The Role of the ADAMTS18 Gene-Induced Immune Microenvironment in Mouse Kidney Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Collection of Embryonic Kidneys
2.3. PAS Staining
2.4. Immunofluorescence
2.5. RT‒PCR
2.6. TUNEL Staining
2.7. Phospho-Histone-3 (PH3) Staining
2.8. Flow Cytometry
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. Morphologic Changes in Mouse Kidney Development and ADAMTS18 Expression
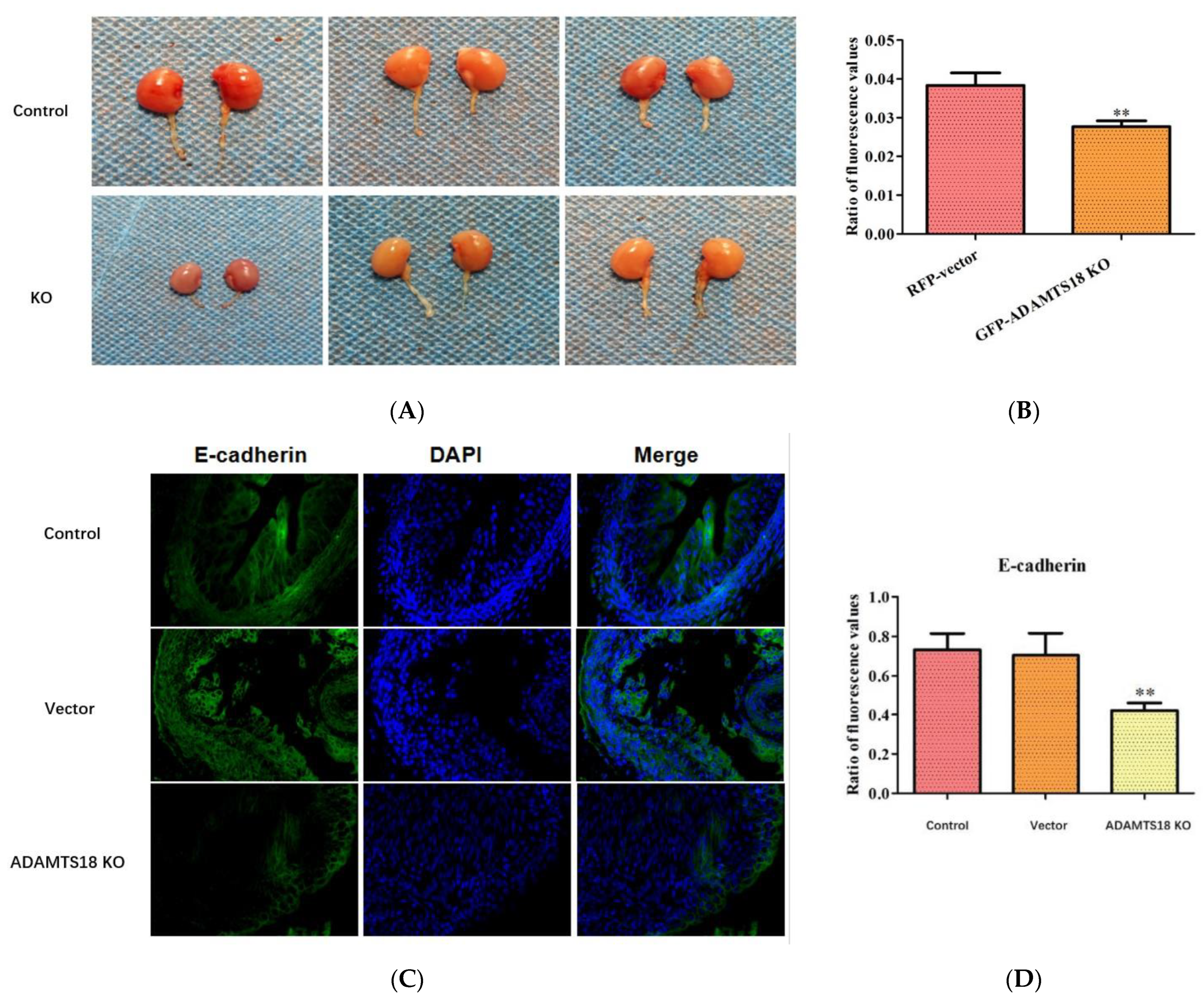
3.2. ADAMTS18 Deletion Induces UB Defects
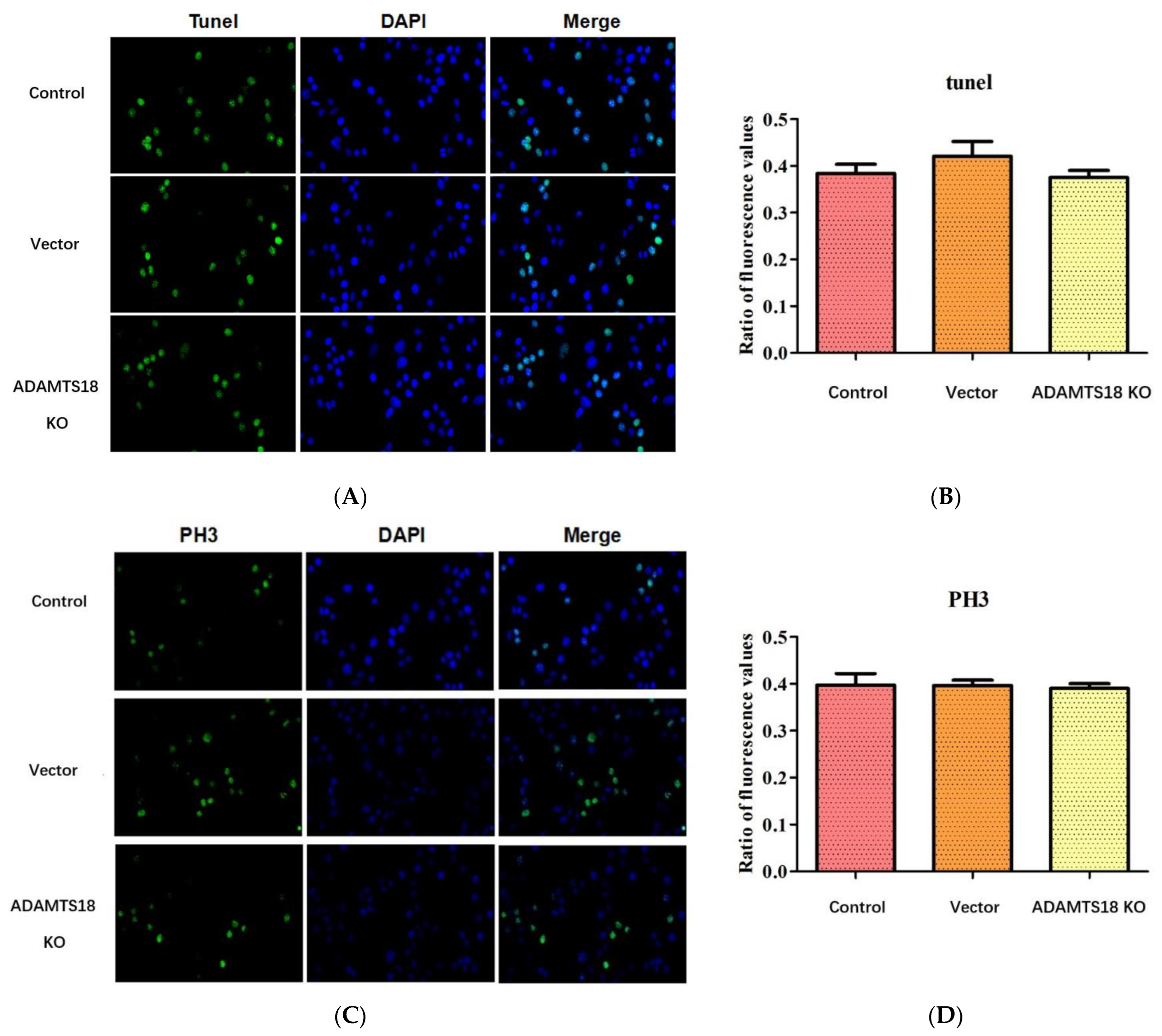
3.3. ADAMTS18 Deletion Did Not Affect Postembryonic Renal Mesenchymal Cell (MSCs) Proliferation or Apoptosis
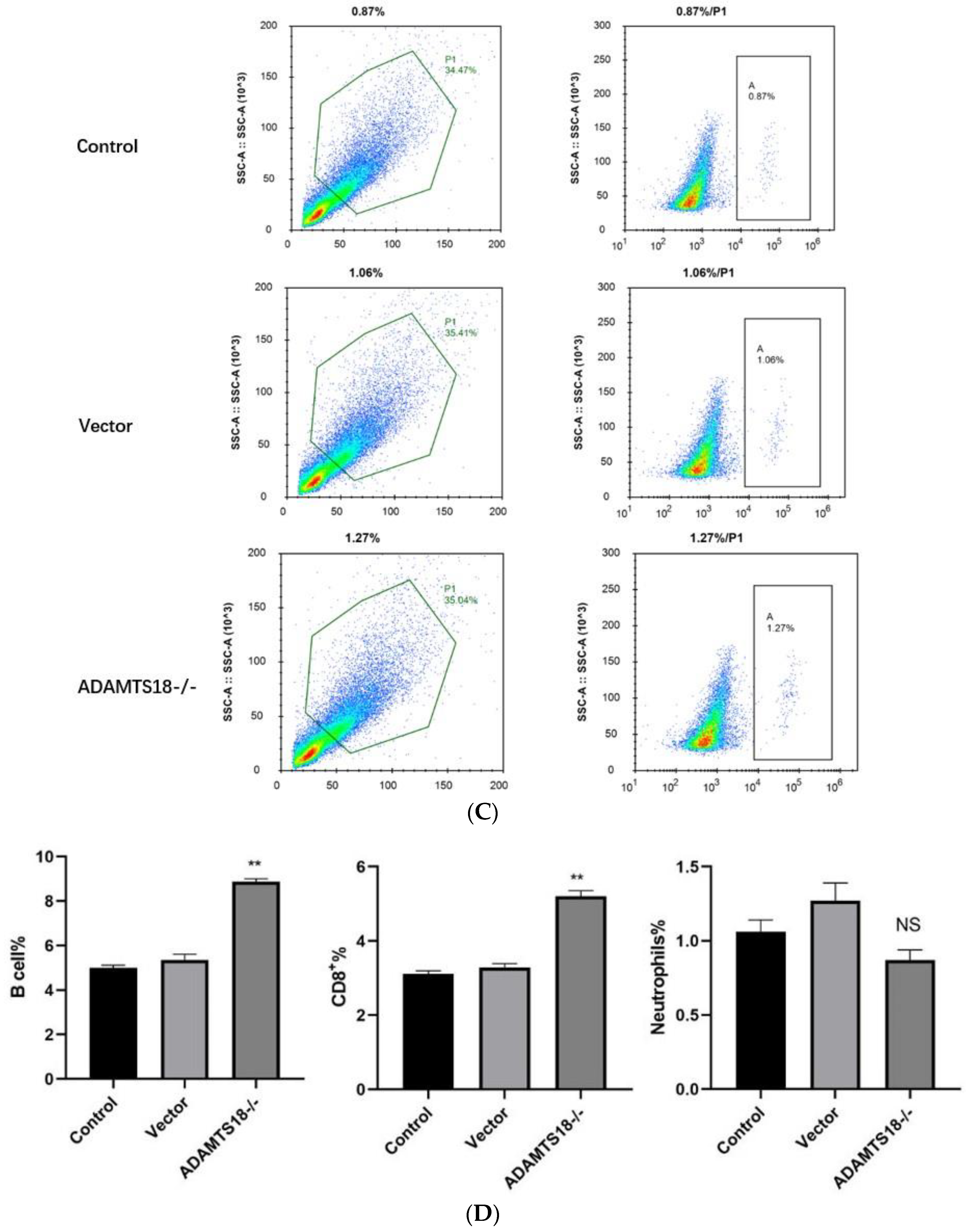
3.4. Analysis of Immune Cell Infiltration
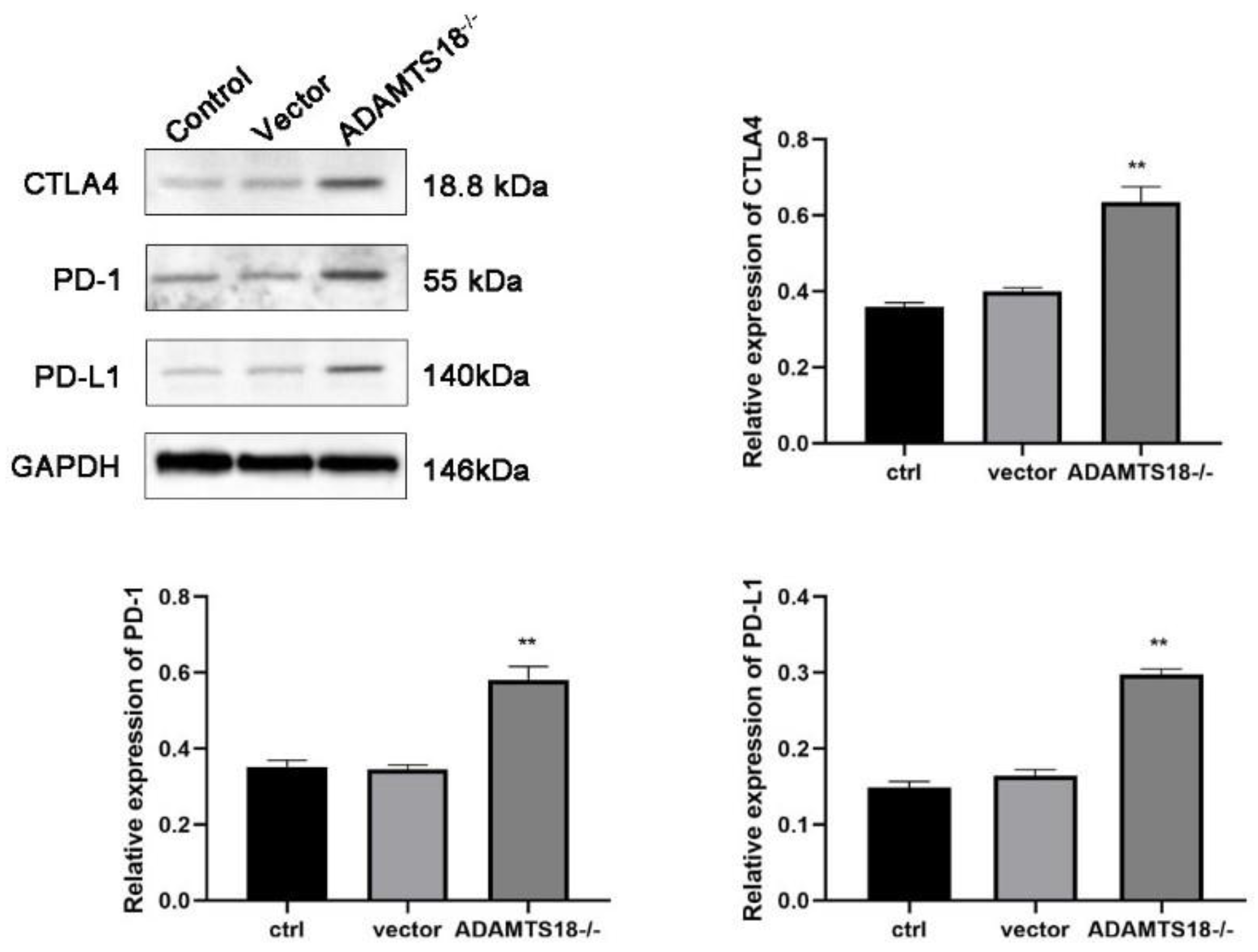
3.5. Analysis of ADAMTS18 Expression and Immune Checkpoint Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Westland, R.; Renkema, K.V.; Knoers, N.V. Clinical integration of genome diagnostics for congenital anomalies of the kidney and urinary tract. Clin. J. Am. Soc. Nephrol. 2020, 16, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Rasouly, H.M.; Lu, W. Lower urinary tract development and Disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 307–342. [Google Scholar] [CrossRef] [PubMed]
- Bebee, T.W.; Sims-Lucas, S.; Park, J.W.; Bushnell, D.; Cieply, B.; Xing, Y.; Bates, C.M.; Carstens, R.P. Ablation of the epithelial-specific splicing factor Esrp1 results in ureteric branching defects and reduced nephron number. Dev. Dyn. 2016, 245, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Dubail, J.; Apte, S.S. Insights on ADAMTS proteases and ADAMTS-like proteins from mammalian genetics. Matrix Biol. 2015, 44–46, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Jaffey, J.A.; Bullock, G.; Guo, J.Y.; Mhlanga-Mutangadura, T.; O’brien, D.P.; Coates, J.R.; Morrissey, R.; Hutchison, R.; Donnelly, K.S.; Cohn, L.A.; et al. Novel homozygous ADAMTS2 variants and associated disease phenotypes in dogs with dermatosparactic Ehlers-Danlos Syndrome. Genes 2022, 13, 2158. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, S.; Groot, R.D. ADAMTS proteases in cardiovascular physiology and disease. Open Biol. 2020, 10, 200333. [Google Scholar] [CrossRef]
- Rutledge, E.A.; Benazet, J.D.; McMahon, A.P. Cellular heterogeneity in the ureteric progenitor niche and distinct profiles of branching morphogenesis in organ development. Development 2017, 144, 3177–3188. [Google Scholar] [CrossRef]
- Ataca, D.; Caikovski, M.; Piersigilli, A.; Moulin, A.; Benarafa, C.; Earp, S.E.; Guri, Y.; Kostic, C.; Arsenijevic, Y.; Soininen, R.; et al. Adamts18 deletion results in distinct developmental defects and provides a model for congenital disorders of lens, lung, and female reproductive tract development. Biol. Open 2016, 5, 1585–1594. [Google Scholar] [CrossRef]
- Lu, T.T.; Dang, S.Y.; Zhu, R.; Wang, Y.; Nie, Z.; Hong, T.; Zhang, W. Adamts18 deficiency promotes colon carcinogenesis by enhancing β-catenin and p38MAPK/ERK1/2 signaling in the mouse model of AOM/DSS-induced colitis-associated colorectal cancer. Oncotarget 2017, 8, 18979–18990. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, Q.; Ye, S.; Lu, T.; Sun, M.; Wang, L.; Wang, M.; Pan, Y.H.; Dang, S.; Zhang, W. Adamts18 deficiency causes spontaneous SMG fibrogenesis in adult mice. J. Dent. Res. 2022, 101, 226–234. [Google Scholar] [CrossRef]
- Munro, D.A.D.; Wineberg, Y.; Tarnick, J.; Vink, C.S.; Li, Z.; Pridans, C.; Dzierzak, E.; Kalisky, T.; Hohenstein, P.; Davies, J.A. Macrophages restrict the nephrogenic field and promote endothelial connections during kidney development. eLife 2019, 8, e43271. [Google Scholar] [CrossRef] [PubMed]
- Song, C.J.; Li, Z.; Ahmed, U.K.B.; Bland, S.J.; Yashchenko, A.; Liu, S.; Aloria, E.J.; Lever, J.M.; Gonzalez, N.M.; Bickel, M.A.; et al. A comprehensive immune cell atlas of cystic kidney disease reveals the involvement of adaptive immune cells in injury-mediated cyst progression in mice. J. Am. Soc. Nephrol. 2022, 33, 747–768. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.Y. Research of Robo2 Regulated the Development of Mouse Kidney and Ureter; Nankai University: Nankai, Japan, 2012; pp. 1–120. [Google Scholar]
- Sainio, K.; Suvanto, P.; Davies, J.; Wartiovaara, J.; Wartiovaara, K.; Saarma, M.; Arumäe, U.; Meng, X.; Lindahl, M.; Pachnis, V.; et al. Glial-cell-line-derived neurotrophic factor isrequired for bud initiation from ureteric epithelium. Development 1997, 124, 4077–4087. [Google Scholar] [CrossRef] [PubMed]
- Mcmahon, A.P. Development of the mammalian kidney. Curr. Top. Dev. Biol. 2016, 117, 31–64. [Google Scholar] [PubMed]
- Hartman, H.A.; Lai, H.L.; Patterson, L.T. Cessation of renal morphogenesis in mice. Dev. Biol. 2007, 310, 379–387. [Google Scholar] [CrossRef]
- Cebrián, C.; Borodo, K.; Charles, N.; Herzlinger, D.A. Morphometric index of the developing murine kidney. Dev. Dyn. 2004, 231, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Short, K.M.; Combes, A.N.; Lefevre, J.; Ju, A.L.; Georgas, K.M.; Lamberton, T.; Cairncross, O.; Rumballe, B.A.; McMahon, A.P.; Hamilton, N.A.; et al. Global quantification of tissue dynamics in the developing mouse kidney. Dev. Cell 2014, 29, 188–202. [Google Scholar] [CrossRef]
- Saxén, L.; Sariola, H. Early organogenesis of the kidney. Pediatr. Nephrol. 1987, 1, 385–392. [Google Scholar] [CrossRef]
- Bertram, J.F.; Young, R.J.; Spencer, K.; Gordon, I. Quantitative analysis of the developing rat kidney: Absolute and relative volumes and growth curves. Anat. Rec. 2000, 258, 128–135. [Google Scholar] [CrossRef]
- Benz, K.; Varga, I.; Neureiter, D.; Campean, V.; Daniel, C.; Heim, C.; Reimann, A.; Weyand, M.; Hilgers, K.F.; Amann, K. Vascular inflammation and media calcification are already present in early stage of chronic kidney disease. Cardiovasc. Pathol. 2017, 27, 57–67. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, B.; Yu, H.Y.; Han, J.; Cui, M.; Zhang, F.; Wang, G.; Guo, L.; Gao, W. Association between plasma ADAMTS-7 levels and severity of disease in patients with stable obstructive coronary artery disease. Medicine 2016, 95, e5523. [Google Scholar] [CrossRef] [PubMed]
- Mittaz, L.; Ricardo, S.; Martinez, G.; Kola, I.; Kelly, D.J.; Little, M.H.; Hertzog, P.J.; Pritchard, M.A. Neonatal calyceal dilation and renal fibrosis resulting from loss of Adamts-1 in mouse kidney is due to a developmental dysgenesis. Nephrol. Dial. Transplant. 2005, 20, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Yu, C.A.; Lu, J.H.; Gao, H.-M.; Li, G.; Kong, W.; Zheng, J. ADAMTS-7 expression increases in the early stage of angiotensin II-induced renal injury in elderly mice. Kidney Blood Press. Res. 2013, 38, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Bramham, K.; Hilton, R.; Horsfield, C.; McDonald, V.; Camilleri, R.; Hunt, B.J. ADAMTS-13 deficiency: Can it cause chronic renal failure? Nephrol. Dial. Transplant. 2011, 26, 742–744. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Winkler, A.; Hovinga, J.A.K.; Bianchi, V.; Studt, J.D.; Lämmle, B. Schistocytic anaemia, severe thrombocytopenia, and renal dysfunction: Thrombotic microangiopathy due to severe acquired ADAMTS-13 deficiency. Case 2. Hamostaseologie 2023, 23, 103–108. [Google Scholar] [CrossRef]
- Sun, M. Role of Metalloproteinase ADAMTS18 in the Formation of Mouse Glomerular Filtration Barrier; East China Normal University: Shanghai, China, 2023; pp. 1–96. [Google Scholar]
- Nagalakshmi, V.K.; Yu, J. The ureteric bud epithelium: Morphogenesis and roles in metanephric kidney patterning. Mol. Reprod. Dev. 2015, 82, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, J.E.; Ye, L.; Yuan, C.-W. Curcumin interferes with TGF-β1-induced fibrosis in NRK-49F cells by reversing ADAMTS18 gene methylation. Chin. J. Integr. Med. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, J.E.; Ye, L.; Yuan, C.W. The progression of obstructive renal fibrosis in rats is regulated by ADAMTS18 gene methylation in the embryonic kidney through the AKT/Notch pathway. J. Biochem. Mol. Toxicol. 2024, 38, e23628. [Google Scholar] [CrossRef]
- Takahashi, M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor. Rev. 2001, 12, 361–373. [Google Scholar] [CrossRef]
- Nigam, S.K. Determinants of branching tubulogenesis. Curr. Opin. Nephrol. Hypertens. 1995, 4, 209. [Google Scholar] [CrossRef]
- Ataca, D.; Aouad, P.; Constantin, C.; Beleut, M.; Shamseddin, M.; Rajaram, R.D.; Jeitziner, R.; Mead, T.J.; Caikovski, M.; Bucher, P. The secreted protease Adamts18 links hormone action to activation of the mammary stem cell niche. Nat. Commun. 2020, 11, 1571. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.T.; Lin, X.T.; Pan, Y.H.; Yang, N.; Ye, S.; Zhang, Q.; Wang, C.; Zhu, R.; Zhang, T.; Wisniewski, T.M.; et al. ADAMTS18 deficiency leads to pulmonary hypoplasia and bronchial microfibril accumulation. iScience 2020, 23, 101472. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.T.; Wu, T.J.; Wang, L.Y.; Dang, S.; Zhang, W. ADAMTS18 deficiency leads to preputial gland hypoplasia and fibrosis in male mice. Reprod. Biol. 2021, 21, 100542. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, E.A.; Lindström, N.O.; Michos, O.; McMahon, A.P. Genetic manipulation of ureteric bud tip progenitors in the mammalian kidney through an Adamts18 enhancer driven tet-on inducible system. Dev. Biol. 2020, 458, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.; Sakurai, H.; Stuart, R.O.; Nigam, S.K. Role of hyaluronan and CD44 in in vitro branching morphogenesis of ureteric bud cells. Dev. Biol. 2000, 224, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, J.E.; Ye, L.; Yuan, C. Chinese herbal compound SanHuang decoction reverses axitinib resistance in ccRCC through regulating immune cell infiltration by affecting ADAMTS18 expression. Am. J. Cancer Res. 2023, 13, 2841–2860. [Google Scholar] [PubMed]
- Pippin, J.W.; Kaverina, N.; Wang, Y.L.; Eng, D.G.; Zeng, Y.; Tran, U.; Loretz, C.J.; Chang, A.; Akilesh, S.; Poudel, C.; et al. Upregulated PD-1 signaling antagonizes glomerular health in aged kidneys and disease. J. Clin. Investig. 2022, 132, e156250. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.H.; Zhang, W. Secreted protease ADAMTS18 in development and disease. Gene 2023, 858, 147169. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Zhang, J.-E.; Ye, L.; Yuan, C.-W. The Role of the ADAMTS18 Gene-Induced Immune Microenvironment in Mouse Kidney Development. Biomedicines 2024, 12, 396. https://doi.org/10.3390/biomedicines12020396
Xu B, Zhang J-E, Ye L, Yuan C-W. The Role of the ADAMTS18 Gene-Induced Immune Microenvironment in Mouse Kidney Development. Biomedicines. 2024; 12(2):396. https://doi.org/10.3390/biomedicines12020396
Chicago/Turabian StyleXu, Ben, Jia-En Zhang, Lin Ye, and Chang-Wei Yuan. 2024. "The Role of the ADAMTS18 Gene-Induced Immune Microenvironment in Mouse Kidney Development" Biomedicines 12, no. 2: 396. https://doi.org/10.3390/biomedicines12020396
APA StyleXu, B., Zhang, J.-E., Ye, L., & Yuan, C.-W. (2024). The Role of the ADAMTS18 Gene-Induced Immune Microenvironment in Mouse Kidney Development. Biomedicines, 12(2), 396. https://doi.org/10.3390/biomedicines12020396






