Potential for New Therapeutic Approaches by Targeting Lactate and pH Mediated Epigenetic Dysregulation in Major Mental Diseases
Abstract
1. Introduction
2. Brain pH Homeostasis: Normal Function and Pathology
3. Association between pH Abnormalities and Neuropsychiatric Diseases
4. Association between Lactate, an Essential Epigenetic Regulatory Molecule, and Neuropsychiatric Diseases
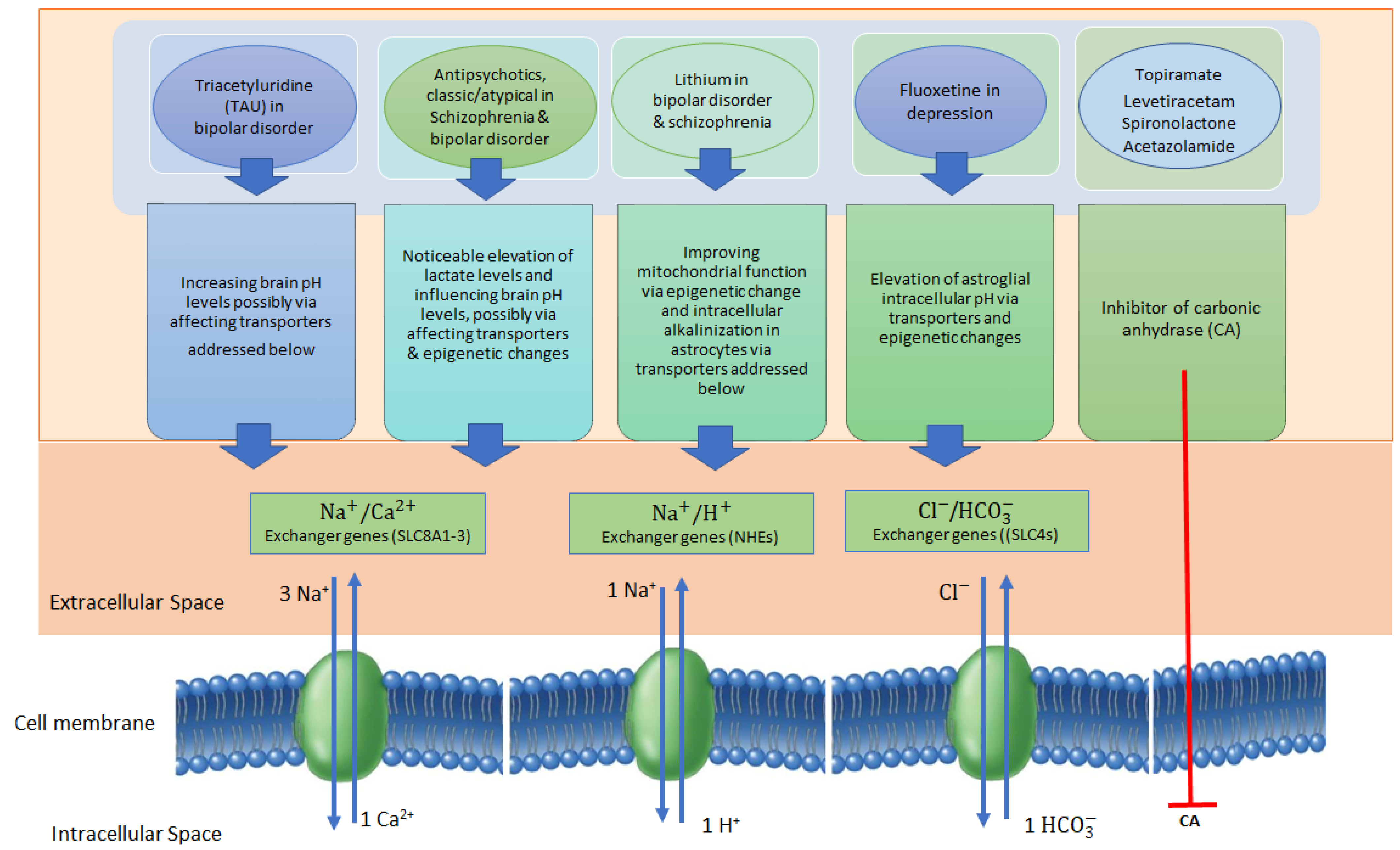
5. pH-Modulating Effects of Neuropsychiatric Drugs via Epigenetic Changes
6. Lactate Producing and Utilizing Gut Microbiota and Their Roles in pH Modulation
7. Opportunities and Challenges in Translating pH-Modulating Therapies to the Clinic for the Treatments of Neuropsychiatric Diseases
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devi, S.; Chaturvedi, M.; Fatima, S.; Priya, S. Environmental factors modulating protein conformations and their role in protein aggregation diseases. Toxicology 2022, 465, 153049. [Google Scholar] [CrossRef]
- Ruffin, V.A.; Salameh, A.I.; Boron, W.F.; Parker, M.D. Intracellular pH regulation by acid-base transporters in mammalian neurons. Front. Physiol. 2014, 5, 43. [Google Scholar] [CrossRef]
- Zhao, H.; Carney, K.E.; Falgoust, L.; Pan, J.W.; Sun, D.; Zhang, Z. Emerging roles of Na+/H+ exchangers in epilepsy and developmental brain disorders. Prog. Neurobiol. 2016, 138, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Gheshlaghi, F.; Eizadi-Mood, N.; Emamikhah-Abarghooeii, S.; Arzani-Shamsabadi, M. Evaluation of serum sodium changes in tricyclic antidepressants toxicity and its correlation with electrocardiography, serum pH, and toxicity severity. Adv. Biomed. Res. 2012, 1, 68. [Google Scholar] [CrossRef]
- Neavyn, M.J.; Boyer, E.W.; Bird, S.B.; Babu, K.M. Sodium acetate as a replacement for sodium bicarbonate in medical toxicology: A review. J. Med. Toxicol. 2013, 9, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Doering, C.; McRory, J. Effects of extracellular pH on neuronal calcium channel activation. Neuroscience 2007, 146, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Sinning, A.; Hübner, C.A. Minireview: pH and synaptic transmission. FEBS Lett. 2013, 587, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, S.; Pekun, T.; Hrynevich, S.; Waseem, T. Extracellular acidification leads to mitochondrial depolarization with following free radical formation in rat brain synaptosomes. SpringerPlus 2015, 4, P12. [Google Scholar] [CrossRef][Green Version]
- Pekun, T.G.; Lemeshchenko, V.V.; Lyskova, T.I.; Waseem, T.V.; Fedorovich, S.V. Influence of intra-and extracellular acidification on free radical formation and mitochondria membrane potential in rat brain synaptosomes. J. Mol. Neurosci. 2013, 49, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, A.; Okonji, E.; Deca, D.; Wei, W.-C.; Glitsch, M.D. Extracellular acidosis impairs P2Y receptor-mediated Ca2+ signalling and migration of microglia. Cell Calcium 2015, 57, 247–256. [Google Scholar] [CrossRef]
- Kumar, A.; Malhotra, P.; Coffing, H.; Priyamvada, S.; Anbazhagan, A.N.; Krishnan, H.R.; Gill, R.K.; Alrefai, W.A.; Gavin, D.P.; Pandey, S.C. Epigenetic modulation of intestinal Na+/H+ exchanger-3 expression. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 314, G309–G318. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Kumar, L.; Sarkar, A.; Srividya, K.; Nazir, A. Targeted downregulation of estradiol binding Na+/H+ exchanger nhx-2, mimics calorie restriction, extends reproductive longevity and ameliorates effects associated with alpha synuclein aggregation in C. elegans. bioRxiv 2020, bioRxiv:2020.07.30.229344. [Google Scholar] [CrossRef]
- González-Nieto, D.; Gómez-Hernández, J.M.; Larrosa, B.; Gutiérrez, C.; Muñoz, M.D.; Fasciani, I.; O’Brien, J.; Zappalà, A.; Cicirata, F.; Barrio, L.C. Regulation of neuronal connexin-36 channels by pH. Proc. Natl. Acad. Sci. USA 2008, 105, 17169–17174. [Google Scholar] [CrossRef] [PubMed]
- Vroman, R.; Klaassen, L.J.; Howlett, M.H.; Cenedese, V.; Klooster, J.; Sjoerdsma, T.; Kamermans, M. Extracellular ATP hydrolysis inhibits synaptic transmission by increasing ph buffering in the synaptic cleft. PLoS Biol. 2014, 12, e1001864. [Google Scholar] [CrossRef]
- Jang, I.-S.; Nakamura, M.; Kubota, H.; Noda, M.; Akaike, N. Extracellular pH modulation of excitatory synaptic transmission in hippocampal CA3 neurons. J. Neurophysiol. 2020, 123, 2426–2436. [Google Scholar] [CrossRef]
- Tresguerres, M.; Buck, J.; Levin, L.R. Physiological carbon dioxide, bicarbonate, and pH sensing. Pflügers Arch.-Eur. J. Physiol. 2010, 460, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases-an overview. Curr. Pharm. Des. 2008, 14, 603–614. [Google Scholar] [CrossRef]
- Shao, Y.; Li, Y.; Zhang, J.; Liu, D.; Liu, F.; Zhao, Y.; Shen, T.; Li, F. Involvement of histone deacetylation in MORC2-mediated down-regulation of carbonic anhydrase IX. Nucleic Acids Res. 2010, 38, 2813–2824. [Google Scholar] [CrossRef]
- Deitmer, J.W.; Theparambil, S.M.; Ruminot, I.; Noor, S.I.; Becker, H.M. Energy dynamics in the brain: Contributions of astrocytes to metabolism and pH homeostasis. Front. Neurosci. 2019, 13, 1301. [Google Scholar] [CrossRef]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.; Li, G. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Christensen, H.L.; Barbuskaite, D.; Rojek, A.; Malte, H.; Christensen, I.B.; Füchtbauer, A.C.; Füchtbauer, E.M.; Wang, T.; Praetorius, J.; Damkier, H.H. The choroid plexus sodium-bicarbonate cotransporter NBCe2 regulates mouse cerebrospinal fluid pH. J. Physiol. 2018, 596, 4709–4728. [Google Scholar] [CrossRef]
- Theparambil, S.M.; Hosford, P.S.; Ruminot, I.; Kopach, O.; Reynolds, J.R.; Sandoval, P.Y.; Rusakov, D.A.; Barros, L.F.; Gourine, A.V. Astrocytes regulate brain extracellular pH via a neuronal activity-dependent bicarbonate shuttle. Nat. Commun. 2020, 11, 5073. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Bunch, L.; Pedersen, S.F. Physiology, pharmacology and pathophysiology of the pH regulatory transport proteins NHE1 and NBCn1: Similarities, differences, and implications for cancer therapy. Curr. Pharm. Des. 2012, 18, 1345–1371. [Google Scholar] [CrossRef]
- Kirischuk, S.; Parpura, V.; Verkhratsky, A. Sodium dynamics: Another key to astroglial excitability? Trends Neurosci. 2012, 35, 497–506. [Google Scholar] [CrossRef]
- Rose, C.R.; Karus, C. Two sides of the same coin: Sodium homeostasis and signaling in astrocytes under physiological and pathophysiological conditions. Glia 2013, 61, 1191–1205. [Google Scholar] [CrossRef]
- Majumdar, A.; Cruz, D.; Asamoah, N.; Buxbaum, A.; Sohar, I.; Lobel, P.; Maxfield, F.R. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol. Biol. Cell 2007, 18, 1490–1496. [Google Scholar] [CrossRef]
- Prasad, H.; Rao, R. Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. Proc. Natl. Acad. Sci. USA 2018, 115, E6640–E6649. [Google Scholar] [CrossRef] [PubMed]
- Gomez Zubieta, D.M.; Hamood, M.A.; Beydoun, R.; Pall, A.E.; Kondapalli, K.C. MicroRNA-135a regulates NHE9 to inhibit proliferation and migration of glioblastoma cells. Cell Commun. Signal. 2017, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kondapalli, K.C.; Prasad, H.; Rao, R. An inside job: How endosomal Na+/H+ exchangers link to autism and neurological disease. Front. Cell. Neurosci. 2014, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Picollo, A.; Pusch, M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 2005, 436, 420–423. [Google Scholar] [CrossRef]
- Lee, C.; Kang, H.J.; Von Ballmoos, C.; Newstead, S.; Uzdavinys, P.; Dotson, D.L.; Iwata, S.; Beckstein, O.; Cameron, A.D.; Drew, D. A two-domain elevator mechanism for sodium/proton antiport. Nature 2013, 501, 573–577. [Google Scholar] [CrossRef]
- Mayburd, A.; Baranova, A. Knowledge-based compact disease models identify new molecular players contributing to early-stage Alzheimer’s disease. BMC Syst. Biol. 2013, 7, 121. [Google Scholar] [CrossRef]
- Prasad, H.; Rao, R. Endosomal acid-base homeostasis in neurodegenerative diseases. In Organelles in Disease; Springer: Cham, Switzerland, 2020; pp. 195–231. [Google Scholar] [CrossRef]
- Rowland, L.; Pradhan, S.; Korenic, S.; Wijtenburg, S.; Hong, L.; Edden, R.; Barker, P. Elevated brain lactate in schizophrenia: A 7 T magnetic resonance spectroscopy study. Transl. Psychiatry 2016, 6, e967. [Google Scholar] [CrossRef]
- Hagihara, H.; Catts, V.S.; Katayama, Y.; Shoji, H.; Takagi, T.; Huang, F.L.; Nakao, A.; Mori, Y.; Huang, K.-P.; Ishii, S. Decreased brain pH as a shared endophenotype of psychiatric disorders. Neuropsychopharmacology 2018, 43, 459–468. [Google Scholar] [CrossRef]
- Pruett, B.S.; Meador-Woodruff, J.H. Evidence for altered energy metabolism, increased lactate, and decreased pH in schizophrenia brain: A focused review and meta-analysis of human postmortem and magnetic resonance spectroscopy studies. Schizophr. Res. 2020, 223, 29–42. [Google Scholar] [CrossRef]
- Hagihara, H.; Murano, T.; Miyakawa, T. The gene expression patterns as surrogate indices of pH in the brain. Front. Psychiatry 2023, 14, 1151480. [Google Scholar] [CrossRef]
- Takahashi, H.; Alves, C.R.; Stanford, K.I.; Middelbeek, R.J.; Nigro, P.; Ryan, R.E.; Xue, R.; Sakaguchi, M.; Lynes, M.D.; So, K. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 2019, 1, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleky, H.M.; Gower, A.C.; Wong, C.K.; Cox, J.W.; Zhang, X.; Thiagalingam, A.; Shafa, R.; Sivaraman, V.; Zhou, J.R.; Thiagalingam, S. Aberrant transcriptomes and DNA methylomes define pathways that drive pathogenesis and loss of brain laterality/asymmetry in schizophrenia and bipolar disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019, 180, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Q.; Lin, J.-R.; Jabalameli, M.R.; Mitra, J.; Nguyen, N.; Zhang, Z.D. Deep post-GWAS analysis identifies potential risk genes and risk variants for Alzheimer’s disease, providing new insights into its disease mechanisms. Sci. Rep. 2021, 11, 20511. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.R.; Chai, Y.L.; Lee, J.H.; Howlett, D.; Attems, J.; Ballard, C.G.; Aarsland, D.; Francis, P.T.; Chen, C.P.; Lai, M.K. Increased transforming growth factor β2 in the neocortex of Alzheimer’s disease and dementia with lewy bodies is correlated with disease severity and soluble Aβ 42 load. J. Alzheimer’s Dis. 2017, 56, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Heath, P.; Eastwood, S.; Burnet, P.; McDonald, B.; Pearson, R. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: Selective mRNA vulnerability and comparison with their encoded proteins. Neurosci. Lett. 1995, 200, 151–154. [Google Scholar] [CrossRef]
- Fillman, S.G.; Sinclair, D.; Fung, S.J.; Webster, M.J.; Shannon Weickert, C. Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl. Psychiatry 2014, 4, e365. [Google Scholar] [CrossRef]
- Hamakawa, H.; Murashita, J.; Yamada, N.; Inubushi, T.; Kato, N.; Kato, T. Reduced intracellular pH in the basal ganglia and whole brain measured by 31P-MRS in bipolar disorder. Psychiatry Clin. Neurosci. 2004, 58, 82–88. [Google Scholar] [CrossRef]
- Wang, J.F.; Shao, L.; Sun, X.; Young, L.T. Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord. 2009, 11, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.A.; Dudley, J.; Lee, J.-H.; Strakowski, S.M.; Adler, C.M.; DelBello, M.P. A pilot study of alterations in high energy phosphoryl compounds and intracellular pH in unmedicated adolescents with bipolar disorder. J. Affect. Disord. 2013, 150, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Magnotta, V.A.; Xu, J.; Fiedorowicz, J.G.; Williams, A.; Shaffer, J.; Christensen, G.; Long, J.D.; Taylor, E.; Sathyaputri, L.; Richards, J.G. Metabolic abnormalities in the basal ganglia and cerebellum in bipolar disorder: A multi-modal MR study. J. Affect. Disord. 2022, 301, 390–399. [Google Scholar] [CrossRef]
- Cheng, K.; Wang, Y.; He, Y.; Tian, Y.; Li, J.; Chen, C.; Xu, X.; Wu, Z.; Yu, H.; Chen, X. Upregulation of carbonic anhydrase 1 beneficial for depressive disorder. Acta Neuropathol. Commun. 2023, 11, 59. [Google Scholar] [CrossRef]
- Glen, A.; Ongley, G.; Robinson, K. Diminished membrane transport in manic-depressive psychosis and recurrent depression. Lancet 1968, 292, 241–243. [Google Scholar] [CrossRef]
- Babu, N.V.; Gudihal, A.; Kekuda, S. Comparative Assessment of Salivary Flow Rate, Buffering Capacity, Resting PH and Dental Caries in Children with Autism. Middle East N. Afr. 2018, 4, 18–22. [Google Scholar]
- Mandal, P.K.; Akolkar, H.; Tripathi, M. Mapping of hippocampal pH and neurochemicals from in vivo multi-voxel 31P study in healthy normal young male/female, mild cognitive impairment, and Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 31, S75–S86. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tanaka, Y.; Kiyono, M.; Chino, M.; Chikuma, T.; Hoshi, K.; Ikeshima, H. Dependence pH and proposed mechanism for aggregation of Alzheimer’s disease-related amyloid-β (1–42) protein. J. Mol. Struct. 2015, 1094, 109–117. [Google Scholar] [CrossRef]
- Lyros, E.; Ragoschke-Schumm, A.; Kostopoulos, P.; Sehr, A.; Backens, M.; Kalampokini, S.; Decker, Y.; Lesmeister, M.; Liu, Y.; Reith, W. Normal brain aging and Alzheimer’s disease are associated with lower cerebral pH: An in vivo histidine 1H-MR spectroscopy study. Neurobiol. Aging 2020, 87, 60–69. [Google Scholar] [CrossRef]
- Decker, Y.; Németh, E.; Schomburg, R.; Chemla, A.; Fülöp, L.; Menger, M.D.; Liu, Y.; Fassbender, K. Decreased pH in the aging brain and Alzheimer’s disease. Neurobiol. Aging 2021, 101, 40–49. [Google Scholar] [CrossRef]
- Shukla, D.; Mandal, P.K.; Mishra, R.; Punjabi, K.; Dwivedi, D.; Tripathi, M.; Badhautia, V. Hippocampal glutathione depletion and ph increment in Alzheimer’s disease: An in vivo MRS study. J. Alzheimer’s Dis. 2021, 84, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K.; Dwivedi, D.; Shukla, D.; Samkaria, A.; Roy, R.G.; Arora, Y.; Jindal, K. Interplay Between Hippocampal Glutathione Depletion and pH Increment in Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 88, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Halim, N.D.; Lipska, B.K.; Hyde, T.M.; Deep-Soboslay, A.; Saylor, E.M.; Herman, M.M.; Thakar, J.; Verma, A.; Kleinman, J.E. Increased lactate levels and reduced pH in postmortem brains of schizophrenics: Medication confounds. J. Neurosci. Methods 2008, 169, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.; Thomas, N.; Scarr, E.; Udawela, M. Evidence for impaired glucose metabolism in the striatum, obtained postmortem, from some subjects with schizophrenia. Transl. Psychiatry 2016, 6, e949. [Google Scholar] [CrossRef]
- Sullivan, C.R.; Mielnik, C.A.; Funk, A.; O’Donovan, S.M.; Bentea, E.; Pletnikov, M.; Ramsey, A.J.; Wen, Z.; Rowland, L.M.; McCullumsmith, R.E. Measurement of lactate levels in postmortem brain, iPSCs, and animal models of schizophrenia. Sci. Rep. 2019, 9, 5087. [Google Scholar] [CrossRef]
- Valiente-Palleja, A.; Torrell, H.; Alonso, Y.; Vilella, E.; Muntané, G.; Martorell, L. Increased blood lactate levels during exercise and mitochondrial DNA alterations converge on mitochondrial dysfunction in schizophrenia. Schizophr. Res. 2020, 220, 61–68. [Google Scholar] [CrossRef]
- Sun, L.; Min, L.; Li, M.; Shao, F. Juvenile social isolation leads to schizophrenia-like behaviors via excess lactate production by astrocytes. Brain Res. Bull. 2021, 174, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Machado-Vieira, R.; Zanetti, M.V.; Otaduy, M.C.; De Sousa, R.T.; Soeiro-de-Souza, M.G.; Costa, A.C.; Carvalho, A.F.; Leite, C.C.; Busatto, G.F.; Zarate, C.A., Jr. Increased brain lactate during depressive episodes and reversal effects by lithium monotherapy in drug-naive bipolar disorder: A 3T 1H-MRS study. J. Clin. Psychopharmacol. 2017, 37, 40. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.A.; Mao, X.; Case, J.A.; Kang, G.; Shungu, D.C.; Gabbay, V. Increased ventricular cerebrospinal fluid lactate in depressed adolescents. Eur. Psychiatry 2016, 32, 1–8. [Google Scholar] [CrossRef]
- Ernst, J.; Hock, A.; Henning, A.; Seifritz, E.; Boeker, H.; Grimm, S. Increased pregenual anterior cingulate glucose and lactate concentrations in major depressive disorder. Mol. Psychiatry 2017, 22, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Kim, S.A.; Yoo, H.J. Higher lactate level and lactate-to-pyruvate ratio in autism spectrum disorder. Exp. Neurobiol. 2020, 29, 314. [Google Scholar] [CrossRef]
- Maier, S.; Nickel, K.; Lange, T.; Oeltzschner, G.; Dacko, M.; Endres, D.; Runge, K.; Schumann, A.; Domschke, K.; Rousos, M. Increased cerebral lactate levels in adults with autism spectrum disorders compared to non-autistic controls: A magnetic resonance spectroscopy study. Mol. Autism 2023, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Stefani, A.; Sancesario, G.; Sancesario, G.; Marciani, M.; Pierantozzi, M. CSF lactate levels, τ proteins, cognitive decline: A dynamic relationship in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 655–659. [Google Scholar] [CrossRef]
- Zebhauser, P.T.; Berthele, A.; Goldhardt, O.; Diehl-Schmid, J.; Priller, J.; Ortner, M.; Grimmer, T. Cerebrospinal fluid lactate levels along the Alzheimer’s disease continuum and associations with blood-brain barrier integrity, age, cognition, and biomarkers. Alzheimer’s Res. Ther. 2022, 14, 61. [Google Scholar] [CrossRef]
- Gupta, G. The lactate and the lactate dehydrogenase in inflammatory diseases and major risk factors in COVID-19 patients. Inflammation 2022, 45, 2091–2123. [Google Scholar] [CrossRef]
- Lezi, E.; Lu, J.; Selfridge, J.E.; Burns, J.M.; Swerdlow, R.H. Lactate administration reproduces specific brain and liver exercise-related changes. J. Neurochem. 2013, 127, 91. [Google Scholar] [CrossRef]
- Newman, L.A.; Korol, D.L.; Gold, P.E. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS ONE 2011, 6, e28427. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ruchti, E.; Petit, J.-M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhao, Y.; Du, J.; Wang, S.; Yang, X.; Li, W.; Song, J.; Zhang, S.; Zhang, Z.; Tan, Y. Exercise improves cognitive dysfunction and neuroinflammation in mice through Histone H3 lactylation in microglia. Immun. Ageing 2023, 20, 63. [Google Scholar] [CrossRef]
- Diskin, C.; Ryan, T.; O’neill, L. Modification of proteins by metabolites in immunity. Immunity 2021, 54, 19–31. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Noe, J.T.; Rendon, B.E.; Geller, A.E.; Conroy, L.R.; Morrissey, S.M.; Young, L.E.; Bruntz, R.C.; Kim, E.J.; Wise-Mitchell, A.; Barbosa de Souza Rizzo, M. Lactate supports a metabolic-epigenetic link in macrophage polarization. Sci. Adv. 2021, 7, eabi8602. [Google Scholar] [CrossRef]
- Miska, J.; Rashidi, A.; Lee-Chang, C.; Gao, P.; Lopez-Rosas, A.; Zhang, P.; Burga, R.; Castro, B.; Xiao, T.; Han, Y. Polyamines drive myeloid cell survival by buffering intracellular pH to promote immunosuppression in glioblastoma. Sci. Adv. 2021, 7, eabc8929. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, E.L.; Yang, C.; Ye, C.Y.; Zeng, P.; Wang, X.M.; Fang, Y.Y.; Cheng, Z.K.; Wang, Q.; Cao, F.Y. Protection of melatonin against acidosis-induced neuronal injuries. J. Cell. Mol. Med. 2020, 24, 6928–6942. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The acidic tumor microenvironment as a driver of cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Choi, I.; Leem, K.-H. Decreased brain PH and pathophysiology in schizophrenia. Int. J. Mol. Sci. 2021, 22, 8358. [Google Scholar] [CrossRef]
- Torrini, C.; Nguyen, T.T.T.; Shu, C.; Mela, A.; Humala, N.; Mahajan, A.; Seeley, E.H.; Zhang, G.; Westhoff, M.-A.; Karpel-Massler, G. Lactate is an epigenetic metabolite that drives survival in model systems of glioblastoma. Mol. Cell 2022, 82, 3061–3076.e6. [Google Scholar] [CrossRef]
- Yu, J.; Chai, P.; Xie, M.; Ge, S.; Ruan, J.; Fan, X.; Jia, R. Histone lactylation drives oncogenesis by facilitating m6A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 2021, 22, 85. [Google Scholar] [CrossRef]
- Li, L.; Chen, K.; Wang, T.; Wu, Y.; Xing, G.; Chen, M.; Hao, Z.; Zhang, C.; Zhang, J.; Ma, B. Glis1 facilitates induction of pluripotency via an epigenome–metabolome–epigenome signalling cascade. Nat. Metab. 2020, 2, 882–892. [Google Scholar] [CrossRef]
- Chen, L.; Huang, L.; Gu, Y.; Cang, W.; Sun, P.; Xiang, Y. Lactate-lactylation hands between metabolic reprogramming and immunosuppression. Int. J. Mol. Sci. 2022, 23, 11943. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.-K.; Liu, P.-P.; Li, X.; Jiao, L.-F.; Teng, Z.-Q.; Liu, C.-M. Dynamic profiling and functional interpretation of histone lysine crotonylation and lactylation during neural development. Development 2022, 149, dev200049. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wang, D.; Qu, Y.; Li, J.; An, K.; Mao, Z.; Li, J.; Xiong, Y.; Min, Z.; Xue, Z. Enhanced glycolysis-derived lactate promotes microglial activation in Parkinson’s disease via histone lactylation. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Xie, J.; Hong, S.; Zhang, X.; Li, Y.; Xie, R. Inhibition of glycolysis prevents behavioural changes in mice with MK801-induced SCZ model by alleviating lactate accumulation and lactylation. Brain Res. 2023, 1812, 148409. [Google Scholar] [CrossRef]
- Stathopoulos, S.; Gaujoux, R.; Lindeque, Z.; Mahony, C.; Van Der Colff, R.; Van Der Westhuizen, F.; O’Ryan, C. DNA methylation associated with mitochondrial dysfunction in a South African autism spectrum disorder cohort. Autism Res. 2020, 13, 1079–1093. [Google Scholar] [CrossRef]
- Sun, J.; Osenberg, S.; Irwin, A.; Ma, L.-H.; Lee, N.; Xiang, Y.; Li, F.; Wan, Y.-W.; Park, I.-H.; Maletic-Savatic, M. Mutations in the transcriptional regulator MeCP2 severely impact key cellular and molecular signatures of human astrocytes during maturation. Cell Rep. 2023, 42, 111942. [Google Scholar] [CrossRef]
- Vuu, Y.M.; Roberts, C.-T.; Rastegar, M. MeCP2 Is an Epigenetic Factor That Links DNA Methylation with Brain Metabolism. Int. J. Mol. Sci. 2023, 24, 4218. [Google Scholar] [CrossRef]
- Turovsky, E.; Karagiannis, A.; Abdala, A.P.; Gourine, A.V. Impaired CO2 sensitivity of astrocytes in a mouse model of Rett syndrome. J. Physiol. 2015, 593, 3159–3168. [Google Scholar] [CrossRef]
- Balicza, P.; Gezsi, A.; Fedor, M.; Sagi, J.C.; Gal, A.; Varga, N.A.; Molnar, M.J. Multilevel evidence of MECP2-associated mitochondrial dysfunction and its therapeutic implications. Front. Psychiatry 2023, 14, 1301272. [Google Scholar] [CrossRef]
- Darwish, M.M. Investigating the Role of Lactate in Regulating Gene Expression through Epigenetic Modifications in Neuronal Cells. Ph.D. Thesis, KAUST University, Thuwal, Saudi Arabia, 2019. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, H. Microglial lactate metabolism as a potential therapeutic target for Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 36. [Google Scholar] [CrossRef]
- Lev-Vachnish, Y.; Cadury, S.; Rotter-Maskowitz, A.; Feldman, N.; Roichman, A.; Illouz, T.; Varvak, A.; Nicola, R.; Madar, R.; Okun, E. L-lactate promotes adult hippocampal neurogenesis. Front. Neurosci. 2019, 13, 403. [Google Scholar] [CrossRef]
- Wang, J.; Lu, T.; Gui, Y.; Zhang, X.; Cao, X.; Li, Y.; Li, C.; Liu, L.; Ding, Z. HSPA12A controls cerebral lactate homeostasis to maintain hippocampal neurogenesis and mood stabilization. Transl. Psychiatry 2023, 13, 280. [Google Scholar] [CrossRef]
- Karnib, N.; El-Ghandour, R.; El Hayek, L.; Nasrallah, P.; Khalifeh, M.; Barmo, N.; Jabre, V.; Ibrahim, P.; Bilen, M.; Stephan, J.S. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology 2019, 44, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, U.; Bingmann, D.; Wiltfang, J.; Scherbaum, N.; Wiemann, M. Modulatory effects of neuropsychopharmaca on intracellular pH of hippocampal neurones in vitro. Br. J. Pharmacol. 2010, 159, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.E.; Daniels, M.; Haws, C.; Bolo, N.R.; Lyoo, I.K.; Yoon, S.J.; Cohen, B.M.; Stoll, A.L.; Rusche, J.R.; Renshaw, P.F. Triacetyluridine (TAU) decreases depressive symptoms and increases brain pH in bipolar patients. Exp. Clin. Psychopharmacol. 2008, 16, 199. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.-L.; Li, H.-D.; Yan, X.-Z.; Sun, B.; Zhang, Q.; Yan, M.; Zhang, W.-Y.; Jiang, P.; Zhu, R.-H.; Liu, Y.-P. Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naive schizophrenia patients after treatment with risperidone. J. Proteome Res. 2012, 11, 4338–4350. [Google Scholar] [CrossRef]
- Elmorsy, E.; Shahda, M.; Mahmoud, E.-H.M.; Rakha, S.A.; Shoaib, M. Blood lactate levels as a biomarker of antipsychotic side effects in patients with schizophrenia. J. Psychopharmacol. 2016, 30, 63–68. [Google Scholar] [CrossRef]
- Kim, D.J.; Lyoo, I.K.; Yoon, S.J.; Choi, T.; Lee, B.; Kim, J.E.; Lee, J.S.; Renshaw, P.F. Clinical response of quetiapine in rapid cycling manic bipolar patients and lactate level changes in proton magnetic resonance spectroscopy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Song, D.; Bai, Q.; Verkhratsky, A.; Peng, L. Fluoxetine induces alkalinization of astroglial cytosol through stimulation of sodium-hydrogen exchanger 1: Dissection of intracellular signaling pathways. Front. Cell. Neurosci. 2015, 9, 61. [Google Scholar] [CrossRef]
- Deitmer, J.W.; Rose, C.R. Ion changes and signalling in perisynaptic glia. Brain Res. Rev. 2010, 63, 113–129. [Google Scholar] [CrossRef]
- Deitmer, J.; Chesler, M. Neuron-glia pH regulation. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 739–747. [Google Scholar] [CrossRef]
- Pruett, B.; Pinner, A.; Meador-Woodruff, J. Altered Interactions and Distribution of Organellar Na+/H+ Exchangers in Schizophrenia Postmortem Dorsolateral Prefrontal Cortex. Biol. Psychiatry 2021, 89, S304–S305. [Google Scholar] [CrossRef]
- Dager, S.R.; Friedman, S.D.; Parow, A.; Demopulos, C.; Stoll, A.L.; Lyoo, I.K.; Dunner, D.L.; Renshaw, P.F. Brain metabolic alterations in medication-free patients with bipolardisorder. Arch. Gen. Psychiatry 2004, 61, 450–458. [Google Scholar] [CrossRef]
- Kato, T.; Takahashi, S.; Shioiri, T.; Inubushi, T. Alterations in brain phosphorous metabolism in bipolar disorder detected by in vivo 31P and 7Li magnetic resonance spectroscopy. J. Affect. Disord. 1993, 27, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Murashita, J.; Kamiya, A.; Shioiri, T.; Kato, N.; Inubushi, T. Decreased brain intracellular pH measured by 31 P-MRS in bipolar disorder: A confirmation in drug-free patients and correlation with white matter hyperintensity. Eur. Arch. Psychiatry Clin. Neurosci. 1998, 248, 301–306. [Google Scholar] [CrossRef]
- Scola, G.; Kim, H.K.; Young, L.T.; Salvador, M.; Andreazza, A.C. Lithium reduces the effects of rotenone-induced complex I dysfunction on DNA methylation and hydroxymethylation in rat cortical primary neurons. Psychopharmacology 2014, 231, 4189–4198. [Google Scholar] [CrossRef]
- Andrabi, M.; Andrabi, M.M.; Kunjunni, R.; Sriwastva, M.K.; Bose, S.; Sagar, R.; Srivastava, A.K.; Mathur, R.; Jain, S.; Subbiah, V. Lithium acts to modulate abnormalities at behavioral, cellular, and molecular levels in sleep deprivation-induced mania-like behavior. Bipolar Disord. 2020, 22, 266–280. [Google Scholar] [CrossRef]
- Song, D.; Du, T.; Li, B.; Cai, L.; Gu, L.; Li, H.; Chen, Y.; Hertz, L.; Peng, L. Astrocytic alkalinization by therapeutically relevant lithium concentrations: Implications for myo-inositol depletion. Psychopharmacology 2008, 200, 187–195. [Google Scholar] [CrossRef]
- Song, D.; Li, B.; Yan, E.; Man, Y.; Wolfson, M.; Chen, Y.; Peng, L. Chronic treatment with anti-bipolar drugs causes intracellular alkalinization in astrocytes, altering their functions. Neurochem. Res. 2012, 37, 2524–2540. [Google Scholar] [CrossRef]
- Wei, L.; Yang, X.; Wang, J.; Wang, Z.; Wang, Q.; Ding, Y.; Yu, A. H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer’s disease through the NFκB signaling pathway. J. Neuroinflamm. 2023, 20, 208. [Google Scholar] [CrossRef]
- Bonnet, U.; Wiemann, M. Topiramate decelerates bicarbonate-driven acid-elimination of human neocortical neurons: Strategic significance for its antiepileptic, antimigraine and neuroprotective properties. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2020, 19, 264–275. [Google Scholar] [CrossRef]
- Eyal, S.; Yagen, B.; Sobol, E.; Altschuler, Y.; Shmuel, M.; Bialer, M. The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia 2004, 45, 737–744. [Google Scholar] [CrossRef]
- Vila-Pueyo, M.; Cuenca-León, E.; Queirós, A.C.; Kulis, M.; Sintas, C.; Cormand, B.; Martín-Subero, J.I.; Pozo-Rosich, P.; Fernàndez-Castillo, N.; Macaya, A. Genome-wide DNA methylation analysis in an antimigraine-treated preclinical model of cortical spreading depolarization. Cephalalgia 2023, 43, 03331024221146317. [Google Scholar] [CrossRef]
- Johanson, C.E.; Parandoosh, Z.; Dyas, M.L. Maturational differences in acetazolamide-altered pH and HCO3 of choroid plexus, cerebrospinal fluid, and brain. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1992, 262, R909–R914. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-C.; Chen, H.-T.; Chang, H.-Y.; Yang, C.-Y.; Hsiao, M.-C.; Cheng, M.-L.; Chen, J.-C. Mineralocorticoid receptor antagonist spironolactone prevents chronic corticosterone induced depression-like behavior. Psychoneuroendocrinology 2013, 38, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Wehr, M.C.; Hinrichs, W.; Brzózka, M.M.; Unterbarnscheidt, T.; Herholt, A.; Wintgens, J.P.; Papiol, S.; Soto-Bernardini, M.C.; Kravchenko, M.; Zhang, M. Spironolactone is an antagonist of NRG 1-ERBB 4 signaling and schizophrenia-relevant endophenotypes in mice. EMBO Mol. Med. 2017, 9, 1448–1462. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.; Sharma, P.; Kulkarni, G.T.; Sharma, B. Spironolactone Alters the Levels of Neuronal Function Markers in Autistic Rats. Bull. Pharm. Sci. Assiut Univ. 2023, 46, 1301–1312. [Google Scholar] [CrossRef]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production–producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Duncan, S.H.; Sheridan, P.O.; Walker, A.W.; Flint, H.J. Microbial lactate utilisation and the stability of the gut microbiome. Gut Microbiome 2022, 3, e3. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Yuan, X.; Pang, L.; Hu, S.; Wang, Y.; Huang, X.; Song, X. The role of butyric acid in treatment response in drug-naive first episode schizophrenia. Front. Psychiatry 2021, 12, 724664. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Sung, Y.-B.; Chung, S.-Y.; Kwon, M.-S. Possible additional antidepressant-like mechanism of sodium butyrate: Targeting the hippocampus. Neuropharmacology 2014, 81, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Nohesara, S.; Abdolmaleky, H.M.; Thiagalingam, S. Epigenetic aberrations in major psychiatric diseases related to diet and gut microbiome alterations. Genes 2023, 14, 1506. [Google Scholar] [CrossRef] [PubMed]
- Nohesara, S.; Abdolmaleky, H.M.; Zhou, J.-R.; Thiagalingam, S. Microbiota-Induced Epigenetic Alterations in Depressive Disorders Are Targets for Nutritional and Probiotic Therapies. Genes 2023, 14, 2217. [Google Scholar] [CrossRef]
- Wang, S.P.; Rubio, L.A.; Duncan, S.H.; Donachie, G.E.; Holtrop, G.; Lo, G.; Farquharson, F.M.; Wagner, J.; Parkhill, J.; Louis, P. Pivotal roles for pH, lactate, and lactate-utilizing bacteria in the stability of a human colonic microbial ecosystem. Msystems 2020, 5, 1110–1128. [Google Scholar] [CrossRef]
- Chen, L.; Li, R.; Wang, Z.; Zhang, Z.; Wang, J.; Qiao, Y.; Huang, Y.; Liu, W. Lactate-utilizing bacteria ameliorates DSS-induced colitis in mice. Life Sci. 2022, 288, 120179. [Google Scholar] [CrossRef]
- Qiu, X.; Wu, G.; Wang, L.; Tan, Y.; Song, Z. Lactobacillus delbrueckii alleviates depression-like behavior through inhibiting toll-like receptor 4 (TLR4) signaling in mice. Ann. Transl. Med. 2021, 9, 366. [Google Scholar] [CrossRef]
- Lee, B.-H.; Hsu, W.-H.; Hou, C.-Y.; Chien, H.-Y.; Wu, S.-C. The protection of lactic acid bacteria fermented-mango peel against neuronal damage induced by amyloid-beta. Molecules 2021, 26, 3503. [Google Scholar] [CrossRef]
- Cecarini, V.; Bonfili, L.; Gogoi, O.; Lawrence, S.; Venanzi, F.M.; Azevedo, V.; Mancha-Agresti, P.; Drumond, M.M.; Rossi, G.; Berardi, S. Neuroprotective effects of p62 (SQSTM1)-engineered lactic acid bacteria in Alzheimer’s disease: A pre-clinical study. Aging 2020, 12, 15995. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, A.; Davis, J.A.; Dawson, S.; Loughman, A.; Collier, F.; O’hely, M.; Simpson, C.; Green, J.; Marx, W.; Hair, C. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 2022, 27, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Naylor, J.M.; Zello, G.A. D-lactate in human and ruminant metabolism. J. Nutr. 2005, 135, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Karcz, P.; Bystrowska, B.; Szwajca, M.; Bryll, A.; Śmierciak, N.; Ligęzka, A.; Turek, A.; Kozicz, T.; Skalniak, A.E. The Association of the Oral Microbiota with the Effects of Acid Stress Induced by an Increase of Brain Lactate in Schizophrenia Patients. Biomedicines 2023, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, P.; Żebrowska-Różańska, P.; Kujawa, D.; Łaczmański, Ł.; Samochowiec, J.; Jabłoński, M.; Plichta, P.; Piotrowski, P.; Bielawski, T.; Misiak, B. Gut microbiota alterations in schizophrenia might be related to stress exposure: Findings from the machine learning analysis. Psychoneuroendocrinology 2023, 155, 106335. [Google Scholar] [CrossRef] [PubMed]
- Castro-Nallar, E.; Bendall, M.L.; Pérez-Losada, M.; Sabuncyan, S.; Severance, E.G.; Dickerson, F.B.; Schroeder, J.R.; Yolken, R.H.; Crandall, K.A. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ 2015, 3, e1140. [Google Scholar] [CrossRef]
- Cuervo-Zanatta, D.; Piña-Escobedo, A.; García-Mena, J.; Perez-Cruz, C. Concentration of short chain fatty acids produced by gut microbiota are related with cognitive dysfunction in a murine model of Alzheimer’ s disease. In Proceedings of the 1st International Electronic Conference on Microbiology, Online, 2–30 November 2020. [Google Scholar] [CrossRef]
- Proia, P.; Di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef]
- Chong, Z.-S.; Khong, Z.J.; Tay, S.H.; Ng, S.-Y. Metabolic contributions to neuronal deficits caused by genomic disruption of schizophrenia risk gene SETD1A. Schizophrenia 2022, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Mecheri, G.; Marie-Cardine, M.; Sappey-Marinier, D.; Bonmartin, H.; Albrand, G.; Ferry, G.; Coppard-Meyer, N.; Courpron, P. In vivo hippocampal 31P NMR metabolites in Alzheimer’s disease and ageing. Eur. Psychiatry 1997, 12, 140–148. [Google Scholar] [CrossRef]

| Diseases | Type of Study | pH Changes | Key Finding | Ref |
|---|---|---|---|---|
| Schizophrenia (SCZ)/bipolar disorder | Post-mortem brains | Lower pH levels (p < 0.05) | Reduced brain pH in patients with high inflammatory/stress vs. controls (attributed to tissue injury or response to an elevated metabolic demand) | [45] |
| Bipolar disorder | Clinical study | Lower intracellular pH (p < 0.05) | Association between altered cellular metabolism and reduced intracellular pH in the brains of patients | [46] |
| Bipolar disorder | Clinical study | Lower pH levels (p < 0.05) | Increasing 4-hydroxynonenal (4-HNE) due to lipid peroxidation in the anterior cingulate cortex, reducing pH in patients vs. controls | [47] |
| Bipolar disorder | Clinical study | Lower pH levels (p < 0.05) | Reduced pH in the anterior cingulate of unmedicated manic adolescents vs. healthy subjects | [48] |
| Bipolar disorder | Clinical study | Lower pH levels (p < 0.05) | Decreased pH levels, and mitochondrial dysfunction in patients | [49] |
| Depression and major depressive disorder (MDD) | Clinical study in MDD and experimental study in mice | Decreased carbonic anhydrase level (p < 0.05) | Decreased carbonic anhydrase 1 (CAR1) level in MDD; depression-like behaviors in CAR1-knockout mice due to lower extracellular bicarbonate (i.e., lower pH) | [50] |
| Manic-depressive or recurrent depression | Clinical study of saliva | Higher pH levels (p < 0·001) | Reduced membrane transport, stronger sodium activity, and higher pH levels vs. controls due to imbalances in sodium and bicarbonate reabsorption | [51] |
| Autism spectrum disorder (ASD) | Clinical study (saliva) | Lower resting pH levels (p < 0.05) | Lower resting pH of saliva in autistic children vs. healthy children | [52] |
| Alzheimer’s Disease (AD) | Clinical study (magnetic resonance spectroscopy) | Higher pH levels (p < 0.05) | Elevated pH to alkaline range in the left hippocampus of AD patients | [53] |
| AD | Experimental study (Human Aβ1–42) | Impact of pH on Aβ1–42 aggregation | Lack of Aβ1–42 aggregation at pH 9.5 | [54] |
| AD | Clinical study | Lower pH levels (p < 0.05) | Lower pH in the hippocampus during normal aging and lower pH in periventricular white matter in AD | [55] |
| AD | Experimental study (mice) | Lower pH levels (p < 0.004) | Reducing brain and CSF pH in AD mice and increasing Aβ plaques load in APP-PS1 mice after CSF infusion with low pH | [56] |
| AD | Clinical study | Higher pH levels (p < 0.05) | Glutathione depletion in the left and right hippocampus and higher pH levels in the left hippocampus in AD vs. controls | [57] |
| AD | Research hypothesis | Higher pH levels | Association between the hippocampal GSH depletion and increased hippocampal pH levels in AD | [58] |
| Disease | Type of Study | Key Finding | Ref |
|---|---|---|---|
| Schizophrenia (SCZ) | Clinical study | Association between elevated lactate level in post-mortem brains of SCZ patients and reduced brain pH | [59] |
| SCZ | Clinical study | Higher levels of lactate and pyruvate, and lower levels of β subunit of pyruvate dehydrogenase, in the striatum of SCZ patients vs. controls | [60] |
| SCZ | Clinical study | Higher lactate level in patients vs. controls; cognitive deficits in patients because of elevated anaerobic glycolysis (likely due to mitochondrial dysfunction) | [35] |
| SCZ/bipolar disorder | Experimental study in mice; clinical study in the patients | Higher lactate level and hence lower pH in the brains of model mice vs. controls; lower brain pH in SCZ and bipolar disorder vs. control | [36] |
| SCZ | Clinical study in SCZ and experimental study in mice | Elevated lactate level in the dorsolateral prefrontal cortex in SCZ, and in iPSC-derived frontal cortical neurons of a SCZ patient with DISC1 mutation; reduced lactate level in astrocytes of mice with “induced expression of mutant human DISC1” | [61] |
| SCZ | Clinical study | Elevated blood level of lactate during exercise, and lower mitochondrial DNA copy numbers vs. control, indicating mitochondrial dysfunction in SCZ | [62] |
| SCZ | Experimental study in male adult rats | Association between high-level lactate production by astrocytes and deficits in auditory sensory gating in isolated rats | [63] |
| Bipolar disorder, depressed | Clinical study | Higher lactate level in the cingulate cortex of patients, which was decreased after 6-weeks lithium monotherapy | [64] |
| MDD | Clinical study | Elevated ventricular lactate in adolescents with MDD vs. controls | [65] |
| MDD | Clinical study | Decreased mitochondrial oxidative clearance of lactate, elevated glucose and lactate levels in patients, linked to increased depression severity | [66] |
| ASD | Clinical study | Higher lactate level and lactate-to-pyruvate ratio in ASD | [67] |
| ASD | Clinical study | Increased cerebral lactate level as a sign of mitochondrial dysfunction in adults with ASD | [68] |
| AD | Clinical study | Elevated level of the CSF lactate vs. controls | [69] |
| AD | Clinical study | Higher CSF lactate levels in earlier stages of AD | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nohesara, S.; Abdolmaleky, H.M.; Thiagalingam, S. Potential for New Therapeutic Approaches by Targeting Lactate and pH Mediated Epigenetic Dysregulation in Major Mental Diseases. Biomedicines 2024, 12, 457. https://doi.org/10.3390/biomedicines12020457
Nohesara S, Abdolmaleky HM, Thiagalingam S. Potential for New Therapeutic Approaches by Targeting Lactate and pH Mediated Epigenetic Dysregulation in Major Mental Diseases. Biomedicines. 2024; 12(2):457. https://doi.org/10.3390/biomedicines12020457
Chicago/Turabian StyleNohesara, Shabnam, Hamid Mostafavi Abdolmaleky, and Sam Thiagalingam. 2024. "Potential for New Therapeutic Approaches by Targeting Lactate and pH Mediated Epigenetic Dysregulation in Major Mental Diseases" Biomedicines 12, no. 2: 457. https://doi.org/10.3390/biomedicines12020457
APA StyleNohesara, S., Abdolmaleky, H. M., & Thiagalingam, S. (2024). Potential for New Therapeutic Approaches by Targeting Lactate and pH Mediated Epigenetic Dysregulation in Major Mental Diseases. Biomedicines, 12(2), 457. https://doi.org/10.3390/biomedicines12020457






