Relationship between Glucagon-like Peptide-1 Receptor Agonists and Cardiovascular Disease in Chronic Respiratory Disease and Diabetes
Abstract
:1. Introduction
2. Methods and Materials
2.1. Operational Exposure and Outcome Definitions (Supplementary Figure S1)
Cohort Identification
2.2. Exclusion Criteria
2.3. Definition of GLP-1 RA Use
2.4. Outcomes
2.5. Definitions of Covariates
2.5.1. Comorbidities Having Impact on the Outcomes of COVID-19
2.5.2. Medications and Psychotherapy Having Impact on the Outcomes of COVID-19
2.6. Statistical Analysis
Propensity Score Matching
2.7. Time-Dependent Analysis
2.8. Fine and Gray Model for Competing Risk
3. Results
4. Discussion
4.1. Summary of the Main Results
4.2. An Explanation of Findings
5. Strengths
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Boubaker, N.; Louhaichi, S.; Khalfallah, I.; Belkhir, S.; Ferchichi, M.; Ammar, J.; Hamdi, B.; Hamzaoui, A. Prevalence and impact of chronic respiratory disease in moderate to severe COVID-19 outcomes. Eur. Respir. J. 2021, 58, PA301. [Google Scholar]
- He, Z.F.; Zhong, N.S.; Guan, W.J. Impact of Chronic Respiratory Diseases on the Outcomes of COVID-19. Arch. Bronconeumol. 2022, 58, 5–7. [Google Scholar] [CrossRef]
- Xie, M.; Liu, X.; Cao, X.; Guo, M.; Li, X. Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir. Res. 2020, 21, 49. [Google Scholar] [CrossRef]
- Fallahzadeh, A.; Sharifnejad Tehrani, Y.; Sheikhy, A.; Ghamari, S.H.; Mohammadi, E.; Saeedi Moghaddam, S.; Esfahani, Z.; Nasserinejad, M.; Shobeiri, P.; Rashidi, M.M.; et al. The burden of chronic respiratory disease and attributable risk factors in North Africa and Middle East: Findings from global burden of disease study (GBD) 2019. Respir. Res. 2022, 23, 268. [Google Scholar] [CrossRef] [PubMed]
- Kopf, S.; Kumar, V.; Kender, Z.; Han, Z.; Fleming, T.; Herzig, S.; Nawroth, P.P. Diabetic Pneumopathy-A New Diabetes-Associated Complication: Mechanisms, Consequences and Treatment Considerations. Front. Endocrinol. 2021, 12, 765201. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.M.; Brok, J.; Backer, V.; Thomsen, S.F.; Meteran, H. Association Between Chronic Obstructive Pulmonary Disease and Type 2 Diabetes: A Systematic Review and Meta-Analysis. COPD 2018, 15, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Ehteshami-Afshar, S.; Mooney, L.; Dewan, P.; Desai, A.S.; Lang, N.N.; Lefkowitz, M.P.; Petrie, M.C.; Rizkala, A.R.; Rouleau, J.L.; Solomon, S.D.; et al. Clinical Characteristics and Outcomes of Patients With Heart Failure With Reduced Ejection Fraction and Chronic Obstructive Pulmonary Disease: Insights From PARADIGM-HF. J. Am. Heart Assoc. 2021, 10, e019238. [Google Scholar] [CrossRef] [PubMed]
- Feary, J.R.; Rodrigues, L.C.; Smith, C.J.; Hubbard, R.B.; Gibson, J.E. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: A comprehensive analysis using data from primary care. Thorax 2010, 65, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Nyland, J.E.; Raja-Khan, N.T.; Bettermann, K.; Haouzi, P.A.; Leslie, D.L.; Kraschnewski, J.L.; Parent, L.J.; Grigson, P.S. Diabetes, Drug Treatment, and Mortality in COVID-19: A Multinational Retrospective Cohort Study. Diabetes 2021, 70, 2903–2916. [Google Scholar] [CrossRef] [PubMed]
- Sazgarnejad, S.; Yazdanpanah, N.; Rezaei, N. Anti-inflammatory effects of GLP-1 in patients with COVID-19. Expert Rev. Anti-Infect. Ther. 2022, 20, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post−COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Chen, J.J.; Wu, C.Y.; Jenq, C.C.; Lee, T.H.; Tsai, C.Y.; Tu, H.T.; Huang, Y.T.; Yen, C.L.; Yen, T.H.; Chen, Y.C.; et al. Association of Glucagon-Like Peptide-1 Receptor Agonist vs. Dipeptidyl Peptidase-4 Inhibitor Use With Mortality Among Patients With Type 2 Diabetes and Advanced Chronic Kidney Disease. JAMA Netw. Open. 2022, 5, e221169. [Google Scholar] [CrossRef]
- Scheen, A.J. GLP-1 receptor agonists and heart failure in diabetes. Diabetes Metab. 2017, 43 (Suppl. S1), 2S13–2S19. [Google Scholar] [CrossRef]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Chen, H.H.; Huang, C.N.; Hsu, C.Y.; Hu, K.C.; Kao, C.H. GLP-1RAs for Ischemic Stroke Prevention in Patients With Type 2 Diabetes Without Established Atherosclerotic Cardiovascular Disease. Diabetes Care 2022, 45, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, R.; Ye, H.; Wang, Y.; Wang, L.; Zhang, X. Effects of GLP-1 receptor agonists on arrhythmias and its subtypes in patients with type 2 diabetes: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 910256. [Google Scholar] [CrossRef] [PubMed]
- Sposito, A.C.; Berwanger, O.; de Carvalho, L.S.F.; Saraiva, J.F.K. GLP-1RAs in type 2 diabetes: Mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc. Diabetol. 2018, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Alkhezi, O.S.; Alsuhaibani, H.A.; Alhadyab, A.A.; Alfaifi, M.E.; Alomrani, B.; Aldossary, A.; Alfayez, O.M. Heart failure outcomes and glucagon-like peptide-1 receptor agonists: A systematic review of observational studies. Prim. Care Diabetes 2021, 15, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.S.; Hobbs, T.M.; Wells, B.J.; Kong, S.X.; Kattan, M.W.; Bouchard, J.; Chagin, K.M.; Yu, C.; Sakurada, B.; Milinovich, A.; et al. Association of glucagon-like peptide-1 receptor agonist use and rates of acute myocardial infarction, stroke and overall mortality in patients with type 2 diabetes mellitus in a large integrated health system. Diabetes Obes. Metab. 2017, 19, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.; Fu, E.L.; Szummer, K.; Norhammar, A.; Lundman, P.; Wanner, C.; Sjölander, A.; Jernberg, T.; Carrero, J.J. Glucagon-like peptide-1 receptor agonists and the risk of cardiovascular events in diabetes patients surviving an acute myocardial infarction. Eur. Heart J.-Cardiovasc. Pharmacother. 2021, 7, 104–111. [Google Scholar] [CrossRef]
- Cazorla-Morallon, D.; Cordero, A.; Pomares Varo, A.; Torroba Balmori, G.; Moreno Garcia, M.J.; Martinez Rey-Ranal, E.; Bertomeu-Gonzalez, V.; Zuazola, P. Stroke and myocardial infarction prevention with GLP1 analogues in high or very-high cardiovascular risk diabetic patients. Eur. Heart J. 2020, 41, ehaa946.2967. [Google Scholar] [CrossRef]
- Boye, K.S.; Stein, D.; Matza, L.S.; Jordan, J.; Yu, R.; Norrbacka, K.; Hassan, S.W.; García-Pérez, L.E. Timing of GLP-1 Receptor Agonist Initiation for Treatment of Type 2 Diabetes in the UK. Drugs RD 2019, 19, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.; Tsao, C.F. Comparison of Glucose Lowering Efficacy of Human GLP-1 Agonist in Taiwan Type 2 Diabetes Patients after Switching from DPP-4 Inhibitor Use or Non-Use. J. Pers. Med. 2022, 12, 1915. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Perez Perez, J.L.; Perez Gandara, B.; Agudelo, C.W.; Rodriguez Ortega, R.; Ahmed, H.; Garcia-Arcos, I.; McCarthy, C.; Geraghty, P. Mechanisms Linking COPD to Type 1 and 2 Diabetes Mellitus: Is There a Relationship between Diabetes and COPD? Medicina 2022, 58, 1030. [Google Scholar] [CrossRef]
- Naseem, S.; Baneen, U. Systemic inflammation in patients of chronic obstructive pulmonary disease with metabolic syndrome. J. Fam. Med. Prim. Care 2019, 8, 3393–3398. [Google Scholar]
- Ghatas, T. The relationship between metabolic syndrome and chronic obstructive pulmonary disease. Egypt. J. Bronchol. 2017, 11, 11–15. [Google Scholar] [CrossRef]
- Watz, H.; Waschki, B.; Kirsten, A.; Müller, K.C.; Kretschmar, G.; Meyer, T.; Holz, O.; Magnussen, H. The Metabolic Syndrome in Patients With Chronic Bronchitis and COPD: Frequency and Associated Consequences for Systemic Inflammation and Physical Inactivity. Chest 2009, 136, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Laghi-Pasini, F.; Acampa, M.; Srivastava, U.; Bertolozzi, I.; Giabbani, B.; Finizola, F.; Vanni, F.; Dokollari, A.; Natale, M.; et al. Systemic Inflammation Rapidly Induces Reversible Atrial Electrical Remodeling: The Role of Interleukin-6–Mediated Changes in Connexin Expression. J. Am. Heart Assoc. 2019, 8, e011006. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Deng, Z.; Li, J.; Ren, Z.; Liu, F. Meta-analysis of the relationship between interleukin-6 levels and the prognosis and severity of acute coronary syndrome. Clinics 2021, 76, e2690. [Google Scholar] [CrossRef]
- Fauchier, G.; Bisson, A.; Bodin, A.; Herbert, J.; Angoulvant, D.; Ducluzeau, P.H.; Lip, G.Y.H.; Fauchier, L. Glucose-lowering drug use and new-onset atrial fibrillation in patients with diabetes mellitus. Diabetologia 2021, 64, 2602–2605. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Tonneijck, L.; Muskiet, M.H.; Hoekstra, T.; Kramer, M.H.; Diamant, M.; van Raalte, D.H. Heart rate acceleration with GLP-1 receptor agonists in type 2 diabetes patients: An acute and 12-week randomised, double-blind, placebo-controlled trial. Eur. J. Endocrinol. 2017, 176, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. 2016, 24, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, L.; Cao, F.; Wang, F.; Wang, H.; Shi, H.; Shen, L.; Zhao, F.; Zhao, Y. Systemic Glycemic Variation Predicts Mortality of Acute Ischemic Stroke After Mechanical Thrombectomy: A Prospective Study Using Continuous Glucose Monitoring. Front. Neurol. 2022, 13, 817033. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S. Cerebrovascular Complications of Diabetes: Alpha Glucosidase Inhibitor as Potential Therapy. Horm. Metab. Res. 2016, 48, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.F.; Sung, S.H.; Cheng, H.M.; Shin, S.J.; Lin, K.D.; Chong, K.; Yen, F.S.; Yu, B.H.; Huang, C.T.; Hsu, C.C. Cardiovascular Benefits of Acarbose vs. Sulfonylureas in Patients With Type 2 Diabetes Treated With Metformin. J. Clin. Endocrinol. Metab. 2018, 103, 3611–3619. [Google Scholar] [CrossRef]
- Sohrabi, F.; Sohrabi, A.; Shams-Alizadeh, N.; Cayoun, B.A. Managing type 2 diabetes and depression with Mindfulness-integrated Cognitive Behavior Therapy (MiCBT). Discov. Psychol. 2022, 2, 10. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Fang, J.; Lee, D.S.; Tu, J.V.; Amir, E.; Austin, P.C.; Anderson, G.M. Importance of Considering Competing Risks in Time-to-Event Analyses: Application to Stroke Risk in a Retrospective Cohort Study of Elderly Patients with Atrial Fibrillation. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004580. [Google Scholar] [CrossRef]

| GLP-1 RA | |||||
|---|---|---|---|---|---|
| No N = 15,801 | Yes N = 15,801 | ||||
| n | % | n | % | p-Value | |
| Age, years | 0.03 * | ||||
| ≤49 | 6153 | 38.9 | 6373 | 40.3 | |
| 50–64 | 6640 | 42.0 | 6521 | 41.3 | |
| ≥65 | 3008 | 19.0 | 2907 | 18.4 | |
| Mean ± SD a | 54.7 | 12.7 | 52.6 | 12.8 | 0.00 * |
| Gender | 0.57 | ||||
| Women | 8481 | 53.7 | 8531 | 54.0 | |
| Men | 7320 | 46.3 | 7270 | 46.0 | |
| Comorbidity | |||||
| Hypertension | 11,013 | 69.7 | 10,957 | 69.3 | 0.49 |
| Hyperlipidemia | 14,388 | 91.7 | 14,376 | 91.0 | 0.03 * |
| Chronic renal disease | 1861 | 11.8 | 1977 | 12.5 | 0.05 |
| Gout | 2430 | 15.4 | 2479 | 15.7 | 0.45 |
| Tobacco dependence related | 341 | 2.16 | 372 | 2.35 | 0.24 |
| Venous thrombosis | 19 | 0.12 | 22 | 0.14 | 0.64 |
| Depression or substance-related disease | 1927 | 12.2 | 1990 | 12.6 | 0.28 |
| Medications | |||||
| AGI | 8856 | 56.1 | 8926 | 56.5 | 0.43 |
| Metformin | 15,737 | 99.6 | 15,660 | 99.1 | 0.00 * |
| Insulin | 13,008 | 82.3 | 12,972 | 82.1 | 0.60 |
| DPP-4 inhibitor | 13,973 | 88.4 | 13,873 | 87.8 | 0.08 |
| Meglitinides | 4878 | 30.9 | 5072 | 32.1 | 0.02 * |
| TZD | 9872 | 62.5 | 9813 | 62.1 | 0.49 |
| Sulphonylurea | 14,731 | 93.2 | 14,503 | 91.8 | 0.00 * |
| Statin | 13,727 | 86.9 | 13,637 | 86.3 | 0.14 |
| Antidepressants | 5311 | 33.6 | 5494 | 34.8 | 0.03 * |
| Antihypertensive | 13,813 | 87.4 | 13,725 | 86.9 | 0.14 |
| Antithrombotic | 7428 | 47.0 | 7312 | 46.3 | 0.19 |
| Psychotherapy | 5300 | 33.5 | 5420 | 34.3 | 0.06 |
| GLP-1 RA | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Outcome | Event | PY | Rate # | Event | PY | Rate # | Crude SHR (95% CI) | Adjusted SHR a (95% CI) |
| All b | 1285 | 43,760 | 29.4 | 1251 | 43,012 | 29.1 | 1.00(0.92, 1.08) | 1.03(0.95, 1.11) |
| Coronary c artery disease | 647 | 44,742 | 14.5 | 667 | 43,990 | 15.2 | 1.06(0.95, 1.18) | 1.09(0.97, 1.21) |
| Arrhythmia d | 271 | 45,452 | 5.96 | 354 | 44,608 | 7.94 | 1.34(1.15, 1.57) *** | 1.36(1.16, 1.59) *** |
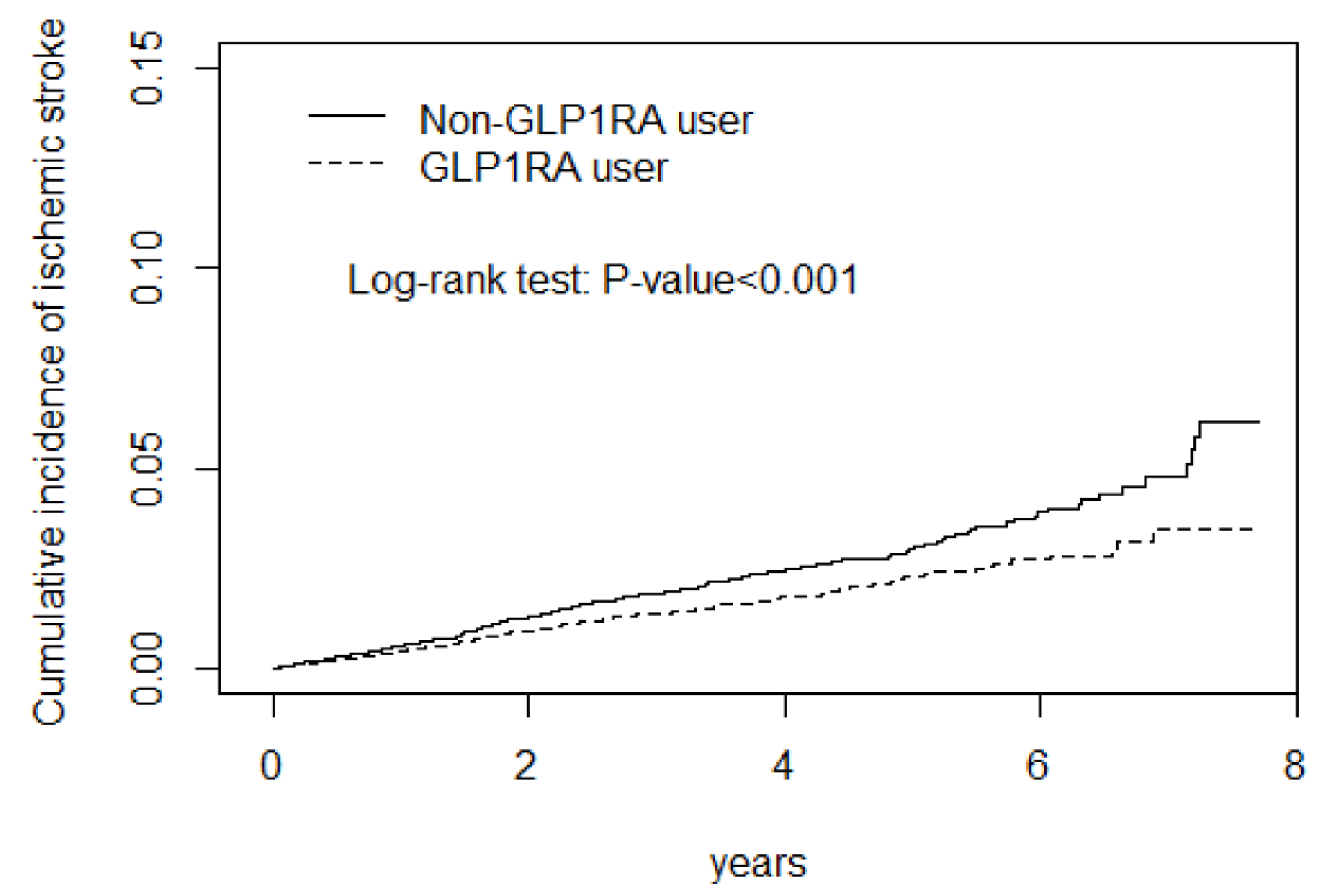
| Stroke e | 354 | 45,756 | 7.74 | 239 | 45,163 | 5.29 | 0.71(0.60, 0.84) *** | 0.76(0.65, 0.90) ** |
| Ischemic f stroke | 291 | 45,398 | 6.41 | 207 | 44,956 | 4.60 | 0.73(0.61, 0.87) *** | 0.77(0.64, 0.92) ** |
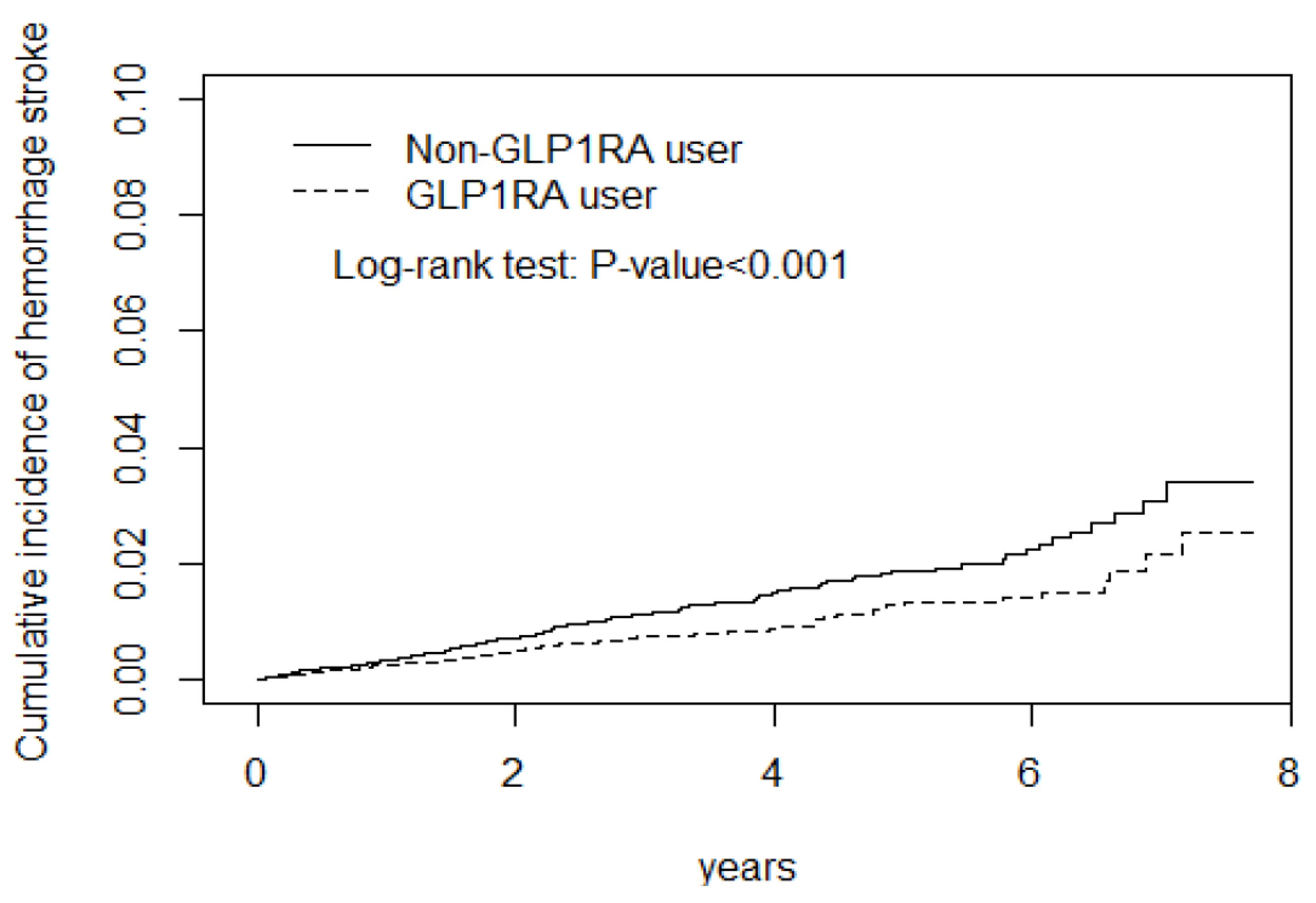
| Hemorrhagic stroke g | 172 | 45,677 | 3.77 | 111 | 45,135 | 2.46 | 0.66(0.52, 0.84) *** | 0.69(0.54, 0.88) ** |
| Heart failure h | 304 | 45,450 | 6.69 | 307 | 44,799 | 6.85 | 1.04(0.88, 1.21) | 1.09(0.93, 1.28) |
| GLP-1 RA | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Variables | Event | PY | Rate # | Event | PY | Rate # | Crude SHR (95% CI) | Adjusted SHR a (95% CI) |
| Age, years | ||||||||
| ≤49 ♥ | 81 | 19,420 | 4.17 | 50 | 19,705 | 2.54 | 0.62(0.44, 0.89) ** | 0.65(0.46, 0.92) * |
| 50–64 ♥♥ | 154 | 19,049 | 8.08 | 106 | 18,399 | 5.76 | 0.75(0.59, 0.96) * | 0.78(0.61, 0.99) * |
| ≥65 ♥♥♥ | 119 | 7287 | 16.3 | 83 | 7059 | 11.8 | 0.75(0.57, 0.99) * | 0.79(0.59, 1.06) |
| p for interaction | 0.43 | |||||||
| Gender | ||||||||
| Women | 175 | 25,053 | 6.99 | 118 | 24,944 | 4.73 | 0.71(0.56, 0.89) ** | 0.76(0.60, 0.96) * |
| Men | 179 | 20,702 | 8.65 | 121 | 20,219 | 5.98 | 0.71(0.57, 0.90) ** | 0.76(0.60, 0.96) * |
| p for interaction | 0.85 | |||||||
| Comorbidity ‡ | ||||||||
| No | 4 | 1347 | 2.97 | 0 | 1133 | 0 | ||
| Yes | 350 | 44,408 | 7.88 | 239 | 44,030 | 5.43 | 0.71(0.61, 0.84) *** | 0.77(0.65, 0.90) ** |
| p for interaction | 0.00 | |||||||
| Medications | ||||||||
| AGI | ||||||||
| No | 118 | 19,580 | 6.03 | 98 | 19,069 | 5.14 | 0.89(0.68, 1.17) | 0.96(0.73, 1.26) |
| Yes | 236 | 26,175 | 9.02 | 141 | 26,094 | 5.40 | 0.62(0.50, 0.77) *** | 0.67(0.54, 0.82) *** |
| p for interaction | 0.04 | |||||||
| Metformin | ||||||||
| No | 3 | 185 | 16.2 | 3 | 340 | 8.83 | 0.64(0.13, 3.20) | 0.30(0.02, 5.69) |
| Yes | 351 | 45,570 | 7.70 | 236 | 44,823 | 5.27 | 0.71(0.60, 0.84) *** | 0.76(0.65, 0.90) ** |
| p for interaction | 0.92 | |||||||
| Insulin | ||||||||
| No | 17 | 7609 | 2.23 | 12 | 7437 | 1.61 | 0.75(0.36, 1.56) | 0.85(0.41, 0.76) |
| Yes | 337 | 38,147 | 8.83 | 227 | 37,726 | 6.02 | 0.70(0.60, 0.83) *** | 0.76(0.64, 0.90) ** |
| p for interaction | 0.83 | |||||||
| DPP-4 inhibitor | ||||||||
| No | 31 | 5115 | 6.06 | 26 | 5245 | 4.96 | 0.85(0.50, 1.42) | 0.91(0.53, 1.54) |
| Yes | 323 | 40,641 | 7.95 | 213 | 39,918 | 5.34 | 0.70(0.59, 0.83) *** | 0.75(0.63, 0.89) ** |
| p for interaction | 0.47 | |||||||
| Meglitinides | ||||||||
| No | 215 | 31,478 | 6.83 | 140 | 30,617 | 4.57 | 0.69(0.56, 0.85) *** | 0.73(0.59, 0.90) ** |
| Yes | 139 | 14,277 | 9.74 | 99 | 14,546 | 6.81 | 0.73(0.57, 0.95) * | 0.79(0.61, 1.02) |
| p for interaction | 0.67 | |||||||
| TZD | ||||||||
| No | 121 | 16,635 | 7.27 | 77 | 16,470 | 4.68 | 0.68(0.51, 0.90) ** | 0.75(0.56, 1.00) * |
| Yes | 233 | 29,121 | 8.00 | 162 | 28,693 | 5.65 | 0.73(0.60, 0.89) ** | 0.77(0.63, 0.94) ** |
| p for interaction | 0.63 | |||||||
| Sulphonylurea | ||||||||
| No | 13 | 2864 | 4.54 | 11 | 3336 | 3.30 | 0.75(0.34, 1.66) | 0.70(0.30, 1.64) |
| Yes | 341 | 42,892 | 7.95 | 228 | 41,827 | 5.45 | 0.71(0.60, 0.84) *** | 0.76(0.64, 0.90) ** |
| p for interaction | 0.95 | |||||||
| Statin | ||||||||
| No | 30 | 5743 | 5.22 | 24 | 5957 | 4.03 | 0.78(0.46, 1.33) | 0.85(0.49, 1.47) |
| Yes | 324 | 40,012 | 8.10 | 215 | 39,206 | 5.48 | 0.70(0.59, 0.83) *** | 0.75(0.63, 0.89) ** |
| p for interaction | 0.68 | |||||||
| Antidepressants | ||||||||
| No | 215 | 30,269 | 7.10 | 129 | 29,424 | 4.38 | 0.65(0.52, 0.80) *** | 0.69(0.55, 0.86) *** |
| Yes | 139 | 15,487 | 8.98 | 110 | 15,739 | 6.99 | 0.80(0.63, 1.03) | 0.86(0.67, 1.11) |
| p for interaction | 0.17 | |||||||
| Antihypertensive | ||||||||
| No | 6 | 5537 | 1.08 | 7 | 5730 | 1.22 | 1.20(0.41, 3.50) | 1.02(0.40, 2.61) |
| Yes | 348 | 40,219 | 8.65 | 232 | 39,433 | 5.88 | 0.71(0.60, 0.83) *** | 0.75(0.64, 0.89) *** |
| p for interaction | 0.38 | |||||||
| Antithrombotic | ||||||||
| No | 40 | 24,106 | 1.66 | 15 | 24,127 | 0.62 | 0.40(0.22.0.71) ** | 0.42(0.24,0.77) ** |
| Yes | 314 | 21,650 | 14.5 | 224 | 21,036 | 10.7 | 0.76(0.64,0.90) ** | 0.80(0.68,0.95) ** |
| P for interaction | 0.02 | |||||||
| Psychological therapy | ||||||||
| No | 47 | 25,106 | 1.77 | 18 | 23,120 | 0.52 | 0.46(0.32, 0.61) ** | 0.43(0.26, 0.98) ** |
| Yes | 414 | 31,620 | 16.5 | 212 | 20,099 | 11.7 | 0.66(0.64, 0.90) ** | 0.79(0.66, 0.90) ** |
| p for interaction | 0.01 | |||||||
| Medication Exposed | N | Event | Person-Year | Rate $ | Crude SHR (95% CI) | Adjusted SHR (95% CI) a |
|---|---|---|---|---|---|---|
| GLP-1 RA | 15,801 | 354 | 45,756 | 7.74 | ||
| No | 1.00 | 1.00 | ||||
| Yes # | ||||||
| <85 days | 4097 | 100 | 11,127 | 8.99 | 1.20(0.96, 1.50) | 1.09(0.87, 1.37) |
| 86–200 days | 3719 | 59 | 8997 | 6.56 | 0.93(0.71, 1.23) | 1.05(0.80, 1.39) |
| 201–350 days | 3961 | 41 | 9745 | 4.21 | 0.66(0.48, 0.91) * | 0.73(0.52, 1.01) |
| ≥351 days | 4024 | 39 | 15,294 | 2.55 | 0.31(0.22, 0.43) *** | 0.35(0.26, 0.49) *** |
| GLP-1 RA | ||
|---|---|---|
| Variables | No (N = 15,801) | Yes (N = 15,801) |
| Stroke | ||
| cSHR (95% CI) | 1 (Reference) | 0.36(0.28, 0.47) *** |
| aSHR (95% CI) a | 1 (Reference) | 0.42(0.32, 0.55) *** |
| Ischemic stroke | ||
| cSHR (95% CI) | 1 (Reference) | 0.51(0.41, 0.64) *** |
| aSHR (95% CI) a | 1 (Reference) | 0.57(0.46, 0.72) *** |
| Hemorrhage stroke | ||
| cSHR (95% CI) | 1 (Reference) | 0.46(0.34, 0.63) *** |
| aSHR (95% CI) a | 1 (Reference) | 0.51(0.37, 0.71) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, J.-J.; Li, C.-C.; Tan, C.-W.; Li, C.-H.; Tsai, T.-H.; Kao, C.-H. Relationship between Glucagon-like Peptide-1 Receptor Agonists and Cardiovascular Disease in Chronic Respiratory Disease and Diabetes. Biomedicines 2024, 12, 488. https://doi.org/10.3390/biomedicines12030488
Yeh J-J, Li C-C, Tan C-W, Li C-H, Tsai T-H, Kao C-H. Relationship between Glucagon-like Peptide-1 Receptor Agonists and Cardiovascular Disease in Chronic Respiratory Disease and Diabetes. Biomedicines. 2024; 12(3):488. https://doi.org/10.3390/biomedicines12030488
Chicago/Turabian StyleYeh, Jun-Jun, Chih-Chien Li, Chang-Wen Tan, Chia-Hsun Li, Tung-Han Tsai, and Chia-Hung Kao. 2024. "Relationship between Glucagon-like Peptide-1 Receptor Agonists and Cardiovascular Disease in Chronic Respiratory Disease and Diabetes" Biomedicines 12, no. 3: 488. https://doi.org/10.3390/biomedicines12030488





