Peripheral Blood B-Cell Subsets Frequency and Distribution and the BSF-2(IL-6) to CSIF:TGIF(IL-10) Ratio as Severity-Associated Signatures in Primary Open-Angle Glaucoma: A Case-Controlled Study
Abstract
:1. Introduction
Research Hypothesis
2. Subjects and Methods
2.1. BSF-2(IL-6) and CSIF:TGIF(IL-10) Assays by ELISA
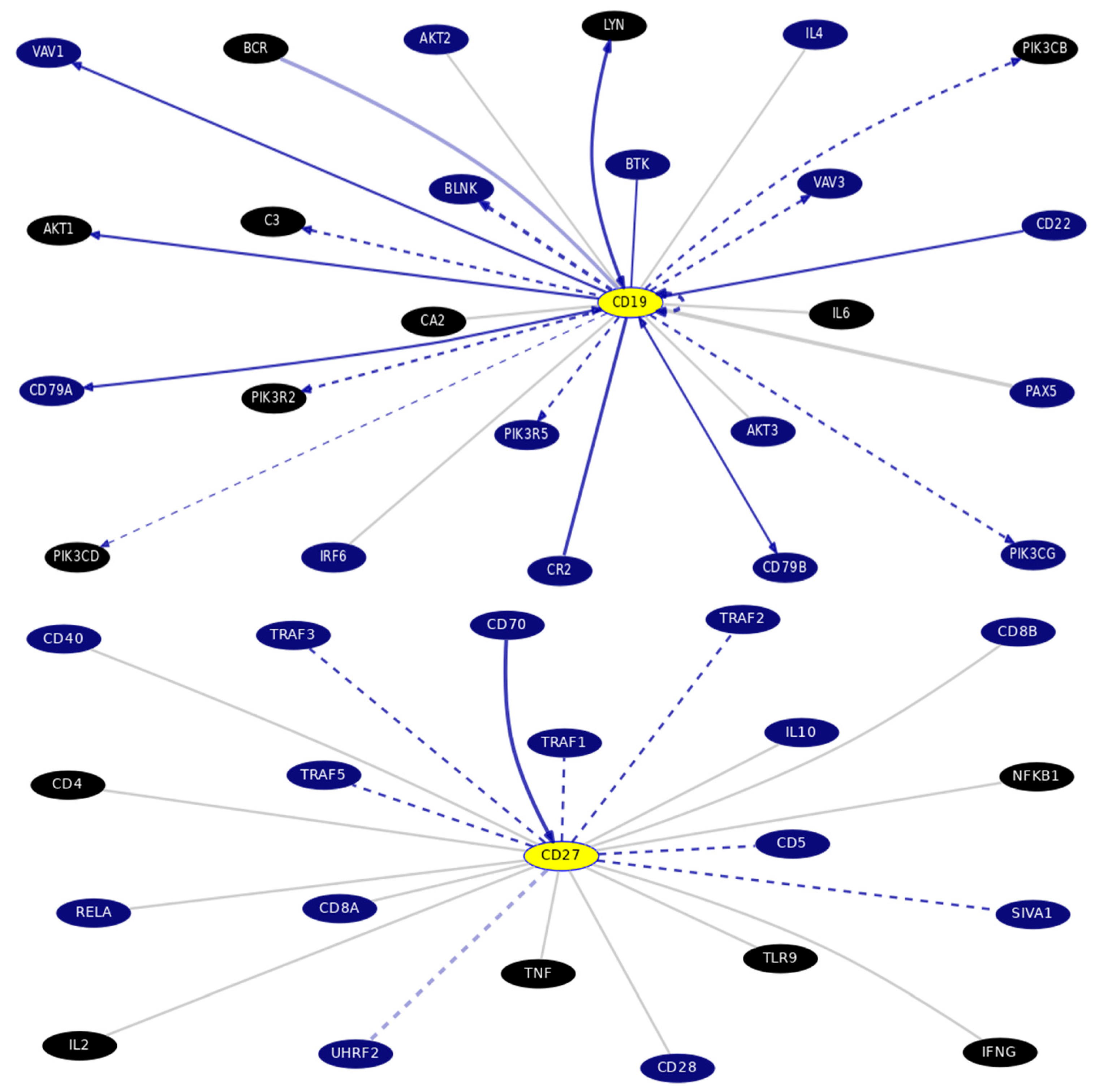
2.2. In Silico Database(s) Search and Bioinformatics Analysis
In Silico Identification of Immune Cells
2.3. PICKLE (Protein InteraCtion KnowLedgebasE)
2.4. Gene–Gene Interactions and Pathways by Bioinformatics Analysis
2.5. Statistical Analysis
3. Results
3.1. POAG Patients and Controls Demographic Characteristics, Clinical and Laboratory Data Results
3.2. In Silico Databases Analysis
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarossy, M.; Crowston, J.; Kumar, D.; Weymouth, A.; Wu, Z. Prediction of glaucoma severity using parameters from the electroretinogram. Sci. Rep. 2021, 11, 23886. [Google Scholar] [CrossRef]
- Nitta, K.; Tachibana, G.; Wajima, R.; Inoue, S.; Ohigashi, T.; Otsuka, N.; Kurashima, H.; Santo, K.; Hashimoto, M.; Shibahara, H.; et al. Predicting Lifetime Transition Risk of Severe Visual Field Defects Using Monte Carlo Simulation in Japanese Patients with Primary Open-Angle Glaucoma. Clin. Ophthalmol. 2020, 14, 1967–1978. [Google Scholar] [CrossRef]
- Shin, Y.J.; Kim, E.; Han, B.K.; Yi, K. Serum biomarkers for the diagnosis of glaucoma. Diagnostics 2020, 11, 20. [Google Scholar] [CrossRef]
- Zimprich, L.; Diedrich, J.; Bleeker, A.; Schweitzer, J.A. Corneal hysteresis as a biomarker of glaucoma: Current insights. Clin. Ophthalmol. 2020, 14, 2255–2264. [Google Scholar] [CrossRef]
- Tapply, I.H.; Bourne, R.R. Epidemiology of glaucoma. In The Science of Glaucoma Management; Academic Press: Cambridge, MA, USA, 2023; pp. 17–34. ISBN 9780323884426. [Google Scholar] [CrossRef]
- Dammak, A.; Sanchez Naves, J.; Huete-Toral, F.; Carracedo, G. New Biomarker Combination Related to Oxidative Stress and Inflammation in Primary Open-Angle Glaucoma. Life 2023, 13, 1455. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, Y.; Xu, X.; Dong, Q.; Xiu, W.; Chen, Q.; Wang, J.; He, C.; Ye, J.; Lu, F. Alterations in peripheral B cell subsets correlate with the disease severity of human glaucoma. J. Inflamm. Res. 2021, 14, 4827. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Hu, F. Double-negative (DN) B cells: An under-recognized effector memory B cell subset in autoimmunity. Clin. Exp. Immunol. 2021, 205, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.C.; Datta, M.; Nicolò, A.; Jumaa, H. Isotype specific assembly of B cell antigen receptors and synergism with chemokine receptor CXCR4. Front. Immunol. 2018, 9, 2988. [Google Scholar] [CrossRef]
- Inoue-Mochita, M.; Inoue, T.; Kojima, S.; Futakuchi, A.; Fujimoto, T.; Sato-Ohira, S.; Tsutsumi, U.; Tanihara, H. Interleukin-6–mediated trans-signaling inhibits transforming growth factor-β signaling in trabecular meshwork cells. J. Biol. Chem. 2018, 293, 10975–10984. [Google Scholar] [CrossRef] [PubMed]
- Ulhaq, Z.S.; Soraya, G.V.; Hasan, Y.T.; Rachma, L.N.; Rachmawati, E.; Shodry, S.; Kusuma, M.A. Serum IL-6/IL-10 ratio as a biomarker for the diagnosis and severity assessment of primary-open angle glaucoma. Eur. J. Ophthalmol. 2022, 32, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, J.; Li, Y.; Jiang, B. Prevalence of primary open angle glaucoma in the last 20 years: A meta-analysis and systematic review. Sci. Rep. 2021, 11, 13762. [Google Scholar] [CrossRef]
- Spaeth, G.L. European Glaucoma Society Terminology and Guidelines for Glaucoma. Br. J. Ophthalmol. 2021, 105 (Suppl. 1), 1–169. [Google Scholar] [CrossRef]
- Shi, X.; Yu, Z.; Ren, P.; Dong, X.; Ding, X.; Song, J.; Zhang, J.; Li, T.; Wang, C. HUSCH: An integrated single-cell transcriptome atlas for human tissue gene expression visualization and analyses. Nucleic Acids Res. 2023, 51, D1029–D1037. [Google Scholar] [CrossRef]
- Dimitrakopoulos, G.N.; Klapa, M.I.; Moschonas, N.K. How Far Are We from the Completion of the Human Protein Interactome Reconstruction? Biomolecules 2022, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.D.; Hinrichs, A.S.; Clawson, H.; Gonzalez, J.N.; Lee, B.T.; Nassar, L.R.; Raney, B.J.; Rosenbloom, K.R.; Nerli, S.; Rao, A.A.; et al. The UCSC SARS-CoV-2 genome browser. Nat. Genet. 2020, 52, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, W.; Lenhard, A. Computation of Effect Sizes. 2022. Available online: https://www.psychometrica.de/effect_size.html (accessed on 4 February 2024). [CrossRef]
- Williams, P.A.; Braine, C.E.; Kizhatil, K.; Foxworth, N.E.; Tolman, N.G.; Harder, J.M.; Scott, R.A.; Sousa, G.L.; Panitch, A.; Howell, G.R.; et al. Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma. Mol. Neurodegener. 2019, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Cho, K.S.; Thee, E.F.; Jager, M.J.; Chen, D.F. Neuroinflammation and microglia in glaucoma: Time for a paradigm shift. J. Neurosci. Res. 2019, 97, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Mendoza, A.; Vannan, D.; Morales, E.G.; González, M.I.; Hernández, J.L. Lymphocytes in Dry Eye Disease. In Dry Eye Syndrome-Modern Diagnostic Techniques and Advanced Treatments; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, Y.L.; Zheng, Y.; Biswal, M.; Zhao, P.Q.; Wang, Z.Y. Analyzing cytokines as biomarkers to evaluate severity of glaucoma. Int. J. Ophthalmol. 2017, 10, 925. [Google Scholar] [PubMed]
- Shestopalov, V.I.; Spurlock, M.; Gramlich, O.W.; Kuehn, M.H. Immune responses in the glaucomatous retina: Regulation and dynamics. Cells 2021, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cho, K.S.; Vu, T.K.; Shen, C.H.; Kaur, M.; Chen, G.; Mathew, R.; McHam, M.L.; Fazelat, A.; Lashkari, K.; et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat. Commun. 2018, 9, 3209. [Google Scholar] [CrossRef]
- Claes, N.; Fraussen, J.; Vanheusden, M.; Hellings, N.; Stinissen, P.; Van Wijmeersch, B.; Hupperts, R.; Somers, V. Age-associated B cells with proinflammatory characteristics are expanded in a proportion of multiple sclerosis patients. J. Immunol. 2016, 197, 4576–4583. [Google Scholar] [CrossRef] [PubMed]
- Sachinidis, A.; Garyfallos, A. Double Negative (DN) B cells: A connecting bridge between rheumatic diseases and COVID-19? Mediterr. J. Rheumatol. 2021, 32, 192. [Google Scholar] [CrossRef] [PubMed]
- Ruschil, C.; Gabernet, G.; Lepennetier, G.; Heumos, S.; Kaminski, M.; Hracsko, Z.; Irmler, M.; Beckers, J.; Ziemann, U.; Nahnsen, S.; et al. Specific induction of double negative B cells during protective and pathogenic immune responses. Front. Immunol. 2020, 11, 606338. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Anolik, J.; Cappione, A.; Zheng, B.; Pugh-Bernard, A.; Brooks, J.; Lee, E.H.; Milner, E.C.; Sanz, I. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J. Immunol. 2007, 178, 6624–6633. [Google Scholar] [CrossRef] [PubMed]
- Jenks, S.A.; Cashman, K.S.; Zumaquero, E.; Marigorta, U.M.; Patel, A.V.; Wang, X.; Tomar, D.; Woodruff, M.C.; Simon, Z.; Bugrovsky, R.; et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 2018, 49, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Moysidou, E.; Lioulios, G.; Christodoulou, M.; Xochelli, A.; Stai, S.; Iosifidou, M.; Iosifidou, A.; Briza, S.; Briza, D.I.; Fylaktou, A.; et al. Increase in Double Negative B Lymphocytes in Patients with Systemic Lupus Erythematosus in Remission and Their Correlation with Early Differentiated T Lymphocyte Subpopulations. Curr. Issues Mol. Biol. 2023, 45, 6667–6681. [Google Scholar] [CrossRef]
- Wu, Y.C.; Kipling, D.; Dunn-Walters, D.K. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front. Immunol. 2011, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- De Gruijter, N.M.; Jebson, B.; Rosser, E.C. Cytokine production by human B cells: Role in health and autoimmune disease. Clin. Exp. Immunol. 2022, 210, 253–262. [Google Scholar] [CrossRef]
- Rutigliani, C.; Tribble, J.R.; Hagström, A.; Lardner, E.; Jóhannesson, G.; Stålhammar, G.; Williams, P.A. Widespread retina and optic nerve neuroinflammation in enucleated eyes from glaucoma patients. Acta Neuropathol. Commun. 2022, 10, 118. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, Q.; Göktaş, E.; Gopal, K.; Al-Aswad, L.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M.; Tezel, G. T-lymphocyte subset distribution and activity in patients with glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 877–888. [Google Scholar] [CrossRef]
- Irkec, M.T.; Bozkurt, B.; Mesci, L.; Bulur, B.; Ersoy, F.; Sanal, O.; Orhan, M.; Arslan, U.; Tezcan, I. TNF–alpha and IL–10 gene polymorphisms in Turkish glaucoma patients. Investig. Ophthalmol. Vis. Sci. 2005, 46, 27. [Google Scholar]
- Chua, J.; Vania, M.; Cheung, C.M.; Ang, M.; Chee, S.P.; Yang, H.; Li, J.; Wong, T.T. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Mol. Vis. 2012, 18, 431. [Google Scholar]
- Gramlich, O.W.; Beck, S.; von Thun und Hohenstein-Blaul, N.; Boehm, N.; Ziegler, A.; Vetter, J.M.; Pfeiffer, N.; Grus, F.H. Enhanced insight into the autoimmune component of glaucoma: IgG autoantibody accumulation and pro-inflammatory conditions in human glaucomatous retina. PLoS ONE 2013, 8, e57557. [Google Scholar] [CrossRef]
- Duddy, M.E.; Alter, A.; Bar-Or, A. Distinct profiles of human B cell effector cytokines: A role in immune regulation? J. Immunol. 2004, 172, 3422–3427. [Google Scholar] [CrossRef]
- Borkenstein, A.; Faschinger, C.; Maier, R.; Weger, M.; Theisl, A.; Demel, U.; Graninger, W.; Irene, H.; Mossböck, G. Measurement of tumor necrosis factor-alpha, interleukin-6, Fas ligand, interleukin-1α, and interleukin-1β in the aqueous humor of patients with open angle glaucoma using multiplex bead analysis. Mol. Vis. 2013, 19, 2306. [Google Scholar]
- Takai, Y.; Tanito, M.; Ohira, A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Investig. Ophthalmol. Vis. Sci. 2012, 53, 241–247. [Google Scholar] [CrossRef]
- Huang, P.; Qi, Y.; Xu, Y.S.; Liu, J.; Liao, D.; Zhang, S.S.; Zhang, C. Serum cytokine alteration is associated with optic neuropathy in human primary open angle glaucoma. J. Glaucoma 2010, 19, 324–330. [Google Scholar] [CrossRef]
- Zenkel, M.; Lewczuk, P.; Jünemann, A.; Kruse, F.E.; Naumann, G.O.; Schlötzer-Schrehardt, U. Proinflammatory cytokines are involved in the initiation of the abnormal matrix process in pseudoexfoliation syndrome/glaucoma. Am. J. Pathol. 2010, 176, 2868–2879. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.; Iserovich, P. Pro-inflammatory cytokines in glaucomatous aqueous and encysted Molteno implant blebs and their relationship to pressure. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4851–4855. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, S.; Gao, X.; Yang, M.; Zhang, J.; Li, X.; Wang, W.; Zhou, M.; Zhang, X.; Zhang, X. Inflammation-related cytokines of aqueous humor in acute primary angle-closure eyes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1088–1094. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Mizoguchi, E.; Takedatsu, H.; Blumberg, R.S.; Bhan, A.K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002, 16, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Gray, D.; Mushtaq, N.; Londei, M. Prevention of arthritis by interleukin 10–producing B cells. J. Exp. Med. 2003, 197, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.A.; Shen, P.; Brown, S.; Lampropoulou, V.; Roch, T.; Lawrie, S.; Fan, B.; O’Connor, R.A.; Anderton, S.M.; Bar-Or, A.; et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6–producing B cells. J. Exp. Med. 2012, 209, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Blair, P.A.; Isenberg, D.A.; Mauri, C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 2016, 44, 683–697. [Google Scholar] [CrossRef]
- Moura, R.A.; Quaresma, C.; Vieira, A.R.; Goncalves, M.J.; Polido-Pereira, J.; Romao, V.C.; Martins, N.; Canhao, H.; Fonseca, J.E. B-cell phenotype and IgD-CD27-memory B cells are affected by TNF-inhibitors and tocilizumab treatment in rheumatoid arthritis. PLoS ONE 2017, 12, e0182927. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, H.; Xu, Y.; Lee, P.; Li, Y.; Wilson, J.C.; Vidal, O.; Choi, H.S.; Sun, Y.; Yang, L.J.; et al. Maintenance of autoantibody production in pristane-induced murine lupus. Arthritis Res. Ther. 2015, 17, 384. [Google Scholar] [CrossRef]




| Group, n | Significance | ||
|---|---|---|---|
| Characteristics (Unit) | Cases, 30 | Control, 30 | p-Value |
| Gender n(%) Male/Female | 17(56.7%)/13(43.3%) | 16(53.3%)/14(46.7%) | NS |
| Age (year) | 55.5 (49.0–59.2) | 51.5 (43.75–55.0) | NS |
| VA (Log MAR) | 0.5 (0.3–0.6) | 0.2 (0.00–0.20) | <0.001 * |
| IOP (mmHg) | 18.0 (15.7–22.2) | 12.0 (11.0–14.2) | <0.001 * |
| C/D | 0.60 (0.51–0.8) | 0.30 (0.20–0.40) | <0.001 * |
| MD (dB) | −13.0 (−20.3–−5.8) | −2.0 (−2.6–−1.67) | <0.001 * |
| PSD (dB) | 5.6 (3.25–8.7) | 2.1 (1.5–2.7) | <0.001 * |
| WBCs (103/μL) | 6.15 (4.6–7.7) | 5.7 (4.8–6.6) | NS |
| Absolute neutrophils count (103/μL) | 3.1 (2.4–4.9) | 3.0 (2.09–4.0) | NS |
| Absolute lymphocytes count (103/μL) | 1.95 (1.7–2.5) | 2.1 (1.7–2.4) | NS |
| NLR | 1.56 (1.08–2.6) | 1.5 (1.1–1.7) | NS |
| Absolute monocytes (103/μL) | 0.52 (0.30–0.8) | 0.4 (0.30–0.42) | 0.019 * |
| MLR | 0.25 (0.14–0.36) | 0.18 (0.14–0.23) | 0.018 * |
| Platelets (103/μL) | 247.0 (203.3–305.3) | 235.0 (204.7–297.2) | NS |
| PLR | 121.7 (95.1–39.4) | 118.0 (98.3–134.3) | NS |
| Total B cells % (CD19+) | 12.8 (9.4–15.1) | 4.5 (3.6–5.6) | <0.001 * |
| DN B cells % (CD19+CD27−IgD−) | 16.45 (9.85–19.5) | 6.7 (4.7–7.92) | <0.001 * |
| Naïve B cells % (CD19+CD27−IgD+) | 59.9 (53.7–70.3) | 32.2 (25.8–42.8) | <0.001 * |
| Unswitched memory B cells % (CD19+CD27+IgD+) | 9.3 (7.95–16.3) | 21.7 (17.6–32.5) | <0.001 * |
| Classical switched memory B cells % (CD19+CD27+IgD−) | 22.4 (16.7–29.3) | 20.6 (10.5–25.7) | NS |
| BSF-2(IL-6) (ng/L) | 58.4 (41.1–76.6) | 38.8(35.9–45.7) | <0.001 * |
| CSIF:TGIF(IL-10) (ng/L) | 73.15 (48.0–101.4) | 100.1 (84.8–149.6) | 0.001 * |
| BSF-2(IL-6) to CSIF:TGIF(IL-10) ratio | 0.76 (0.58–1.32) | 0.36 (0.23–0.48) | <0.001 * |
| Variable | MD | BSF-2(IL-6) (ng/L) | CSIF:TGIF(IL-10) (ng/L) | BSF-2(IL-6) to CSIF:TGIF(IL-10) Ratio | Cohen’s q/Effect Size | ||||
|---|---|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | ||
| MD | - | - | 0.85 | <0.001 * | −0.03 | NS | 0.684 | <0.001 * | 0.42/medium |
| DN B cells % (CD19+CD27−IgD−) | 0.876 | <0.001 * | 0.96 | <0.001 * | 0.065 | NS | 0.641 | <0.001 * | 0.588–1.186/large |
| naïve B cells (CD19+CD27−IgD+) | −0.29 | NS | −0.18 | NS | −0.045 | NS | −0.13 | NS | no or small effect |
| Unswitched memory B cells % (CD19+CD27+IgD+) | −0.84 | <0.001 * | −0.97 | <0.001 * | −0.129 | NS | −0.61 | NS | 0.871/large |
| Classical switched memory B cells % (CD19+CD27+IgD−) | 0.146 | NS | 0.22 | NS | −0.175 # | NS | 0.353 # | NS | 0.546 #/large |
| Cohen’s q/effect size | 2.579/large | 0.69–4/large | no or small effect | no effect | - | ||||
| % | 95% C.I. | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Cut-Off | AUC | SN | SP | p-Value | Lower | Upper |
| Total B cells % (CD19+) | >7.6 | 0.997 | 96.7 | 100 | <0.001 * | 0.989 | 1.005 |
| DN B cells % (CD19+CD27−IgD−) | >8.15 | 0.994 | 90.0 | 83.3 | <0.001 * | 0.889 | 0.997 |
| Naïve B cells % (CD19+CD27−IgD+) | >44.1 | 0.951 | 90.0 | 83.3 | <0.001 * | 0.904 | 0.998 |
| Unswitched memory B cells % (CD19+CD27+IgD+) | <14.45 | 0.855 | 73.3 | 90.0 | <0.001 * | 0.049 | 0.241 |
| BSF-2(IL-6) (ng/L) | >47.0 | 0.805 | 73.3 | 83.3 | <0.001 * | 0.683 | 0.927 |
| CSIF:TGIF(IL-10) (ng/L) | <87.8 | 0.754 | 73.3 | 73.3 | 0.001 * | 0.630 | 0.878 |
| BSF-2(IL-6) to CSIF:TGIF(IL-10) ratio | >0.55 | 0.859 | 80.0 | 90.0 | <0.001 * | 0.763 | 0.956 |
| POAG Group (n = 30) Subclass, n | Significance | ||
|---|---|---|---|
| Characteristics (Unit) | Mild-to-Moderate, 12 | Severe, 18 | p-Value |
| Total B cells % (CD19+) | 12.7 (9.5–14.7) | 12.8 (9.12–15.3) | NS |
| DN B cells % (CD19+CD27−IgD−) | 9.0 (8.12–10.4) | 18.9 (16.9–22.9) | <0.001 * |
| Naïve B cells % (CD19+CD27−IgD+) | 65.9 (56.27–76.6) | 58.8 (44.8–68.2) | NS |
| Unswitched memory B cells % (CD19+CD27+IgD+) | 18.6 (13.5–24.8) | 8.3 (6.6–9.07) | <0.001 * |
| Classical switched memory B cells % (CD19+CD27+IgD−) | 22.2 (11.6–27.7) | 22.9 (17.4–36.6) | NS |
| BSF-2(IL-6) (ng/L) | 38.55 (29.02–50.6) | 73.2 (61.1–80.8) | <0.001 * |
| CSIF:TGIF(IL-10) (ng/L) | 71.7 (59.15–104.2) | 76.7 (45.4–90.9) | NS |
| BSF-2(IL-6) to CSIF:TGIF(IL-10) ratio | 0.54 (0.35–0.68) | 1.0 (0.75–1.7) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, E.R.; Elmadbouly, A.A.; Abo Elkheir, O.I.; Mansour, M.N.; El Attar, S.; Heiba, M.A.; Mohamed, M.N.; Elhakeem, H.; Gad, L.A.; Abdelrahman, H.M.; et al. Peripheral Blood B-Cell Subsets Frequency and Distribution and the BSF-2(IL-6) to CSIF:TGIF(IL-10) Ratio as Severity-Associated Signatures in Primary Open-Angle Glaucoma: A Case-Controlled Study. Biomedicines 2024, 12, 485. https://doi.org/10.3390/biomedicines12030485
Mokhtar ER, Elmadbouly AA, Abo Elkheir OI, Mansour MN, El Attar S, Heiba MA, Mohamed MN, Elhakeem H, Gad LA, Abdelrahman HM, et al. Peripheral Blood B-Cell Subsets Frequency and Distribution and the BSF-2(IL-6) to CSIF:TGIF(IL-10) Ratio as Severity-Associated Signatures in Primary Open-Angle Glaucoma: A Case-Controlled Study. Biomedicines. 2024; 12(3):485. https://doi.org/10.3390/biomedicines12030485
Chicago/Turabian StyleMokhtar, Entsar R., Asmaa A. Elmadbouly, Omaima I. Abo Elkheir, Mona Nabeh Mansour, Shahinaz El Attar, Mohamed A. Heiba, Mennatullah N. Mohamed, Heba Elhakeem, Lamia A. Gad, Heba Mahmoud Abdelrahman, and et al. 2024. "Peripheral Blood B-Cell Subsets Frequency and Distribution and the BSF-2(IL-6) to CSIF:TGIF(IL-10) Ratio as Severity-Associated Signatures in Primary Open-Angle Glaucoma: A Case-Controlled Study" Biomedicines 12, no. 3: 485. https://doi.org/10.3390/biomedicines12030485
APA StyleMokhtar, E. R., Elmadbouly, A. A., Abo Elkheir, O. I., Mansour, M. N., El Attar, S., Heiba, M. A., Mohamed, M. N., Elhakeem, H., Gad, L. A., Abdelrahman, H. M., Kamel, R. M., El Magdoub, H. M., Hamdy, N. M., & Abd El-Fattah, D. A. (2024). Peripheral Blood B-Cell Subsets Frequency and Distribution and the BSF-2(IL-6) to CSIF:TGIF(IL-10) Ratio as Severity-Associated Signatures in Primary Open-Angle Glaucoma: A Case-Controlled Study. Biomedicines, 12(3), 485. https://doi.org/10.3390/biomedicines12030485







