]
- Lam, C.M.; Lo, C.M.; Liu, C.L.; Fan, S.T. Biliary complications during liver resection. World J. Surg. 2001, 25, 1273–1276. [Google Scholar] [CrossRef]
- Lock, J.F.; Westphal, T.; Rubin, T.; Malinowski, M.; Schulz, A.; Jara, M.; Bednarsch, J.; Stockmann, M. LiMAx Test Improves Diagnosis of Chemotherapy-Associated Liver Injury Before Resection of Colorectal Liver Metastases. Ann. Surg. Oncol. 2017, 24, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.; Bednarsch, J.; Malinowski, M.; Pratschke, J.; Stockmann, M. Effects of oxaliplatin-based chemotherapy on liver function—An analysis of impact and functional recovery using the LiMAx test. Langenbecks Arch. Surg. 2016, 401, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, M.; Lock, J.F.; Seehofer, D.; Gebauer, B.; Schulz, A.; Demirel, L.; Bednarsch, J.; Stary, V.; Neuhaus, P.; Stockmann, M. Preliminary study on liver function changes after trisectionectomy with versus without prior portal vein embolization. Surg. Today 2016, 46, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, B.; Menger, M.D. The hepatic microcirculation: Mechanistic contributions and therapeutic targets in liver injury and repair. Physiol. Rev. 2009, 89, 1269–1339. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.F.; Reinhold, T.; Malinowski, M.; Pratschke, J.; Neuhaus, P.; Stockmann, M. The costs of postoperative liver failure and the economic impact of liver function capacity after extended liver resection—A single-center experience. Langenbecks Arch. Surg. 2009, 394, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Lodewick, T.; Alizai, P.; van Dam, R.; Roeth, A.; Schmeding, M.; Heidenhain, C.; Andert, A.; Gassler, N.; Dejong, C.; Neumann, U. Effect of Age on Liver Function in Patients Undergoing Partial Hepatectomy. Dig. Surg. 2017, 34, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Tomassini, F.; Giglio, M.C.; De Simone, G.; Montalti, R.; Troisi, R.I. Hepatic function assessment to predict post-hepatectomy liver failure: What can we trust? A systematic review. Updates Surg. 2020, 72, 925–938. [Google Scholar] [CrossRef]
- Sumiyoshi, T.; Shima, Y.; Tokorodani, R.; Okabayashi, T.; Kozuki, A.; Hata, Y.; Noda, Y.; Murata, Y.; Nakamura, T.; Uka, K. CT/99mTc-GSA SPECT fusion images demonstrate functional differences between the liver lobes. World J. Gastroenterol. 2013, 19, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, H.; Karlgren, S.; Blomqvist, L.; Jonas, E. The inhomogeneous distribution of liver function: Possible impact on the prediction of post-operative remnant liver function. HPB 2015, 17, 272–277. [Google Scholar] [CrossRef]
- Vauthey, J.-N.; Chaoui, A.; Do, K.-A.; Bilimoria, M.M.; Fenstermacher, M.J.; Charnsangavej, C.; Hicks, M.; Alsfasser, G.; Lauwers, G.; Hawkins, I.F.; et al. Standardized measurement of the future liver remnant prior to extended liver resection: Methodology and clinical associations. Surgery 2000, 127, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Martel, G.; Cieslak, K.P.; Huang, R.; van Lienden, K.P.; Wiggers, J.K.; Belblidia, A.; Dagenais, M.; Lapointe, R.; van Gulik, T.M.; Vandenbroucke-Menu, F. Comparison of techniques for volumetric analysis of the future liver remnant: Implications for major hepatic resections. HPB 2015, 17, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Truant, S.; Baillet, C.; Fulbert, M.; Olivier, A.; Sergent, G.; Turpin, A.; Boleslawski, E.; El Amrani, M.; Huglo, D.; Pruvot, F.-R. Asymmetric kinetics of volume and function of the remnant liver after major hepatectomy as a key for postoperative outcome—A case-matched study. HPB 2020, 22, 855–863. [Google Scholar] [CrossRef]
- Sparrelid, E.; Jonas, E.; Tzortzakakis, A.; Dahlén, U.; Murquist, G.; Brismar, T.; Axelsson, R.; Isaksson, B. Dynamic Evaluation of Liver Volume and Function in Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. J. Gastrointest. Surg. 2017, 21, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.; Bednarsch, J.; Lock, J.F.; Malinowski, M.; Schulz, A.; Seehofer, D.; Stockmann, M. Enhancing safety in liver surgery using a new diagnostic tool for evaluation of actual liver function capacity—The LiMAx test. Dtsch. Med. Wochenschr. 2014, 139, 387–391. [Google Scholar] [PubMed]
- Koonrungsesomboon, N.; Khatsri, R.; Wongchompoo, P.; Teekachunhatean, S. The impact of genetic polymorphisms on CYP1A2 activity in humans: A systematic review and meta-analysis. Pharmacogenom. J. 2018, 18, 760–768. [Google Scholar] [CrossRef]
| Patients | n = 40 |
|---|---|
| Age (years, median (min-max)) | 67 (22–81) |
| Sex (female/male) | 21 (52.5%)/19 (47.5%) |
| Aetiology | |
| Benign, n (%) | 2 (5.0%) |
| HCC, n (%) | 6 (15.0%) |
| Intrahepatic CCC, n (%) | 8 (20.0%) |
| CRLM, n (%) | 14 (35.0%) |
| Klatskin tumour, n (%) | 2 (5.0%) |
| Gallbladder carcinoma, n (%) | 2 (5.0%) |
| Other (GIST, NET, MM, adenoma of adrenal gland), n (%) | 6 (15.0%) |
| Stay in ICU/IMC, days, median | 3 |
| Total days in hospital, days, median | 17 |
| In-house mortality | 1 (2.5%) |
| Preoperative PVE, n (%) | 11 (27.5%) |
| Preoperative chemotherapy, n (%) | 19 (47.5%) |
| Nicotine abuse | 7 (17.5%) |
| Alcohol abuse | 4 (10%) |
| Patient | Age | Volumetry Required | Disease | PVE | Preop. CTx | Surgery | Bilirubin (mg/dL) | Prothrombin Time (%) | Preop. LiMAx |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | no | HCC | no | no | RHH | 0.3 | 122 | 627 |
| 2 | 55 | no | HCC | no | no | RHH | 0.3 | 95 | 531 |
| 3 | 22 | yes | benign | yes | no | RTRI | 0.4 | 90 | 426 |
| 4 | 73 | yes | HCC | no | yes | RTRI | 0.4 | 94 | 732 |
| 5 | 81 | yes | NET | no | yes | ERHH | 0.4 | 87 | 531 |
| 6 | 77 | yes | CRLM | no | no | ERHH | <0.3 | 90 | 368 |
| 7 | 72 | no | CRLM | no | yes | RHH | 0.4 | 97 | 325 |
| 8 | 71 | yes | CRLM | yes | yes | RTRI | 0.6 | 68 | 408 |
| 9 | 37 | yes | benign | no | no | RHH | 0.7 | 82 | 297 |
| 10 | 65 | no | CRLM | yes | yes | RHH | 0.8 | 87 | 534 |
| 11 | 50 | yes | CRLM | yes | yes | ELHH | n/a | 100 | 616 |
| 12 | 73 | yes | GIST | yes | yes | ERHH | 0.3 | 122 | 509 |
| 13 | 48 | yes | CCC | yes | no | RTRI | 0.7 | 106 | 415 |
| 14 | 64 | no | NET | no | no | LHH | 0.6 | 98 | 468 |
| 15 | 53 | no | CRLM | no | yes | RHH | 0.3 | 150 | 939 |
| 16 | 80 | no | CCC | no | no | RHH | 0.8 | 89 | 612 |
| 17 | 71 | no | CCC | no | no | RHH | 0.3 | 85 | 683 |
| 18 | 62 | yes | CRLM | yes | yes | ERHH | 0.6 | 122 | 157 |
| 19 | 72 | no | HCC | no | no | LHH | 0.8 | 95 | 465 |
| 20 | 69 | no | MM | no | no | ERHH | 0.6 | 108 | 425 |
| 21 | 64 | yes | CRLM | no | yes | RHH | 0.3 | 113 | 337 |
| Pat. | Total Liver Vol. | Tumour Vol. # | Resected Vol. | FLR | Preop. LiMAx | Expected LiMAx (Post-Op) | Measured LiMAx (POD 1) | Difference Expected vs. Measured LiMAx (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | n/a | n/a | n/a | n/a | 627 | n/a | 267 | / |
| 2 | n/a | n/a | n/a | n/a | 531 | n/a | 33 | / |
| 3 | 2444 | 733 | 1672 | 772 | 426 | 191 | 146 | 31 |
| 4 | 1971 | 591 | 1338 | 633 | 732 | 329 | 340 | 3 |
| 5 | n/a | n/a | n/a | n/a | 531 | n/a | 137 | / |
| 6 | n/a | n/a | n/a | n/a | 368 | n/a | 29 | / |
| 7 | 2970 | 891 | 2077 | 893 | 325 | 136 | 149 | 9 |
| 8 | 1514 | 151 | 1117 | 397 | 408 | 118 | 121 | 2 |
| 9 | 1022 | 102 | 665 | 357 | 297 | 112 | 141 | 21 |
| 10 | 1745 | 523 | 1084 | 661 | 534 | 288 | 192 | 50 |
| 11 | 1937 | 193 | 757 | 1180 | 616 | 412 | 169 | 144 |
| 12 | 1512 | 1058 | 1023 | 489 | 509 | 544 | 38 | 1332 |
| 13 | 1917 | 958 | 1414 | 503 | 415 | 215 | 135 * | / |
| 14 | 1640 | 164 | 559 | 1081 | 468 | 341 | 347 | 2 |
| 15 | 1464 | 146 | 961 | 503 | 939 | 356 | 381 | 7 |
| 16 | 1525 | 152 | 1048 | 477 | 612 | 208 | 94 | 121 |
| 17 | n/a | n/a | n/a | n/a | 683 | n/a | 293 | / |
| 18 | 1453 | 145 | 844 | 609 | 157 | 72 | 224 | 68 |
| 19 | 1815 | 181 | 651 | 1164 | 465 | 330 | 323 | 2 |
| 20 | n/a | n/a | n/a | n/a | 425 | n/a | n/a | / |
| 21 | n/a | n/a | n/a | n/a | 337 | n/a | n/a | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rühlmann, F.; Azizian, A.; Moosmann, C.; Bernhardt, M.; Keck, J.; Flebbe, H.; Al-Bourini, O.; Hosseini, A.S.A.; Grade, M.; Lorf, T.; et al. Perioperative LiMAx Test Analysis: Impact of Portal Vein Embolisation, Chemotherapy and Major Liver Resection. Biomedicines 2024, 12, 254. https://doi.org/10.3390/biomedicines12020254
Rühlmann F, Azizian A, Moosmann C, Bernhardt M, Keck J, Flebbe H, Al-Bourini O, Hosseini ASA, Grade M, Lorf T, et al. Perioperative LiMAx Test Analysis: Impact of Portal Vein Embolisation, Chemotherapy and Major Liver Resection. Biomedicines. 2024; 12(2):254. https://doi.org/10.3390/biomedicines12020254
Chicago/Turabian StyleRühlmann, Felix, Azadeh Azizian, Christian Moosmann, Markus Bernhardt, Jan Keck, Hannah Flebbe, Omar Al-Bourini, Ali Seif Amir Hosseini, Marian Grade, Thomas Lorf, and et al. 2024. "Perioperative LiMAx Test Analysis: Impact of Portal Vein Embolisation, Chemotherapy and Major Liver Resection" Biomedicines 12, no. 2: 254. https://doi.org/10.3390/biomedicines12020254
APA StyleRühlmann, F., Azizian, A., Moosmann, C., Bernhardt, M., Keck, J., Flebbe, H., Al-Bourini, O., Hosseini, A. S. A., Grade, M., Lorf, T., Ghadimi, M., Perl, T., & Gaedcke, J. (2024). Perioperative LiMAx Test Analysis: Impact of Portal Vein Embolisation, Chemotherapy and Major Liver Resection. Biomedicines, 12(2), 254. https://doi.org/10.3390/biomedicines12020254





