Quantitative Evaluation by Digital Pathology of Immunohistochemical Expression of CK7, CK19, and EpCAM in Advanced Stages of NASH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Liver Biopsies
2.2. R-Ratio Calculation
2.3. Histological and Immunohistochemical Analysis
2.4. Digital Image Analysis
2.5. Semi-Quantitative Scoring System for Histological Analysis
2.6. Statistical Analysis
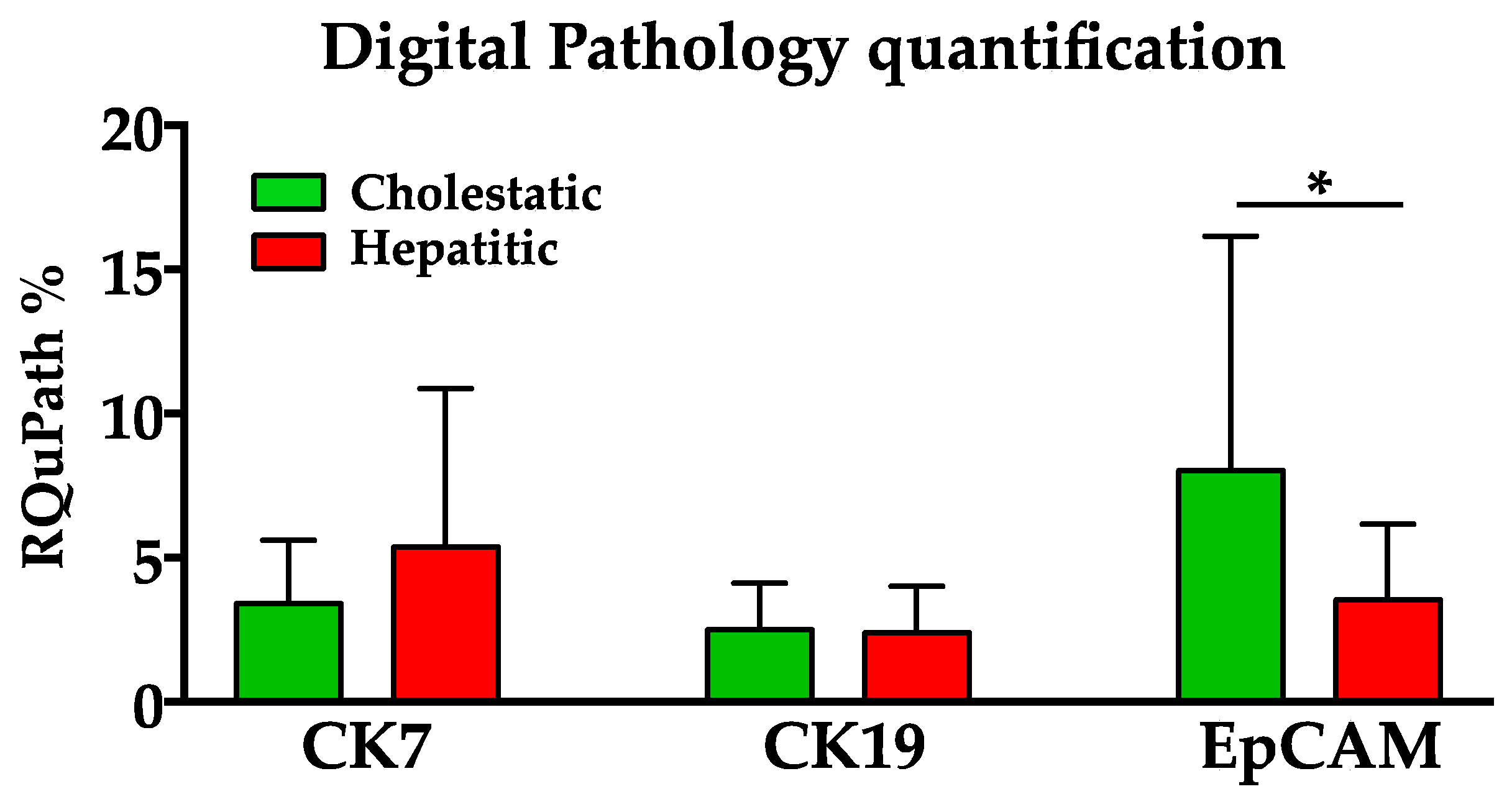
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Farrell, G.C.; Larter, C.Z. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006, 43, S99–S112. [Google Scholar] [CrossRef]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wai-Sun Wong, V.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e1612. [Google Scholar] [CrossRef]
- Portillo-Sanchez, P.; Bril, F.; Maximos, M.; Lomonaco, R.; Biernacki, D.; Orsak, B.; Subbarayan, S.; Webb, A.; Hecht, J.; Cusi, K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J. Clin. Endocrinol. Metab. 2015, 100, 2231–2238. [Google Scholar] [CrossRef]
- National Guideline Center. National Institute for Health and Care Excellence: Guidelines. In Non-Alcoholic Fatty Liver Disease: Assessment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2016. [Google Scholar]
- Maurice, J.; Manousou, P. Non-alcoholic fatty liver disease. Clin. Med. 2018, 18, 245–250. [Google Scholar] [CrossRef]
- Shipovskaya, A.A.; Dudanova, O.P. Intrahepatic cholestasis in nonalcoholic fatty liver disease. Ther. Arkh. 2018, 90, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, P.; Tarantino, G.; Perrella, A.; Micheli, P.; Perrella, O.; Conca, P. A clinical-morphological study on cholestatic presentation of nonalcoholic fatty liver disease. Dig. Dis. Sci. 2005, 50, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Gadd, V.L.; Skoien, R.; Powell, E.E.; Fagan, K.J.; Winterford, C.; Horsfall, L.; Irvine, K.; Clouston, A.D. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 2014, 59, 1393–1405. [Google Scholar] [CrossRef]
- Jüngst, C.; Berg, T.; Cheng, J.; Green, R.M.; Jia, J.; Mason, A.L.; Lammert, F. Intrahepatic cholestasis in common chronic liver diseases. Eur. J. Clin. Investig. 2013, 43, 1069–1083. [Google Scholar] [CrossRef]
- DeLeve, L.D.; Kaplowitz, N. Mechanisms of drug-induced liver disease. Gastroenterol. Clin. North Am. 1995, 24, 787–810. [Google Scholar] [CrossRef] [PubMed]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Shirin, D.; Peleg, N.; Sneh-Arbib, O.; Cohen-Naftaly, M.; Braun, M.; Shochat, T.; Issachar, A.; Shlomai, A. The Pattern of Elevated Liver Function Tests in Nonalcoholic Fatty Liver Disease Predicts Fibrosis Stage and Metabolic-Associated Comorbidities. Dig. Dis. 2019, 37, 69–76. [Google Scholar] [CrossRef]
- Pennisi, G.; Pipitone, R.M.; Cabibi, D.; Enea, M.; Romero-Gomez, M.; Viganò, M.; Bugianesi, E.; Wong, V.W.; Fracanzani, A.L.; Sebastiani, G.; et al. A cholestatic pattern predicts major liver-related outcomes in patients with non-alcoholic fatty liver disease. Liver Int. 2022, 42, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Franke, W.W.; Schiller, D.L.; Geiger, B.; Krepler, R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.G.; Weiss, L.M. Keratin expression in human tissues and neoplasms. Histopathology 2002, 40, 403–439. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.C.; Hübscher, S.G. Cytokeratin expression as an aid to diagnosis in medical liver biopsies. Histopathology 2010, 56, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Matsukuma, S.; Takeo, H.; Kono, T.; Nagata, Y.; Sato, K. Aberrant cytokeratin 7 expression of centrilobular hepatocytes: A clinicopathological study. Histopathology 2012, 61, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Schnell, U.; Cirulli, V.; Giepmans, B.N. EpCAM: Structure and function in health and disease. Biochim. Biophys. Acta 2013, 1828, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Herlyn, M.; Steplewski, Z.; Herlyn, D.; Koprowski, H. Colorectal carcinoma-specific antigen: Detection by means of monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1979, 76, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Pavšič, M.; Gunčar, G.; Djinović-Carugo, K.; Lenarčič, B. Crystal structure and its bearing towards an understanding of key biological functions of EpCAM. Nat. Commun. 2014, 5, 4764. [Google Scholar] [CrossRef]
- Strnad, J.; Hamilton, A.E.; Beavers, L.S.; Gamboa, G.C.; Apelgren, L.D.; Taber, L.D.; Sportsman, J.R.; Bumol, T.F.; Sharp, J.D.; Gadski, R.A. Molecular cloning and characterization of a human adenocarcinoma/epithelial cell surface antigen complementary DNA. Cancer Res. 1989, 49, 314–317. [Google Scholar]
- Litvinov, S.V.; Bakker, H.A.; Gourevitch, M.M.; Velders, M.P.; Warnaar, S.O. Evidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epithelial cell-cell adhesion. Cell Adhes. Commun. 1994, 2, 417–428. [Google Scholar] [CrossRef]
- de Boer, C.J.; van Krieken, J.H.; Janssen-van Rhijn, C.M.; Litvinov, S.V. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J. Pathol. 1999, 188, 201–206. [Google Scholar] [CrossRef]
- Yoon, S.M.; Gerasimidou, D.; Kuwahara, R.; Hytiroglou, P.; Yoo, J.E.; Park, Y.N.; Theise, N.D. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology 2011, 53, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Safarikia, S.; Carpino, G.; Overi, D.; Cardinale, V.; Venere, R.; Franchitto, A.; Onori, P.; Alvaro, D.; Gaudio, E. Distinct EpCAM-Positive Stem Cell Niches Are Engaged in Chronic and Neoplastic Liver Diseases. Front. Med. 2020, 7, 479. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, F.; Birk, G.; Stierstorfer, B. Deep learning enables pathologist-like scoring of NASH models. Sci. Rep. 2019, 9, 18454. [Google Scholar] [CrossRef]
- Heinemann, F.; Gross, P.; Zeveleva, S.; Qian, H.S.; Hill, J.; Höfer, A.; Jonigk, D.; Diehl, A.M.; Abdelmalek, M.; Lenter, M.C.; et al. Deep learning-based quantification of NAFLD/NASH progression in human liver biopsies. Sci. Rep. 2022, 12, 19236. [Google Scholar] [CrossRef]
- Shirin, D.; Tobar, A.; Bendersky, A.G.; Velders, M.P.; Harif, Y.; Naamneh, R.; Shlomai, A. Liver test-derived R factor is associated with portal hypertension in patients with non-alcoholic fatty liver disease. In Proceedings of the Easl ILC, London, UK, 22–26 June 2022. [Google Scholar]
- Desmet, V.J. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011, 458, 251–259. [Google Scholar] [CrossRef]
- Onofrio, F.Q.; Hirschfield, G.M. The Pathophysiology of Cholestasis and Its Relevance to Clinical Practice. Clin. Liver Dis. 2020, 15, 110–114. [Google Scholar] [CrossRef]
- Desmet, V.J. Ductal plates in hepatic ductular reactions. Hypothesis and implications. II. Ontogenic liver growth in childhood. Virchows Arch. 2011, 458, 261–270. [Google Scholar] [CrossRef]
- Desmet, V.J. Ductal plates in hepatic ductular reactions. Hypothesis and implications. III. Implications for liver pathology. Virchows Arch. 2011, 458, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Manco, R.; Clerbaux, L.A.; Verhulst, S.; Bou Nader, M.; Sempoux, C.; Ambroise, J.; Bearzatto, B.; Gala, J.L.; Horsmans, Y.; van Grunsven, L.; et al. Reactive cholangiocytes differentiate into proliferative hepatocytes with efficient DNA repair in mice with chronic liver injury. J. Hepatol. 2019, 70, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Marzioni, M.; Meng, F.; Francis, H.; Glaser, S.; Alpini, G. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019, 69, 420–430. [Google Scholar] [CrossRef]
- Carpino, G.; Cardinale, V.; Folseraas, T.; Overi, D.; Floreani, A.; Franchitto, A.; Onori, P.; Cazzagon, N.; Berloco, P.B.; Karlsen, T.H.; et al. Hepatic Stem/Progenitor Cell Activation Differs between Primary Sclerosing and Primary Biliary Cholangitis. Am. J. Pathol. 2018, 188, 627–639. [Google Scholar] [CrossRef]
- Weber, S.; Allgeier, J.; Denk, G.; Gerbes, A.L. Marked Increase of Gamma-Glutamyltransferase as an Indicator of Drug-Induced Liver Injury in Patients without Conventional Diagnostic Criteria of Acute Liver Injury. Visc. Med. 2022, 38, 223–228. [Google Scholar] [CrossRef]
- Irie, M.; Suzuki, N.; Sohda, T.; Anan, A.; Iwata, K.; Takeyama, Y.; Watanabe, H.; Fischer, P.; Scherberich, J.E.; Sakisaka, S. Hepatic expression of gamma-glutamyltranspeptidase in the human liver of patients with alcoholic liver disease. Hepatol. Res. 2007, 37, 966–973. [Google Scholar] [CrossRef]
- Bulle, F.; Mavier, P.; Zafrani, E.S.; Preaux, A.M.; Lescs, M.C.; Siegrist, S.; Dhumeaux, D.; Guellaën, G. Mechanism of gamma-glutamyl transpeptidase release in serum during intrahepatic and extrahepatic cholestasis in the rat: A histochemical, biochemical and molecular approach. Hepatology 1990, 11, 545–550. [Google Scholar] [CrossRef]
- Trauner, M.; Fuchs, C.D. Novel therapeutic targets for cholestatic and fatty liver disease. Gut 2022, 71, 194–209. [Google Scholar] [CrossRef]
- Marchianò, S.; Biagioli, M.; Morretta, E.; Di Giorgio, C.; Roselli, R.; Bordoni, M.; Bellini, R.; Urbani, G.; Massa, C.; Monti, M.C.; et al. Combinatorial therapy with BAR502 and UDCA resets FXR and GPBAR1 signaling and reverses liver histopathology in a model of NASH. Sci. Rep. 2023, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Chen, Y.P.; Ma, K.F.; Ye, Y.F.; Zheng, L.; Yang, Y.D.; Li, Y.M.; Jin, X. The role of ursodeoxycholic acid in non-alcoholic steatohepatitis: A systematic review. BMC Gastroenterol. 2013, 13, 140. [Google Scholar] [CrossRef]


| N | Gender | Age | ALP (U/I) | γGT (U/I) | ALT (U/I) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M/F% | Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
| C Pattern | 22 | 62/38 | 63 | 54–65 | 94 | 75–108 | 59 | 43–97 | 33 | 29–50 |
| H Pattern | 25 | 48/52 | 58 | 51.5–62 | 62 | 55.5–80.5 | 76 | 51.5–154 | 91 | 70–128.5 |
| Total | 47 | 53/47 | 58.5 | 56–98 | 67.50 | 56–98 | 76 | 48–113 | 70 | 42–119.75 |
| S-CK7 | S-CK19 | S-EpCAM | Q-CK7 | Q-CK19 | Q-EpCAM | |
|---|---|---|---|---|---|---|
| S-CK7 | 1.000 | 0.737** 0.000 | 0.561 ** 0.001 | 0.468 ** 0.006 | 0.496 ** 0.003 | 0.453 ** 0.008 |
| S-CK19 | 0.737 ** 0.000 | 1.000 | 0.369 * 0.029 | 0.433 ** 0.009 | 0.420 * 0.012 | 0.382 * 0.024 |
| S-EpCAM | 0.561 ** 0.001 | 0.369 * 0.029 | 1.000 | 0.597 ** 0.000 | 0.533 ** 0.001 | 0.816 ** 0.000 |
| Q-CK7 | 0.468 ** 0.006 | 0.433 ** 0.009 | 0.597 ** 0.000 | 1.000 | 0.726 ** 0.000 | 0.586 ** 0.000 |
| Q-CK19 | 0.496 ** 0.003 | 0.420 * 0.012 | 0.533 ** 0.001 | 0.726 ** 0.000 | 1.000 | 0.744 ** 0.000 |
| Q-EpCAM | 0.453 ** 0.008 | 0.382 * 0.024 | 0.816 ** 0.000 | 0.586 ** 0.000 | 0.744 ** 0.000 | 1.000 |
| Q-CK7 | Q-CK19 | Q-EpCAM | Pattern C b | Fibrosis Stage 4 | ALP | γGT | ALT | |
|---|---|---|---|---|---|---|---|---|
| Fibrosis stage 4 | 0.620 ** | 0.538 ** | 0.417 * | 0.112 | 1.000 | 0.329 * | 0.313 | −0.036 |
| 0.000 | 0.001 | 0.011 | 0.515 | 0.050 | 0.063 | 0.836 | ||
| ALP | 0.218 | 0.230 | 0.387 * | 0.379 * | 0.329 * | 1.000 | 0.391 * | 0.006 |
| 0.201 | 0.177 | 0.020 | 0.023 | 0.050 | . | 0.018 | 0.971 | |
| γGT | 0.347 * | 0.103 | −0.013 | −0.251 | 0.313 | 0.391 * | 1.000 | 0.394 * |
| 0.038 | 0.549 | 0.938 | 0.140 | 0.063 | 0.018 | . | 0.017 | |
| ALT | 0.094 | 0.007 | −0.070 | −0.724 ** | −0.036 | 0.006 | 0.394 * | 1.000 |
| 0.585 | 0.970 | 0.684 | 0.000 | 0.836 | 0.971 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabibi, D.; Giannone, A.G.; Quattrocchi, A.; Calvaruso, V.; Porcasi, R.; Di Grusa, D.; Pavone, A.M.; Comelli, A.; Petta, S. Quantitative Evaluation by Digital Pathology of Immunohistochemical Expression of CK7, CK19, and EpCAM in Advanced Stages of NASH. Biomedicines 2024, 12, 440. https://doi.org/10.3390/biomedicines12020440
Cabibi D, Giannone AG, Quattrocchi A, Calvaruso V, Porcasi R, Di Grusa D, Pavone AM, Comelli A, Petta S. Quantitative Evaluation by Digital Pathology of Immunohistochemical Expression of CK7, CK19, and EpCAM in Advanced Stages of NASH. Biomedicines. 2024; 12(2):440. https://doi.org/10.3390/biomedicines12020440
Chicago/Turabian StyleCabibi, Daniela, Antonino Giulio Giannone, Alberto Quattrocchi, Vincenza Calvaruso, Rossana Porcasi, Domenico Di Grusa, Anna Maria Pavone, Albert Comelli, and Salvatore Petta. 2024. "Quantitative Evaluation by Digital Pathology of Immunohistochemical Expression of CK7, CK19, and EpCAM in Advanced Stages of NASH" Biomedicines 12, no. 2: 440. https://doi.org/10.3390/biomedicines12020440






