Effects of Physical Cues on Stem Cell-Derived Extracellular Vesicles toward Neuropathy Applications
Abstract
:1. Introduction
2. Mechanisms of Peripheral Neuropathies
2.1. Diabetic Peripheral Neuropathy
2.2. Chemotherapy-Induced Peripheral Neuropathy
2.3. Peripheral Neuropathy via Physical Injury
2.4. Pathophysiology of Axonal Injury
3. Approaches to Peripheral Nerve Injury Treatment
4. Neurotrophic Support in Neuropathy Treatment
4.1. Stem Cell Differentiation
4.2. The Application of Stem Cells in Neuropathy Treatment
4.3. Potential of MSCs Secretome in Nerve Regeneration
4.3.1. EV Biogenesis and Transport
4.3.2. Targeted Transplantation of EVs in Neuropathy Treatment
4.4. Methods to Increase EV Production
Electrical Stimulation Promoting Transdifferentiation and EV Production
| Title of Study | Cell Culture | ES | ES Duration | ES Method | Reference |
|---|---|---|---|---|---|
| Intermittent electrical stimuli for guidance of human mesenchymal stem cell lineage commitment towards neural-like cells on electroconductive substrates. | MSCs | DC; 1 mV–2 V | 10 min/day, 3 days | Parallel stainless-steel electrodes PANI film | [289] |
| Neurogenesis-on-Chip: Electric field modulated transdifferentiation of human mesenchymal stem cell and mouse muscle precursor cell coculture. | hMSCs Murine myoblast | DC ~8 ± 0.06 mV/mm | 20 h/day for 9 days | Microfluidic device; graphene oxide (GO) microfiber | [287] |
| Effectiveness of electrical stimulation on nerve regeneration after crush injury: Comparison between invasive and non-invasive stimulation. | Sciatic nerve crush injury | 25 Hz, 1–3 mA, 0.1 ms pulse width | 30 min/day 5 times/week for 6 weeks | Implanted wireless cuff electrodes | [298] |
| Low level electricity increases the secretion of extracellular vesicles from cultured cells. | Murine melanoma cell line, B16F1 | 0.34 mA/cm2 | 60 min Immediate EV isolation | Two Ag–AgCl electrodes with 2.5 cm2 surface areas | [288] |
| The frequency-dependent effect of electrical fields on the mobility of intracellular vesicles in astrocytes. | Rat astrocytes | 5 mV/mm; 2 Hz | 5 min of constant voltage; 0.1 nms pulse 600 total pulses | Stimulus isolator A365 with 1 KΩ resistor | [299] |
| Electrical stimulation increases the secretion of cardioprotective extracellular vesicles from cardiac mesenchymal stem cells. | Cardiac MSC | 1.5 V/1.8 cm | 2–72 h; 1.5 V/1.8 cm voltage, 0.5 Hz frequency, pulse width at 5 ms | Cultured-cell pacer system (IonOptix) | [292] |
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Alternating current | NAD+ | Oxidative nicotinamide adenine dinucleotide |
| hASC | Human adipose derived MSC | NADH | Reductive nicotinamide adenine dinucleotide |
| AKT | Protein kinase B | NAM | Nicotinamide |
| ATP | Adenosine triphosphate | NEFL | Neurofilament light polypeptide |
| Aβ | Alpha beta | NEFM | Neurofilament medium polypeptide |
| Aδ | Alpha delta | NESTIN | Neuroepithelial stem cell protein |
| BBB | Blood–brain barrier | NK-kB | Nuclear factor-kappa beta |
| BDNF | Brain-derived neurotrophic factor | NGF | Nerve growth factor |
| b-FGF | Fibroblast growth factor | nm | Nanometers |
| hBM-MSC | Human bone marrow derived MSC | NMDA | N-methyl-D-aspartate |
| Ca2+ | Calcium | NMN | Nicotinamide mononucleotide |
| CD44 | Cluster of differentiation 44 | NMNAT | Nicotinamide mononucleotide adenylyltransferase |
| CHMP4C | Chromatin modified protein 4C | NP | Neuropathic pain |
| CIPN | Chemotherapy-induced peripheral neuropathy | NR | Nicotinamide riboside |
| CNS | Central nervous system | nSMase2 | Neutral sphingomyelinase 2 |
| CT | Computerized tomography | ||
| DC | Direct current | NT-3 | Neurotrophin-3 |
| DHT | Dihydrotestosterone | NURR1 | Dopaminergic neuron marker |
| DPN | Diabetic peripheral neuropathy | OCT-3/4 | Octamer transcription factor 3 and 4 |
| DSN | Distal symmetric neuropathy | P0 | Protein zero |
| ECM | Extracellular matrix | PI3K | Phosphatidylinositol 3-kinase |
| ER | Endoplasmic reticulum | PAX6 | Paired box 6 |
| ESCs | Embryonic stem cells | PC | Phosphatidylcholine |
| ESCRT | Endosomal sorting complex required for transport | PCL | Polycaprolactone |
| EVs | Extracellular vesicles | PE | Phosphatidylethanolamine |
| FasL | Fas ligand | PGA | Poly-glycolic acid |
| FBS | Fetal bovine serum | PI | Phosphatidylinositol |
| FDA | U.S. Food and Drug Administration | PLA | Polylactic acid |
| FK506 | Tacrolimus | PLGA | Poly-dl-lactic-co-glycolic acid |
| GABA | Gamma-aminobutyric acid | PMP22 | Peripheral myelin protein-22 |
| GAP-43 | Growth-associated protein 43 | PN | Peripheral neuropathy |
| GDF3 | Growth and differentiation factor 3 | PNS | Peripheral nervous system |
| GDNF | Glial cell-derived neurotrophic factor | PS | Phosphatidylserine |
| GFAP | Glial fibrillary acidic protein | PSA | Pressure sensitive adhesive tape |
| GluN1 | Glycine-binding subunits | PVA | Polyvinyl acetate |
| GM | Gangliosides | qPCR | Quantitative polymerase chain reaction |
| GPCR | G protein-coupled receptor | ||
| GPI | Glycosylphosphatidylinositol | RA | Retinoic acid |
| GTPase | Guanosine triphosphate | Rab GTPases | Ras-associated binding guanosine triphosphates |
| HA | Hyaluronic acid | REX1 | Reduced expression 1 |
| hASC | Human ASC | ROS | Reactive oxygen species |
| hBM-MSC | Human BM-MSC | rSCs | Repair Schwann cells |
| Hrs | Hepatocyte growth factor-regulated tyrosine kinase substrate | RT-PCR | Reverse transcription polymerase chain reaction |
| HF | High frequency | S100B | Calcium-binding protein B |
| HLA-DR | Human leukocyte antigen | SARM1 | Sterile alpha and toll/interleukin-1 receptor motif-containing 1 |
| HPL | Human platelet lysate | SC | Schwann cell |
| Hsc | Heat shock cognate | SHH | Sonic Hedgehog protein |
| Hsp | Heat shock protein | SMPD2 | Sphingomyelin phosphodiesterase 2 |
| hUC | Human umbilical cord | SOX2 | Sex determining region Y-box 2 |
| Hz | Hertz | STAM1 | Signal transducing adaptor molecule |
| IGF-1 | Insulin-like growth factor 1 | STZ | Streptozotocin |
| IL | Interleukin | TENS | Transcutaneous electrical nerve stimulation |
| ILV | Intraluminal vesicles | TfR | Transferrin receptor |
| iPSCs | Induced pluripotent stem cells | TGF-β1 | Transforming growth factor-beta 1 |
| JNK | C-Jun N-terminal kinase | TH | Tyrosine hydroxylase |
| K+ | Potassium | TLR4 | Toll-like receptor member 4 |
| KLF4 | Kruppel-like factor 4 | TNF-α | Tumor necrosis factor |
| kPa | Kilopascal | ||
| LAMP1 | Lysosomal-associated membrane protein 1 | Trp | Tryptophan |
| LF | Low frequency | TRAIL | TNF related apoptosis-inducing ligand |
| mA | Milliamps | TSG | Tumor susceptibility gene |
| MAL | Myelin and lymphocyte protein | Tuj1 | Class III beta-tubulin |
| MAPK | Mitogen-activated protein kinase | hUM-MSC | Human umbilical cord derived MSC |
| MEK/ERK | Kinase extracellular signaling regulation pathway | VEGF | Vascular endothelial growth factor |
| MHC | Major histocompatibility complex | VPS | Vascular protein sorting-associated protein |
| miRNA | MicroRNA | VTA1 | Vacuolar protein sorting-associated protein |
| MITF | Melanocyte including transcription factor | WD | Wallerian degeneration |
| MM | Multiple myeloma | WHO | World Health Organization |
| MRI | Magnetic resonance imaging | Wlds | Wallerian degeneration slow |
| mRNA | Messenger RNA | μs | Microseconds |
| MSCs | Mesenchymal stromal cells | µm | Micrometers |
| MVB | Multivesicular bodies | 2D | Two-dimensional |
| NA | Nicotinic acid | 3D | Three-dimensional |
| Na+ | Sodium |
References
- Jakob, M.O.; Kofoed-Branzk, M.; Deshpande, D.; Murugan, S.; Klose, C.S.N. An Integrated View on Neuronal Subsets in the Peripheral Nervous System and Their Role in Immunoregulation. Front. Immunol. 2021, 12, 679055. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.-L.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef]
- Butler, S.J.; Bronner, M.E. From classical to current: Analyzing peripheral nervous system and spinal cord lineage and fate. Dev. Biol. 2015, 398, 135–146. [Google Scholar] [CrossRef]
- Jortner, B.S. Preparation and Analysis of the Peripheral Nervous System. Toxicol. Pathol. 2010, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- He, C.-W.; Liao, C.-P.; Pan, C.-L. Wnt signalling in the development of axon, dendrites and synapses. Open Biol. 2018, 8, 180116. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.; Tadi, P. Histology, Schwann Cells. In BTI—StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Cha, J.Y.; Maan, Z.N.; Duscher, D.; Jew, O.S.; Siemers, F.; van Schoonhoven, J. The Role of Current Techniques and Concepts in Peripheral Nerve Repair. Plast. Surg. Int. 2016, 2016, 4175293. [Google Scholar] [CrossRef] [PubMed]
- Catala, M.; Kubis, N. Chapter 3—Gross anatomy and development of the peripheral nervous system. In Handbook of Clinical Neurology; Said, G., Krarup, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 29–41. [Google Scholar]
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef]
- Lehmann, H.C.; Wunderlich, G.; Fink, G.R.; Sommer, C. Diagnosis of peripheral neuropathy. Neurol. Res. Pract. 2020, 2, 20. [Google Scholar] [CrossRef]
- Hanewinckel, R.; Drenthen, J.; van Oijen, M.; Hofman, A.; van Doorn, P.A.; Ikram, M.A. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology 2016, 87, 1892–1898. [Google Scholar] [CrossRef]
- Hanewinckel, R.; van Oijen, M.; Ikram, M.A.; van Doorn, P.A. The epidemiology and risk factors of chronic polyneuropathy. Eur. J. Epidemiol. 2016, 31, 5–20. [Google Scholar] [CrossRef]
- Kneis, S.; Wehrle, A.; Müller, J.; Maurer, C.; Ihorst, G.; Gollhofer, A.; Bertz, H. It’s never too late—Balance and endurance training improves functional performance, quality of life, and alleviates neuropathic symptoms in cancer survivors suffering from chemotherapy-induced peripheral neuropathy: Results of a randomized controlled trial. BMC Cancer 2019, 19, 414. [Google Scholar] [CrossRef]
- Zakin, E.; Abrams, R.; Simpson, D.M. Diabetic Neuropathy. Semin. Neurol. 2019, 39, 560–569. [Google Scholar] [CrossRef]
- Nesbit, S.A.; Sharma, R.; Waldfogel, J.M.; Zhang, A.; Bennett, W.L.; Yeh, H.-C.; Chelladurai, Y.; Feldman, D.; Robinson, K.A.; Dy, S.M. Non-pharmacologic treatments for symptoms of diabetic peripheral neuropathy: A systematic review. Curr. Med Res. Opin. 2019, 35, 15–25. [Google Scholar] [CrossRef]
- Khdour, M.R. Treatment of diabetic peripheral neuropathy: A review. J. Pharm. Pharmacol. 2020, 72, 863–872. [Google Scholar] [CrossRef]
- Reeves, N.D.; Orlando, G.; Brown, S.J. Sensory-Motor Mechanisms Increasing Falls Risk in Diabetic Peripheral Neuropathy. Medicina 2021, 57, 457. [Google Scholar] [CrossRef]
- Staszel, J.P.; Miknevich, M. Not Always as It Seems: A Case of Ascending Paralysis. Am. J. Phys. Med. Rehabil. 2020, 99, e32–e34. [Google Scholar] [CrossRef]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef]
- Stassart, R.M.; Woodhoo, A. Axo-glial interaction in the injured PNS. Dev. Neurobiol. 2021, 81, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Jiang, D.; Liu, W.; Li, H.; Li, Z. Urine-derived Stem Cells, A New Source of Seed Cells for Tissue Engineering. Curr. Stem Cell Res. Ther. 2016, 11, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Powell, R.; Phillips, J.B.; Haastert-Talini, K. Perspective on Schwann Cells Derived from Induced Pluripotent Stem Cells in Peripheral Nerve Tissue Engineering. Cells 2020, 9, 2497. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Gewandter, J.S.; Faber, C.G.; Gibbons, C.; Haroutounian, S.; Lauria, G.; Levine, T.; Malik, R.A.; Singleton, J.R.; Smith, A.G.; et al. Idiopathic distal sensory polyneuropathy. Neurology 2020, 95, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Said, G. Diabetic neuropathy—A review. Nat. Clin. Pract. Neurol. 2007, 3, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Maser, R.E.; Mitchell, B.D.; Freeman, R. Diabetic Autonomic Neuropathy. Diabetes Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, H.; Osonoi, S. Pathogenesis and Molecular Treatment Strategies of Diabetic Neuropathy Collateral Glucose-Utilizing Pathways in Diabetic Polyneuropathy. Int. J. Mol. Sci. 2020, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Raza, C.; Riaz, H.A.; Anjum, R.; Shakeel, N.U.A. Repair strategies for injured peripheral nerve: Review. Life Sci. 2020, 243, 117308. [Google Scholar] [CrossRef] [PubMed]
- Hogg, P.J.; McLachlan, E.M. Blood vessels and nerves: Together or not? Lancet 2002, 360, 1714. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.-J. Sources and implications of NADH/NAD(+) redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. 2016, 9, 145–153. [Google Scholar]
- Casem, M.L. Chapter 11—Cell Metabolism. In Case Studies in Cell Biology; Casem, M.L., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 263–281. [Google Scholar]
- Bouché, C.; Serdy, S.; Kahn, C.R.; Goldfine, A.B. The Cellular Fate of Glucose and Its Relevance in Type 2 Diabetes. Endocr. Rev. 2004, 25, 807–830. [Google Scholar] [CrossRef]
- Kopp, W.A.-O. How Western Diet and Lifestyle Drive The Pandemic of Obesity and Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Huang, T.-J.; Price, S.A.; Chilton, L.; Calcutt, N.A.; Tomlinson, D.R.; Verkhratsky, A.; Fernyhough, P. Insulin Prevents Depolarization of the Mitochondrial Inner Membrane in Sensory Neurons of Type 1 Diabetic Rats in the Presence of Sustained Hyperglycemia. Diabetes 2003, 52, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.W.; Golovoy, D.; Vincent, A.M.; Mahendru, P.; Olzmann, J.A.; Mentzer, A.; Feldman, E.L. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002, 16, 1738–1748. [Google Scholar] [CrossRef]
- Samakidou, G.; Eleftheriadou, I.; Tentolouris, A.; Papanas, N.; Tentolouris, N. Rare diabetic neuropathies: It is not only distal symmetrical polyneuropathy. Diabetes Res. Clin. Pract. 2021, 177, 108932. [Google Scholar] [CrossRef] [PubMed]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.; Frank, B.; Marshall, A.; Khalil, R.S.; Ponirakis, G.; Petropoulos, I.N.; Cuthbertson, D.J.; Malik, R.A.; Alam, U. Early Detection of Diabetic Peripheral Neuropathy: A Focus on Small Nerve Fibres. Diagnostics 2021, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Mandeville, R.; Wali, A.; Park, C.; Groessl, E.; Walker, F.O.; Cartwright, M.S. Cost-effectiveness of neuromuscular ultrasound in focal neuropathies. Neurology 2019, 92, e2674–e2678. [Google Scholar] [CrossRef]
- Bansal, V.; Kalita, J.; Misra, U.K. Diabetic neuropathy. Postgrad. Med. J. 2006, 82, 95. [Google Scholar] [CrossRef]
- Sharma, D.; Jaggi, A.S.; Bali, A. Clinical evidence and mechanisms of growth factors in idiopathic and diabetes-induced carpal tunnel syndrome. Eur. J. Pharmacol. 2018, 837, 156–163. [Google Scholar] [CrossRef]
- Said, G. Focal and multifocal diabetic neuropathies. Arq. De Neuro-Psiquiatr. 2007, 65, 1272–1278. [Google Scholar] [CrossRef]
- Watson, J.C.; Dyck, P.J.B. Peripheral Neuropathy: A Practical Approach to Diagnosis and Symptom Management. Mayo Clin. Proc. 2015, 90, 940–951. [Google Scholar] [CrossRef]
- Singh, J.; Aslam, A.; Rajbhandari, S. Diagnosis and Treatment of a Typical Painful Neuropathy Due to “Insulin Neuritis” in Patients with Diabetes. Open Access J. Neurol. Neurosurg. 2018, 9, 555759. [Google Scholar] [CrossRef]
- Kim, S.H.; Baek, C.-O.; Lee, K.A.; Jin, H.Y.; Baek, H.S.; Park, T.S. Neuropathic truncal pain in patients with type 2 diabetes mellitus relieved by topical cream and nerve block. Korean J. Intern. Med. 2014, 29, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Cascella, M. Multifocal Motor Neuropathy. In BTI—StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Spallone, V.A.-O.X. Update on the Impact, Diagnosis and Management of Cardiovascular Autonomic Neuropathy in Diabetes: What Is Defined, What Is New, and What Is Unmet. Diabetes Metab. J. 2019, 43, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Spallone, V.; Ziegler, D.; Freeman, R.; Bernardi, L.; Frontoni, S.; Pop-Busui, R.; Stevens, M.; Kempler, P.; Hilsted, J.; Tesfaye, S.; et al. Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes/Metab. Res. Rev. 2011, 27, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.K.; Inglis, J.T.; Madden, K.M.; Boyd, L.A. Brain and Body: A Review of Central Nervous System Contributions to Movement Impairments in Diabetes. Diabetes 2019, 69, 3–11. [Google Scholar] [CrossRef]
- Azhary, H.; Farooq, M.U.; Bhanushali, M.; Majid, A.; Kassab, M.Y. Peripheral neuropathy: Differential diagnosis and management. Am. Fam. Physician 2010, 81, 887–892. [Google Scholar]
- Zis, P.; Sarrigiannis, P.G.; Rao, D.G.; Hadjivassiliou, M. Gluten neuropathy: Prevalence of neuropathic pain and the role of gluten-free diet. J. Neurol. 2018, 265, 2231–2236. [Google Scholar] [CrossRef]
- Robinson, D.J.; Coons, M.; Haensel, H.; Vallis, M.; Yale, J.F. Diabetes and Mental Health. Can. J. Diabetes 2018, 42, S130–S141. [Google Scholar] [CrossRef]
- Garrett, C.; Doherty, A. Diabetes and mental health. Clin. Med. 2014, 14, 669. [Google Scholar] [CrossRef]
- Kiyani, M.; Yang, Z.; Charalambous, L.T.; Adil, S.M.; Lee, H.J.; Yang, S.; Pagadala, P.; Parente, B.; Spratt, S.E.; Lad, S.P. Painful diabetic peripheral neuropathy: Health care costs and complications from 2010 to 2015. Neurol. Clin. Pract. 2020, 10, 47–57. [Google Scholar] [CrossRef]
- O’Connor, A.B. Neuropathic Pain. Pharm. Econ. 2009, 27, 95–112. [Google Scholar] [CrossRef]
- Hex, N.; Bartlett, C.; Wright, D.; Taylor, M.; Varley, D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet. Med. 2012, 29, 855–862. [Google Scholar] [CrossRef]
- Chapman, D.; Foxcroft, R.; Dale-Harris, L.; Ronte, H.; Bidgoli, F.; Bellary, S. Insights for Care: The Healthcare Utilisation and Cost Impact of Managing Type 2 Diabetes-Associated Microvascular Complications. Diabetes Ther. 2019, 10, 575–585. [Google Scholar] [CrossRef]
- Sadosky, A.; Schaefer, C.; Mann, R.; Bergstrom, F.; Baik, R.; Parsons, B.; Nalamachu, S.; Nieshoff, E.; Stacey, B.R.; Anschel, A.; et al. Burden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: Results from a retrospective chart review and cross-sectional survey. Diabetes Metab. Syndr. Obes. 2013, 6, 79–92. [Google Scholar] [CrossRef]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Selvy, M.; Kerckhove, N.; Pereira, B.; Barreau, F.; Nguyen, D.; Busserolles, J.; Giraudet, F.; Cabrespine, A.; Chaleteix, C.; Soubrier, M.; et al. Prevalence of Chemotherapy-Induced Peripheral Neuropathy in Multiple Myeloma Patients and its Impact on Quality of Life: A Single Center Cross-Sectional Study. Front. Pharmacol. 2021, 12, 637593. [Google Scholar] [CrossRef] [PubMed]
- Pike, C.T.; Birnbaum, H.G.; Muehlenbein, C.E.; Pohl, G.M.; Natale, R.B. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother. Res. Pract. 2012, 2012, 913848. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Almohammadi, A.; Alqarni, A.; Alraddadi, R.; Alzahrani, F. Assessment of Patients’ Knowledge in Managing Side Effects of Chemotherapy: Case of King Abdul-Aziz University Hospital. J. Cancer Educ. 2019, 35, 334–338. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Behranvand, N.; Nasri, F.; Emameh, R.Z.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef]
- Chortkoff, B.; Stenehjem, D. 38—Chemotherapy, Immunosuppression, and Anesthesia. In Pharmacology and Physiology for Anesthesia, 2nd ed.; Hemmings, H.C., Egan, T.D., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 753–768. [Google Scholar]
- Fallon, M.T. Neuropathic pain in cancer. Br. J. Anaesth. 2013, 111, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Walton, R.; Kataria, S.P. Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Recommendations, and New Trends. Cancer Treat. Res. Commun. 2021, 26, 100278. [Google Scholar] [CrossRef] [PubMed]
- Nurgalieva, Z.; Liu, C.-C.; Du, X.L. Chemotherapy use and risk of bone marrow suppression in a large population-based cohort of older women with breast and ovarian cancer. Med. Oncol. 2011, 28, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, G.; Monfrini, M.; Scuteri, A. Axonal Transport Impairment in Chemotherapy-Induced Peripheral Neuropathy. Toxics 2015, 3, 322–341. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Kavallaris, M.; McCarroll, J.A. Microtubules and Their Role in Cellular Stress in Cancer. Front. Oncol. 2014, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, F.; Taglieri, L.; Salerno, G.; Lanza, R.; Scarpa, S. Taxane induced neuropathy in patients affected by breast cancer: Literature review. Crit. Rev. Oncol. 2015, 96, 34–45. [Google Scholar] [CrossRef]
- Rizo, J. Mechanism of neurotransmitter release coming into focus. Protein Sci. 2018, 27, 1364–1391. [Google Scholar] [CrossRef]
- Costanzi, S.; Machado, J.-H.; Mitchell, M. Nerve Agents: What They Are, How They Work, How to Counter Them. ACS Chem. Neurosci. 2018, 9, 873–885. [Google Scholar] [CrossRef]
- Südhof, T.C. Calcium Control of Neurotransmitter Release. Cold Spring Harb. Perspect. Biol. 2012, 4, a011353. [Google Scholar] [CrossRef]
- Lipscombe, D.; Toro, C.P. Chapter 13—Biophysics of Voltage-Gated Ion Channels. In From Molecules to Networks, 3rd ed.; Byrne, J.H., Heidelberger, R., Waxham, M.N., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 377–407. [Google Scholar]
- Hyman, S.E. Neurotransmitters. Curr. Biol. 2005, 15, R154–R158. [Google Scholar] [CrossRef]
- Lines, J.; Martin, E.D.; Kofuji, P.; Aguilar, J.; Araque, A. Astrocytes modulate sensory-evoked neuronal network activity. Nat. Commun. 2020, 11, 3689. [Google Scholar] [CrossRef]
- Hammond, E.A.; Pitz, M.; Shay, B. Neuropathic Pain in Taxane-Induced Peripheral Neuropathy: Evidence for Exercise in Treatment. Neurorehabilit. Neural Repair 2019, 33, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, A.; Meregalli, C.; Rodriguez-Menendez, V.; Nicolini, G. Chemotherapy-Induced Peripheral Neuropathy and Changes in Cytoskeleton. Int. J. Mol. Sci. 2019, 20, 2287. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Sunada, Y. Mechanism of taxane neurotoxicity. Breast Cancer 2004, 11, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef]
- Chen, J.; Ye, C.; Wan, C.; Li, G.; Peng, L.; Peng, Y.; Fang, R. The Roles of c-Jun N-Terminal Kinase (JNK) in Infectious Diseases. Int. J. Mol. Sci. 2021, 22, 9640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Bubici, C.; Papa, S. JNK signalling in cancer: In need of new, smarter therapeutic targets. Br. J. Pharmacol. 2013, 171, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Li, Y.; Segal, R.A. A Mechanistic Understanding of Axon Degeneration in Chemotherapy-Induced Peripheral Neuropathy. Front. Neurosci. 2017, 11, 481. [Google Scholar] [CrossRef]
- Tian, N.; Hanson, K.A.; Canty, A.J.; Vickers, J.C.; King, A.E. Microtubule-dependent processes precede pathological calcium influx in excitotoxin-induced axon degeneration. J. Neurochem. 2020, 152, 542–555. [Google Scholar] [CrossRef]
- Feinberg, J.H.; Nadler, S.F.; Krivickas, L.S. Peripheral nerve injuries in the athlete. Sports Med. 1997, 24, 385–408. [Google Scholar] [CrossRef] [PubMed]
- Tosti, R.; Rossy, W.; Sanchez, A.; Lee, S.G. Burners, Stingers, and Other Brachial Plexus Injuries in the Contact Athlete. Oper. Tech. Sports Med. 2016, 24, 273–277. [Google Scholar] [CrossRef]
- Campbell, W.W. Evaluation and management of peripheral nerve injury. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral nerve injury, scarring, and recovery. Connect. Tissue Res. 2019, 60, 3–9. [Google Scholar] [CrossRef]
- Barral, J.-P.; Croibier, A. Chapter 1—Some preliminary thoughts. In Manual Therapy for the Cranial Nerves; Barral, J.-P., Croibier, A., Eds.; Churchill Livingstone: Edinburgh, UK, 2009; pp. 1–5. [Google Scholar]
- Liu, Q.; Wang, X.; Yi, S. Pathophysiological Changes of Physical Barriers of Peripheral Nerves After Injury. Front. Neurosci. 2018, 12, 597. [Google Scholar] [CrossRef]
- Reina, M.A.; Boezaart, A.P.; Tubbs, R.S.; Zasimovich, Y.; Fernández-Domínguez, M.; Fernández, P.; Sala-Blanch, X. Another (Internal) Epineurium: Beyond the Anatomical Barriers of Nerves. Clin. Anat. 2020, 33, 199–206. [Google Scholar] [CrossRef]
- Grinsell, D.; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. BioMed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef]
- Chaney, B.; Nadi, M. Axonotmesis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef]
- Rasband, M.N.; Peles, E. Mechanisms of node of Ranvier assembly. Nat. Rev. Neurosci. 2021, 22, 7–20. [Google Scholar] [CrossRef]
- Arancibia-Carcamo, I.L.; Attwell, D. The node of Ranvier in CNS pathology. Acta Neuropathol. 2014, 128, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R. Physiology of conduction block in multifocal motor neuropathy and other demyelinating neuropathies. Muscle Nerve 2003, 27, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Debanne, D.; Campanac, E.; Bialowas, A.; Carlier, E.; Alcaraz, G. Axon Physiology. Physiol. Rev. 2011, 91, 555–602. [Google Scholar] [CrossRef]
- Sunderland, S.S. The anatomy and physiology of nerve injury. Muscle Nerve 1990, 13, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Seddon, H.J. THREE TYPES OF NERVE INJURY. Brain 1943, 66, 237–288. [Google Scholar] [CrossRef]
- Seddon, H.J. A Classification of Nerve Injuries. Br. Med. J. 1942, 2, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Andrei, M.; Ioana, M.R.; Mircea, E.D. Underlying histopathology of peripheral nerve injury and the classical nerve repair techniques. Romanian Neurosurg. 2019, 33, 17–22. [Google Scholar] [CrossRef]
- Lee, D.-H. Clinical Efficacy of Electroneurography in Acute Facial Paralysis. J. Audiol. Otol. 2016, 20, 8–12. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Chandrasekaran, S.; Davis, J.; Bersch, I.; Goldberg, G. Electrical stimulation and denervated muscles after spinal cord injury. Neural Regen. Res. 2020, 15, 1397–1407. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef]
- Bhandari, P.S. Management of peripheral nerve injury. J. Clin. Orthop. Trauma 2019, 10, 862–866. [Google Scholar] [CrossRef]
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus 2004, 16, E1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jiang, M.; Fang, Y. The Drama of Wallerian Degeneration: The Cast, Crew, and Script. Annu. Rev. Genet. 2021, 55, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Straube, A. Intracellular cargo transport by kinesin-3 motors. Biochemistry 2017, 82, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Pillai, M.M.; Sathishkumar, G.; Houshyar, S.; Senthilkumar, R.; Quigley, A.F.; Shanthakumari, S.; Padhye, R.; Bhattacharyya, A. Nanocomposite-Coated Silk-Based Artificial Conduits: The Influence of Structures on Regeneration of the Peripheral Nerve. ACS Appl. Bio Mater. 2020, 3, 4454–4464. [Google Scholar] [CrossRef]
- Garcin, C.; Straube, A. Microtubules in cell migration. Essays Biochem. 2019, 63, 509–520. [Google Scholar]
- Bandyopadhyay, D.; Cyphersmith, A.; Zapata, J.A.; Kim, Y.J.; Payne, C.K. Lysosome Transport as a Function of Lysosome Diameter. PLoS ONE 2014, 9, e86847. [Google Scholar] [CrossRef]
- Guedes-Dias, P.; Holzbaur, E.L.F. Axonal transport: Driving synaptic function. Science 2019, 366, eaaw9997. [Google Scholar] [CrossRef]
- Peters, O.M.; Weiss, A.; Metterville, J.; Song, L.; Logan, R.; Smith, G.A.; Schwarzschild, M.A.; Mueller, C.; Brown, R.H.; Freeman, M. Genetic diversity of axon degenerative mechanisms in models of Parkinson’s disease. Neurobiol. Dis. 2021, 155, 105368. [Google Scholar] [CrossRef]
- Coleman, M.P.; Freeman, M.R. Wallerian Degeneration, WldS, and Nmnat. Annu. Rev. Neurosci. 2010, 33, 245–267. [Google Scholar] [CrossRef]
- Freeman, M.R. Signaling mechanisms regulating Wallerian degeneration. Curr. Opin. Neurobiol. 2014, 27, 224–231. [Google Scholar] [CrossRef]
- Liu, H.-W.; Smith, C.B.; Schmidt, M.S.; Cambronne, X.A.; Cohen, M.S.; Migaud, M.E.; Brenner, C.; Goodman, R.H. Pharmacological bypass of NAD+ salvage pathway protects neurons from chemotherapy-induced degeneration. Proc. Natl. Acad. Sci. USA 2018, 115, 10654–10659. [Google Scholar] [CrossRef] [PubMed]
- Brazill, J.M.; Li, C.; Zhu, Y.; Zhai, R.G. NMNAT: It’s an NAD+ synthase… It’s a chaperone… It’s a neuroprotector. Curr. Opin. Genet. Dev. 2017, 44, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2020, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Sheng, R.; Qin, Z.-H. Pharmacology and Potential Implications of Nicotinamide Adenine Dinucleotide Precursors. Aging Dis. 2021, 12, 1879–1897. [Google Scholar] [CrossRef] [PubMed]
- Giroud-Gerbetant, J.; Joffraud, M.; Giner, M.P.; Cercillieux, A.; Bartova, S.; Makarov, M.V.; Zapata-Pérez, R.; Sánchez-García, J.L.; Houtkooper, R.H.; Migaud, M.E.; et al. A reduced form of nicotinamide riboside defines a new path for NAD+ biosynthesis and acts as an orally bioavailable NAD+ precursor. Mol. Metab. 2019, 30, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Harder, J.M.; Cardozo, B.H.; Foxworth, N.E.; John, S.W.M. Nicotinamide treatment robustly protects from inherited mouse glaucoma. Commun. Integr. Biol. 2018, 11, e1356956. [Google Scholar] [CrossRef]
- Kennedy, B.E.; Sharif, T.; Martell, E.; Dai, C.; Kim, Y.; Lee, P.W.; Gujar, S.A. NAD+ salvage pathway in cancer metabolism and therapy. Pharmacol. Res. 2016, 114, 274–283. [Google Scholar] [CrossRef]
- Johnson, S.; Imai, S.I. NAD (+) biosynthesis, aging, and disease. F1000Research 2018, 7, 132. [Google Scholar] [CrossRef]
- Bignon, Y.; Rinaldi, A.; Nadour, Z.; Poindessous, V.; Nemazanyy, I.; Lenoir, O.; Fohlen, B.; Weill-Raynal, P.; Hertig, A.; Karras, A.; et al. Cell stress response impairs de novo NAD+ biosynthesis in the kidney. J. Clin. Investig. 2022, 7, e153019. [Google Scholar] [CrossRef]
- Sasaki, Y.; Nakagawa, T.; Mao, X.; DiAntonio, A.; Milbrandt, J. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD+ depletion. eLife 2016, 5, e19749. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.J.; Summers, D.W.; Sasaki, Y.; Brace, E.; Milbrandt, J.; DiAntonio, A. MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. eLife 2017, 6, e22540. [Google Scholar] [CrossRef] [PubMed]
- Rigby, M.J.; Gomez, T.M.; Puglielli, L. Glial Cell-Axonal Growth Cone Interactions in Neurodevelopment and Regeneration. Front. Neurosci. 2020, 14, 203. [Google Scholar] [CrossRef]
- Ullah, R.; Yin, Q.; Snell, A.H.; Wan, L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin. Cancer Biol. 2022, 85, 123–154. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef] [PubMed]
- Rios, R.; Jablonka-Shariff, A.; Broberg, C.; Snyder-Warwick, A.K. Macrophage roles in peripheral nervous system injury and pathology: Allies in neuromuscular junction recovery. Mol. Cell. Neurosci. 2021, 111, 103590. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Duan, Z.; Kang, J.; Chen, K.; Li, G.; Weng, C.; Zhang, D.; Zhang, L.; Wang, J.; et al. The effects of the M2a macrophage-induced axonal regeneration of neurons by arginase 1. Biosci. Rep. 2020, 40, BSR20193031. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Piao, X.; Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Kawabuchi, M. Myelin phagocytosis by macrophages and nonmacrophages during Wallerian degeneration. Microsc. Res. Tech. 2002, 57, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Cattin, A.-L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Calavia, N.G.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Wahyuningtyas, R.; Aui, S.; Chang, K. Autocrine VEGF signalling on M2 macrophages regulates PD-L1 expression for immunomodulation of T cells. J. Cell. Mol. Med. 2019, 23, 1257–1267. [Google Scholar] [CrossRef]
- de Ruiter, G.C.; Spinner, R.J.; Verhaagen, J.; Malessy, M.J. Misdirection and guidance of regenerating axons after experimental nerve injury and repair. J. Neurosurg. 2014, 120, 493–501. [Google Scholar] [CrossRef]
- Juel, V.C. Evaluation of Neuromuscular Junction Disorders in the Electromyography Laboratory. Neurol. Clin. 2012, 30, 621–639. [Google Scholar] [CrossRef]
- Jimsheleishvili, S.; Marwaha, K.; Sherman, A.L. Physiology, Neuromuscular Transmission. In BTI—StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sommer, C.; Cruccu, G. Topical Treatment of Peripheral Neuropathic Pain: Applying the Evidence. J. Pain Symptom Manag. 2016, 53, 614–629. [Google Scholar] [CrossRef]
- Dreibati, B.; Lavet, C.; Pinti, A.; Poumarat, G. Influence of electrical stimulation frequency on skeletal muscle force and fatigue. Ann. Phys. Rehabil. Med. 2010, 53, 266–277. [Google Scholar] [CrossRef]
- Gibson, W.; Wand, B.M.; O’Connell, N.E. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 9, CD011976. [Google Scholar] [PubMed]
- Kulikova, N.; Khalilovich, A.-Z.M.; Konchugova, T.; Rachin, A.; Chkheidze, T.; Kulchitskaya, D.; Anatoliy, F.; Sanina, N.P.; Ivanova, E. Analgesic effects of high-frequency and low-frequency TENS currents in patients with distal neuropathy. Eur. J. Transl. Myol. 2022, 32, 10687. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, M.; Minenko, I.A.; Kulikova, N.G.; Alade, M.; Petrova, M.M.; Pronina, E.A.; Romanova, I.V.; Narodova, E.A.; Nasyrova, R.F.; Shnayder, N.A. Clinical Experience of High Frequency and Low Frequency TENS in Treatment of Diabetic Neuropathic Pain in Russia. Healthcare 2022, 10, 250. [Google Scholar] [CrossRef]
- Baptista, A.F.; Gomes, J.R.; Oliveira, J.T.; Santos, S.M.; Vannier-Santos, M.A.; Martinez, A.M. High- and low-frequency transcutaneous electrical nerve stimulation delay sciatic nerve regeneration after crush lesion in the mouse. J. Peripher. Nerv. Syst. 2008, 13, 71–80. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Magnaghi, V.; Martini, L. Steroid metabolism and effects in central and peripheral glial cells. J. Neurobiol. 1999, 40, 471–483. [Google Scholar] [CrossRef]
- Coronel, M.F.; Labombarda, F.; González, S.L. Neuroactive steroids, nociception and neuropathic pain: A flashback to go forward. Steroids 2016, 110, 77–87. [Google Scholar] [CrossRef]
- Rupprecht, R. Neuroactive steroids: Mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 2003, 28, 139–168. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, S.; Di Giannuario, A.; Di Giovannandrea, R.; Marzoli, F.; Piccaro, G.; Minosi, P.; Aloisi, A.M. Gender differences in pain and its relief. Ann. Dell’istituto Super. Di Sanita 2016, 52, 184–189. [Google Scholar]
- Calabrese, D.; Giatti, S.; Romano, S.; Porretta-Serapiglia, C.; Bianchi, R.; Milanese, M.; Bonanno, G.; Caruso, D.; Viviani, B.; Gardoni, F.; et al. Diabetic neuropathic pain: A role for testosterone metabolites. J. Endocrinol. 2014, 221, 1–13. [Google Scholar] [CrossRef]
- Piao, Y.; Gwon, D.H.; Kang, D.-W.; Hwang, T.W.; Shin, N.; Kwon, H.H.; Shin, H.J.; Yin, Y.; Kim, J.-J.; Hong, J.; et al. TLR4-mediated autophagic impairment contributes to neuropathic pain in chronic constriction injury mice. Mol. Brain 2018, 11, 11. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Sindrup, S.H.; Jensen, T.S. The evidence for pharmacological treatment of neuropathic pain. Pain 2010, 150, 573–581. [Google Scholar] [CrossRef]
- Yang, K.; Wang, Y.; Li, Y.-W.; Chen, Y.-G.; Xing, N.; Lin, H.-B.; Zhou, P.; Yu, X.-P. Progress in the treatment of diabetic peripheral neuropathy. Biomed. Pharmacother. 2022, 148, 112717. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.Y.; Kim, Y.T. Food-Derived Natural Compounds for Pain Relief in Neuropathic Pain. BioMed Res. Int. 2016, 2016, 7917528. [Google Scholar] [CrossRef] [PubMed]
- Keswani, S.C.; Chander, B.; Hasan, C.; Griffin, J.W.; McArthur, J.C.; Hoke, A. FK506 is neuroprotective in a model of antiretroviral toxic neuropathy. Ann. Neurol. 2003, 53, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zuber, M.; Donnerer, J. Effect of FK506 on Neurotransmitter Content and Expression of GAP-43 in Neurotoxin-Lesioned Peripheral Sensory and Sympathetic Neurons. Pharmacology 2002, 66, 44–50. [Google Scholar] [CrossRef]
- Su, Y.-C.; Shen, Y.-P.; Li, T.-Y.; Ho, T.-Y.; Chen, L.-C.; Wu, Y.-T. The Efficacy of Hyaluronic Acid for Carpal Tunnel Syndrome: A Randomized Double-Blind Clinical Trial. Pain Med. 2021, 22, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Jou, I.; Wu, T.; Hsu, C.; Yang, C.; Huang, J.; Tu, Y.; Lee, J.; Su, F.; Kuo, Y. High molecular weight form of hyaluronic acid reduces neuroinflammatory response in injured sciatic nerve via the intracellular domain of CD44. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Julian, T.; Syeed, R.; Glascow, N.; Angelopoulou, E.; Zis, P. B12 as a Treatment for Peripheral Neuropathic Pain: A Systematic Review. Nutrients 2020, 12, 2221. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- Kang, N.-U.; Lee, S.-J.; Gwak, S.-J. Fabrication Techniques of Nerve Guidance Conduits for Nerve Regeneration. Yonsei Med. J. 2022, 63, 114–123. [Google Scholar] [CrossRef]
- Costa, F.; Silva, R.; Boccaccini, A.R. 7—Fibrous protein-based biomaterials (silk, keratin, elastin, and resilin proteins) for tissue regeneration and repair. In Peptides and Proteins As Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Woodhead Publishing: Thorston, UK, 2018; pp. 175–204. [Google Scholar]
- Nelson, D.W.; Gilbert, R.J. Extracellular Matrix-Mimetic Hydrogels for Treating Neural Tissue Injury: A Focus on Fibrin, Hyaluronic Acid, and Elastin-Like Polypeptide Hydrogels. Adv. Healthc. Mater. 2021, 10, 2101329. [Google Scholar] [CrossRef]
- Chang, W.; Shah, M.B.; Zhou, G.; Walsh, K.; Rudraiah, S.; Kumbar, S.G.; Yu, X. Polymeric nanofibrous nerve conduits coupled with laminin for peripheral nerve regeneration. Biomed. Mater. 2020, 15, 035003. [Google Scholar] [CrossRef]
- Neal, R.A.; Tholpady, S.S.; Foley, P.L.; Swami, N.; Ogle, R.C.; Botchwey, E.A. Alignment and composition of laminin–polycaprolactone nanofiber blends enhance peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2012, 100A, 406–423. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.R.; Chang, W.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M.; Kohn, J. Engineering Silk Fibroin-Based Nerve Conduit with Neurotrophic Factors for Proximal Protection after Peripheral Nerve Injury. Adv. Health Mater. 2021, 10, e2000753. [Google Scholar] [CrossRef] [PubMed]
- Magaz, A.; Faroni, A.; Gough, J.E.; Reid, A.J.; Li, X.; Blaker, J.J. Bioactive Silk-Based Nerve Guidance Conduits for Augmenting Peripheral Nerve Repair. Adv. Health Mater. 2018, 7, e1800308. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Sensharma, P.; Madhumathi, G.; Jayant, R.D.; Jaiswal, A.K. Biomaterials and cells for neural tissue engineering: Current choices. Mater. Sci. Eng. C 2017, 77, 1302–1315. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Nune, M.; Bhat, M.; Nagarajan, A. Design of ECM Functionalized Polycaprolactone Aligned Nanofibers for Peripheral Nerve Tissue Engineering. J. Med. Biol. Eng. 2022, 42, 147–156. [Google Scholar] [CrossRef]
- Soucy, J.; Sani, E.S.; Portillo-Lara, R.; Diaz, D.; Dias, F.; Weiss, A.S.; Koppes, A.; Koppes, R.A.; Annabi, N. Photocrosslinkable Gelatin/Tropoelastin Hydrogel Adhesives for Peripheral Nerve Repair. Tissue Eng. Part A 2018, 24, 1393–1405. [Google Scholar] [CrossRef]
- Rebowe, R.; Rogers, A.; Yang, X.; Kundu, S.C.; Smith, T.L.; Li, Z. Nerve Repair with Nerve Conduits: Problems, Solutions, and Future Directions. J. Hand Microsurg. 2018, 10, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, K.; Wang, Y.; Zhao, G.; Jiang, J. Stem Cells in the Treatment of Neuropathic Pain: Research Progress of Mechanism. Stem Cells Int. 2020, 2020, 8861251. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Chen, J.; Li, Y.-Q.; Yang, J.; Hsu, C.-C.; Cao, T.-T. A hyaluronic acid granular hydrogel nerve guidance conduit promotes regeneration and functional recovery of injured sciatic nerves in rats. Neural Regen. Res. 2023, 18, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Pabari, A.; Lloyd-Hughes, H.; Seifalian, A.M.; Mosahebi, A. Nerve conduits for peripheral nerve surgery. Plast. Reconstr. Surg. 2014, 133, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, C.A.; Grochmal, J.; Kung, T.A.; Cederna, P.S.; Midha, R.; Kemp, S.W.P. Stem-cell–based therapies to enhance peripheral nerve regeneration. Muscle Nerve 2020, 61, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Sobhani, A.; Khanlarkhani, N.; Baazm, M.; Mohammadzadeh, F.; Najafi, A.; Mehdinejadiani, S.; Sargolzaei Aval, F. Multipotent Stem Cell and Current Application. Acta Medica Iran. 2017, 55, 6–23. [Google Scholar]
- Tatullo, M.; Gargiulo, I.C.; Dipalma, G.; Ballini, A.; Inchingolo, A.M.; Paduanelli, G.; Nguyen, C.đ.K.; Inchingolo, A.D.; Makeeva, I.; Scacco, S.; et al. 17—Stem cells and regenerative medicine. In Translational Systems Medicine and Oral Disease; Sonis, S.T., Villa, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 387–407. [Google Scholar]
- Poliwoda, S.; Noor, N.; Downs, E.; Schaaf, A.; Cantwell, A.; Ganti, L.; Kaye, A.D.; Mosel, L.I.; Carroll, C.B.; Viswanath, O.; et al. Stem cells: A comprehensive review of origins and emerging clinical roles in medical practice. Orthop. Rev. 2022, 14, 37498. [Google Scholar] [CrossRef]
- Lu, V.; Roy, I.J.; Teitell, M.A. Nutrients in the fate of pluripotent stem cells. Cell Metab. 2021, 33, 2108–2121. [Google Scholar] [CrossRef]
- Kiecker, C.; Bates, T.; Bell, E. Molecular specification of germ layers in vertebrate embryos. Cell. Mol. Life Sci. 2016, 73, 923–947. [Google Scholar] [CrossRef]
- Guasti, L.; New, S.E.; Hadjidemetriou, I.; Palmiero, M.; Ferretti, P. Plasticity of human adipose-derived stem cells—Relevance to tissue repair. Int. J. Dev. Biol. 2018, 62, 431–439. [Google Scholar] [CrossRef]
- Guo, N.-N.; Liu, L.-P.; Zheng, Y.-W.; Li, Y.-M. Inducing human induced pluripotent stem cell differentiation through embryoid bodies: A practical and stable approach. World J. Stem Cells 2020, 12, 25–34. [Google Scholar] [CrossRef]
- Priester, C.; MacDonald, A.; Dhar, M.; Bow, A. Examining the Characteristics and Applications of Mesenchymal, Induced Pluripotent, and Embryonic Stem Cells for Tissue Engineering Approaches across the Germ Layers. Pharmaceuticals 2020, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Saffari, T.; Saffari, S.; Vyas, K.; Mardini, S. Role of adipose tissue grafting and adipose-derived stem cells in peripheral nerve surgery. Neural Regen. Res. 2022, 17, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ferrer, S.; Scadden, D.T.; Sánchez-Aguilera, A. Bone marrow stem cells: Current and emerging concepts. Ann. New York Acad. Sci. 2015, 1335, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Visweswaran, M.; Pohl, S.; Arfuso, F.; Newsholme, P.; Dilley, R.; Pervaiz, S.; Dharmarajan, A. Multi-lineage differentiation of mesenchymal stem cells—To Wnt, or not Wnt. Int. J. Biochem. Cell Biol. 2015, 68, 139–147. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, D.N.; Caviedes, P.; Mardones, R.; Minguell, J.J.; Vega-Letter, A.M.; Jofre, C.M. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLoS ONE 2019, 14, e0213032. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiao, Y.; Nie, W.; Lian, B.; Wang, B. In vitro proliferation and differentiation potential of bone marrow-derived mesenchymal stem cells from ovariectomized rats. Tissue Cell 2014, 46, 450–456. [Google Scholar] [CrossRef]
- Jang, S.; Cho, H.-H.; Cho, Y.-B.; Park, J.-S.; Jeong, H.-S. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010, 11, 25. [Google Scholar] [CrossRef]
- Mildmay-White, A.; Khan, W. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2017, 12, 484–492. [Google Scholar] [CrossRef]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Strategies and New Developments in the Generation of Patient-Specific Pluripotent Stem Cells. Cell Stem Cell 2007, 1, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Wilmut, I. The First Direct Reprogramming of Adult Human Fibroblasts. Cell Stem Cell 2007, 1, 593–594. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Chen, X.; Gong, S.; Yu, P.; Yau, S.; Su, Z.; Zhou, L.; Yu, J.; Pan, G.; Shi, L. Characteristic analyses of a neural differentiation model from iPSC-derived neuron according to morphology, physiology, and global gene expression pattern. Sci. Rep. 2017, 7, 12233. [Google Scholar] [CrossRef]
- Pajer, K.; Bellák, T.; Nógrádi, A. Stem Cell Secretome for Spinal Cord Repair: Is It More than Just a Random Baseline Set of Factors? Cells 2021, 10, 3214. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Xu, X.; Zhang, L.; Mao, F. Mesenchymal stem cell-derived exosome: The likely game-changer in stem cell research. Biocell 2022, 46, 1169–1172. [Google Scholar] [CrossRef]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, X.; Yu, X.; Wang, S.; Qiu, J.; Tang, W.; Li, L.; Liu, H.; Wang, Z.L. Self-Powered Electrical Stimulation for Enhancing Neural Differentiation of Mesenchymal Stem Cells on Graphene-Poly(3,4-ethylenedioxythiophene) Hybrid Microfibers. ACS Nano 2016, 10, 5086–5095. [Google Scholar] [CrossRef]
- Al-Massri, K.F.; Ahmed, L.A.; El-Abhar, H.S. Mesenchymal stem cells in chemotherapy-induced peripheral neuropathy: A new challenging approach that requires further investigations. J. Tissue Eng. Regen. Med. 2020, 14, 108–122. [Google Scholar] [CrossRef]
- Gama, K.B.; Santos, D.S.; Evangelista, A.F.; Silva, D.N.; De Alcântara, A.C.; Dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Conditioned Medium of Bone Marrow-Derived Mesenchymal Stromal Cells as a Therapeutic Approach to Neuropathic Pain: A Preclinical Evaluation. Stem Cells Int. 2018, 2018, 8179013. [Google Scholar] [CrossRef]
- Gao, L.; Xu, W.; Li, T.; Chen, J.; Shao, A.; Yan, F.; Chen, G. Stem Cell Therapy: A Promising Therapeutic Method for Intracerebral Hemorrhage. Cell Transplant. 2018, 27, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Xiang, E.; Han, B.; Zhang, Q.; Rao, W.; Wang, Z.; Chang, C.; Zhang, Y.; Tu, C.; Li, C.; Wu, D. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res. Ther. 2020, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Xu, Y.; Xiong, X.; Yin, C.; Lei, S.; Cheng, X. The bone marrow-derived mesenchymal stem cells (BMSCs) alleviate diabetic peripheral neuropathy induced by STZ via activating GSK-3β/β-catenin signaling pathway. Environ. Toxicol. Pharmacol. 2020, 79, 103432. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Cheng, Y.; Zhang, L.; Yin, Y.; Xue, J.; Li, B.; Gong, Z.; Gao, J.; Mu, Y. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res. Ther. 2019, 10, 333. [Google Scholar] [CrossRef]
- Zamorano, M.; Alexander, J.F.; Catania, D.; Dharmaraj, S.; Kavelaars, A.; Heijnen, C.J. Nasal administration of mesenchymal stem cells prevents accelerated age-related tauopathy after chemotherapy in mice. Immun. Ageing 2023, 20, 5. [Google Scholar] [CrossRef]
- Sezer, G.; Yay, A.H.; Sarica, Z.S.; Gonen, Z.B.; Onder, G.O.; Alan, A.; Yilmaz, S.; Saraymen, B.; Bahar, D. Bone marrow-derived mesenchymal stem cells alleviate paclitaxel-induced mechanical allodynia in rats. J. Biochem. Mol. Toxicol. 2022, 36, e23207. [Google Scholar] [CrossRef]
- Boukelmoune, N.; Laumet, G.; Tang, Y.; Ma, J.; Mahant, I.; Singh, S.K.; Nijboer, C.; Benders, M.; Kavelaars, A.; Heijnen, C.J. Nasal administration of mesenchymal stem cells reverses chemotherapy-induced peripheral neuropathy in mice. Brain Behav. Immun. 2021, 93, 43–54. [Google Scholar] [CrossRef]
- Mannelli, L.D.C.; Tenci, B.; Micheli, L.; Vona, A.; Corti, F.; Zanardelli, M.; Lapucci, A.; Clemente, A.M.; Failli, P.; Ghelardini, C. Adipose-derived stem cells decrease pain in a rat model of oxaliplatin-induced neuropathy: Role of VEGF-A modulation. Neuropharmacology 2017, 131, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Miyano, K.; Ikehata, M.; Ohshima, K.; Yoshida, Y.; Nose, Y.; Yoshihara, S.-I.; Oki, K.; Shiraishi, S.; Uzu, M.; Nonaka, M.; et al. Intravenous administration of human mesenchymal stem cells derived from adipose tissue and umbilical cord improves neuropathic pain via suppression of neuronal damage and anti-inflammatory actions in rats. PLoS ONE 2022, 17, e0262892. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, A.; Ojansivu, M.; van der Koog, L.; Whittaker, T.E.; Cunnane, E.M.; Silva, A.M.; Dekker, N.; Stevens, M.M. Extracellular vesicles for tissue repair and regeneration: Evidence, challenges and opportunities. Adv. Drug Deliv. Rev. 2021, 175, 113775. [Google Scholar] [CrossRef] [PubMed]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marbán, E. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J. Nanobiotechnol. 2018, 16, 61. [Google Scholar] [CrossRef]
- Fu, P.; Zhang, J.; Li, H.; Mak, M.; Xu, W.; Tao, Z. Extracellular vesicles as delivery systems at nano-/micro-scale. Adv. Drug Deliv. Rev. 2021, 179, 113910. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Peferoen, L.; Amor, S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130516. [Google Scholar] [CrossRef]
- Toribio, V.; Morales, S.; López-Martín, S.; Cardeñes, B.; Cabañas, C.; Yáñez-Mó, M. Development of a quantitative method to measure EV uptake. Sci. Rep. 2019, 9, 10522. [Google Scholar] [CrossRef]
- Takeda, Y.S.; Xu, Q. Neuronal Differentiation of Human Mesenchymal Stem Cells Using Exosomes Derived from Differentiating Neuronal Cells. PLoS ONE 2015, 10, e0135111. [Google Scholar] [CrossRef]
- Hercher, D.; Nguyen, M.Q.; Dworak, H. Extracellular vesicles and their role in peripheral nerve regeneration. Exp. Neurol. 2022, 350, 113968. [Google Scholar] [CrossRef]
- Raposo, G.; Stahl, P.D. Extracellular vesicles: A new communication paradigm? Nat. Rev. Mol. Cell Biol. 2019, 20, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bonacquisti, E.E.; Brown, A.D.; Nguyen, J. Boosting the Biogenesis and Secretion of Mesenchymal Stem Cell-Derived Exosomes. Cells 2020, 9, 660. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Tancini, B.; Emiliani, C. Signaling Pathways in Exosomes Biogenesis, Secretion and Fate. Genes 2013, 4, 152–170. [Google Scholar] [CrossRef]
- Fan, B.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Treatment of diabetic peripheral neuropathy with engineered mesenchymal stromal cell-derived exosomes enriched with microRNA-146a provide amplified therapeutic efficacy. Exp. Neurol. 2021, 341, 113694. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Qin, Y.; Cepparulo, P.; Millman, M.; Chopp, M.; Kemper, A.; Szalad, A.; Lu, X.; Wang, L.; et al. Small extracellular vesicles ameliorate peripheral neuropathy and enhance chemotherapy of oxaliplatin on ovarian cancer. J. Extracell. Vesicles 2021, 10, e12073. [Google Scholar] [CrossRef]
- Wong, F.C.; Ye, L.; Demir, I.E.; Kahlert, C. Schwann cell-derived exosomes: Janus-faced mediators of regeneration and disease. Glia 2021, 70, 20–34. [Google Scholar] [CrossRef]
- Yu, M.; Liu, W.; Li, J.; Lu, J.; Lu, H.; Jia, W.; Liu, F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res. Ther. 2020, 11, 350. [Google Scholar] [CrossRef]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Chen, R.; Miao, J.; Wang, L.; Cui, L.; Ji, H.; Liu, Y. Cortical Neuron-Derived Exosomal MicroRNA-181c-3p Inhibits Neuroinflammation by Downregulating CXCL1 in Astrocytes of a Rat Model with Ischemic Brain Injury. Neuroimmunomodulation 2019, 26, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Han, Z.; Hu, T.; Zhang, S.; Ge, X.; Huang, S.; Wang, L.; Yu, J.; Li, W.; Wang, Y.; et al. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain, Behav. Immun. 2020, 83, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Ochiya, T. Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res. Ther. 2015, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, Q.; Yin, Y. miR-133b is a potential diagnostic biomarker for Alzheimer’s disease and has a neuroprotective role. Exp. Ther. Med. 2019, 18, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chopp, M.; Szalad, A.; Lu, X.; Zhang, Y.; Wang, X.; Cepparulo, P.; Lu, M.; Li, C.; Zhang, Z.G. Exosomes Derived From Schwann Cells Ameliorate Peripheral Neuropathy in Type 2 Diabetic Mice. Diabetes 2020, 69, 749–759. [Google Scholar] [CrossRef] [PubMed]
- El-Derany, M.O.; Noureldein, M.H. Bone marrow mesenchymal stem cells and their derived exosomes resolve doxorubicin-induced chemobrain: Critical role of their miRNA cargo. Stem Cell Res. Ther. 2021, 12, 322. [Google Scholar] [CrossRef]
- Gao, X.; Gao, L.-F.; Zhang, Y.-N.; Kong, X.-Q.; Jia, S.; Meng, C.-Y. Huc-MSCs-derived exosomes attenuate neuropathic pain by inhibiting activation of the TLR2/MyD88/NF-κB signaling pathway in the spinal microglia by targeting Rsad2. Int. Immunopharmacol. 2023, 114, 109505. [Google Scholar] [CrossRef]
- Gao, X.; Gao, L.-F.; Kong, X.-Q.; Zhang, Y.-N.; Jia, S.; Meng, C.-Y. Mesenchymal stem cell-derived extracellular vesicles carrying miR-99b-3p restrain microglial activation and neuropathic pain by stimulating autophagy. Int. Immunopharmacol. 2023, 115, 109695. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, G.; Zhao, J.; Chen, Y.; Kong, L.; Sheng, C.; Yuan, L. Exosomes carried miR-181c-5p alleviates neuropathic pain in CCI rat models. An. da Acad. Bras. de Cienc. 2022, 94, e20210564. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Ouyang, F.; Su, M.; Li, W.; Chen, J.; Xiao, H.; Zhou, X.; Liu, B. Extracellular vesicles derived from mesenchymal stem cells alleviate neuroinflammation and mechanical allodynia in interstitial cystitis rats by inhibiting NLRP3 inflammasome activation. J. Neuroinflammation 2022, 19, 80. [Google Scholar] [CrossRef]
- Hua, T.; Yang, M.; Song, H.; Kong, E.; Deng, M.; Li, Y.; Li, J.; Liu, Z.; Fu, H.; Wang, Y.; et al. Huc-MSCs-derived exosomes attenuate inflammatory pain by regulating microglia pyroptosis and autophagy via the miR-146a-5p/TRAF6 axis. J. Nanobiotechnology 2022, 20, 324. [Google Scholar] [CrossRef]
- Anakor, E.; Le Gall, L.; Dumonceaux, J.; Duddy, W.J.; Duguez, S. Exosomes in Ageing and Motor Neurone Disease: Biogenesis, Uptake Mechanisms, Modifications in Disease and Uses in the Development of Biomarkers and Therapeutics. Cells 2021, 10, 2930. [Google Scholar] [CrossRef] [PubMed]
- French, K.C.; Antonyak, M.A.; Cerione, R.A. Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin. Cell Dev. Biol. 2017, 67, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ren, W.; Wang, W.; Han, W.; Jiang, L.; Zhang, D.; Guo, M. Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 2022, 12, 2385–2402. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Liu, Y.; Yang, Y.; Wang, H.; Xu, Y.; Zhang, Z. MSC-Derived Exosomes-Based Therapy for Peripheral Nerve Injury: A Novel Therapeutic Strategy. BioMed Res. Int. 2019, 2019, 6458237. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, C.; Díaz, P.; López-Leal, R.; Manque, P.; Court, F.A. Purification of Exosomes from Primary Schwann Cells, RNA Extraction, and Next-Generation Sequencing of Exosomal RNAs. In Schwann Cells: Methods and Protocols; Monje, P.V., Kim, H.A., Eds.; Springer: New York, NY, USA, 2018; pp. 299–315. [Google Scholar]
- López-Leal, R.; Díaz-Viraqué, F.; Catalan, R.J.; Saquel, C.; Enright, A.; Iraola, G.; Court, F.A. Schwann cell reprogramming into repair cells increases exosome-loaded miRNA-21 promoting axonal growth. J. Cell Sci. 2020, 133, jcs239004. [Google Scholar] [CrossRef] [PubMed]
- Long, H.-Z.; Cheng, Y.; Zhou, Z.-W.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Wang, L.; Liu, P.; Zhao, S.; Li, H.; Jiang, Y.; Guo, Y.; Wang, X. Resveratrol inhibits paclitaxel-induced neuropathic pain by the activation of PI3K/Akt and SIRT1/PGC1α pathway. J. Pain Res. 2019, 12, 879–890. [Google Scholar] [CrossRef]
- Figlia, G.; Norrmén, C.; Pereira, J.A.; Gerber, D.; Suter, U. Dual function of the PI3K-Akt-mTORC1 axis in myelination of the peripheral nervous system. eLife 2017, 6, e29241. [Google Scholar] [CrossRef]
- Sengupta, S.; Peterson, T.R.; Sabatini, D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 2010, 40, 310–322. [Google Scholar] [CrossRef]
- Dibble, C.C.; Cantley, L.C. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015, 25, 545–555. [Google Scholar] [CrossRef]
- Magnaghi, V.; Castelnovo, L.F.; Bonalume, V.; Melfi, S.; Ballabio, M.; Colleoni, D. Schwann cell development, maturation and regeneration: A focus on classic and emerging intracellular signaling pathways. Neural Regen. Res. 2017, 12, 1013–1023. [Google Scholar] [CrossRef]
- Ishii, A.; Furusho, M.; Bansal, R. Mek/ERK1/2-MAPK and PI3K/Akt/mTOR signaling plays both independent and cooperative roles in Schwann cell differentiation, myelination and dysmyelination. Glia 2021, 69, 2429–2446. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Lu, L.; Liu, Y. SPIONs mediated magnetic actuation promotes nerve regeneration by inducing and maintaining repair-supportive phenotypes in Schwann cells. J. Nanobiotechnol. 2022, 20, 159. [Google Scholar] [CrossRef]
- Phan, J.; Kumar, P.; Hao, D.; Gao, K.; Farmer, D.; Wang, A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J. Extracell. Vesicles 2018, 7, 1522236. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, L.; Jeske, R.; Nkosi, D.; York, S.B.; Liu, Y.; Grant, S.C.; Meckes, D.G.; Li, Y. Engineering extracellular vesicles by three-dimensional dynamic culture of human mesenchymal stem cells. J. Extracell. Vesicles 2022, 11, e12235. [Google Scholar] [CrossRef]
- Jeske, R.; Liu, C.; Duke, L.; Canonicco Castro, M.L.; Muok, L.; Arthur, P.; Singh, M.; Jung, S.; Sun, L.; Li, Y. Upscaling human mesenchymal stromal cell production in a novel vertical-wheel bioreactor enhances extracellular vesicle secretion and cargo profile. Bioact. Mater. 2023, 25, 732–747. [Google Scholar] [CrossRef]
- Gobin, J.; Muradia, G.; Mehic, J.; Westwood, C.; Couvrette, L.; Stalker, A.; Bigelow, S.; Luebbert, C.C.; Bissonnette, F.S.-D.; Johnston, M.J.W.; et al. Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immuno-modulatory antigenic signature of the producer cell. Stem Cell Res. Ther. 2021, 12, 127. [Google Scholar] [CrossRef]
- Ghasemian, M.; Layton, C.; Nampe, D.; Nieden, N.I.Z.; Tsutsui, H.; Princevac, M. Hydrodynamic characterization within a spinner flask and a rotary wall vessel for stem cell culture. Biochem. Eng. J. 2020, 157, 107533. [Google Scholar] [CrossRef]
- de Sousa Pinto, D.; Bandeiras, C.; de Almeida Fuzeta, M.; Rodrigues, C.A.V.; Jung, S.; Hashimura, Y.; Tseng, R.J.; Milligan, W.; Lee, B.; Ferreira, F.C.; et al. Scalable Manufacturing of Human Mesenchymal Stromal Cells in the Vertical-Wheel Bioreactor System: An Experimental and Economic Approach. Biotechnol. J. 2019, 14, e1800716. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Kawai-Harada, Y.; El Itawi, H.; Komuro, H.; Harada, M. Evaluation of EV Storage Buffer for Efficient Preservation of Engineered Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 12841. [Google Scholar] [CrossRef]
- Görgens, A.; Corso, G.; Hagey, D.W.; Wiklander, R.J.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The impact of storage on extracellular vesicles: A systematic study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef]
- Luhtala, N.; Aslanian, A.; Yates, J.R., 3rd; Hunter, T. Secreted Glioblastoma Nanovesicles Contain Intracellular Signaling Proteins and Active Ras Incorporated in a Farnesylation-dependent Manner. J. Biol. Chem. 2017, 292, 611–628. [Google Scholar] [CrossRef]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef]
- Cheng, Y.; Zeng, Q.; Han, Q.; Xia, W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell 2018, 10, 295–299. [Google Scholar] [CrossRef]
- Buccitelli, C.; Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef]
- Knotkova, H.; Hamani, C.; Sivanesan, E.; Le Beuffe, M.F.E.; Moon, J.Y.; Cohen, S.P.; Huntoon, M.A. Neuromodulation for chronic pain. Lancet 2021, 397, 2111–2124. [Google Scholar] [CrossRef]
- Naskar, S.; Kumaran, V.; Markandeya, Y.S.; Mehta, B.; Basu, B. Neurogenesis-on-Chip: Electric field modulated transdifferentiation of human mesenchymal stem cell and mouse muscle precursor cell coculture. Biomaterials 2020, 226, 119522. [Google Scholar] [CrossRef]
- Fukuta, T.; Nishikawa, A.; Kogure, K. Low level electricity increases the secretion of extracellular vesicles from cultured cells. Biochem. Biophys. Rep. 2020, 21, 100713. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Madras, G.; Basu, B. Intermittent electrical stimuli for guidance of human mesenchymal stem cell lineage commitment towards neural-like cells on electroconductive substrates. Biomaterials 2014, 35, 6219–6235. [Google Scholar] [CrossRef]
- Campana, W.M.; Mantuano, E.; Azmoon, P.; Henry, K.; Banki, M.A.; Kim, J.H.; Pizzo, D.P.; Gonias, S.L. Ionotropic glutamate receptors activate cell signaling in response to glutamate in Schwann cells. FASEB J. 2016, 31, 1744–1755. [Google Scholar] [CrossRef]
- Hu, M.; Hong, L.; Liu, C.; Hong, S.; He, S.; Zhou, M.; Huang, G.; Chen, Q. Electrical stimulation enhances neuronal cell activity mediated by Schwann cell derived exosomes. Sci. Rep. 2019, 9, 4206. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Y.; Kim, I.-M.; Liu, Y.; Cai, J.; Berman, A.E.; Nilsson, K.R.; Weintraub, N.L.; Tang, Y. Electrical Stimulation Increases the Secretion of Cardioprotective Extracellular Vesicles from Cardiac Mesenchymal Stem Cells. Cells 2023, 12, 875. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological Interactions of Graphene-Family Nanomaterials: An Interdisciplinary Review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef]
- Kalbacova, M.; Broz, A.; Kong, J.; Kalbac, M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 2010, 48, 4323–4329. [Google Scholar] [CrossRef]
- Bidez, P.R.; Li, S.; MacDiarmid, A.G.; Venancio, E.C.; Wei, Y.; Lelkes, P.I. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci. Polym. Ed. 2006, 17, 199–212. [Google Scholar] [CrossRef]
- Wang, H.-J.; Ji, L.-W.; Li, D.-F.; Wang, J.-Y. Characterization of Nanostructure and Cell Compatibility of Polyaniline Films with Different Dopant Acids. J. Phys. Chem. B 2008, 112, 2671–2677. [Google Scholar] [CrossRef]
- Oren, R.; Sfez, R.; Korbakov, N.; Shabtai, K.; Cohen, A.; Erez, H.; Dormann, A.; Cohen, H.; Shappir, J.; Spira, M.; et al. Electrically conductive 2D-PAN-containing surfaces as a culturing substrate for neurons. J. Biomater. Sci. Polym. Ed. 2004, 15, 1355–1374. [Google Scholar] [CrossRef]
- Ju, C.; Park, E.; Kim, T.; Kim, T.; Kang, M.; Lee, K.-S.; Park, S.-M. Effectiveness of electrical stimulation on nerve regeneration after crush injury: Comparison between invasive and non-invasive stimulation. PLoS ONE 2020, 15, e0233531. [Google Scholar] [CrossRef]
- Wang, Y.; Burghardt, T.P.; Worrell, G.A.; Wang, H.-L. The frequency-dependent effect of electrical fields on the mobility of intracellular vesicles in astrocytes. Biochem. Biophys. Res. Commun. 2020, 534, 429–435. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, Y.; Yue, H.; Fan, Y. Electrical Stimulation Promotes Stem Cell Neural Differentiation in Tissue Engineering. Stem Cells Int. 2021, 2021, 6697574. [Google Scholar] [CrossRef]
- Leng, T.; Fishman, H.A. Carbon nanotube bucky paper as an artificial support membrane for retinal cell transplantation. Ophthalmic Surg. Lasers Imaging Retin. 2013, 44, 73–76. [Google Scholar] [CrossRef]
- Lee, M.H.; Park, Y.J.; Hong, S.H.; Koo, M.-A.; Cho, M.; Park, J.-C. Pulsed Electrical Stimulation Enhances Consistency of Directional Migration of Adipose-Derived Stem Cells. Cells 2021, 10, 2846. [Google Scholar] [CrossRef]
- Banks, T.A.; Luckman, P.S.B.; Frith, J.E.; Cooper-White, J.J. Effects of electric fields on human mesenchymal stem cell behaviour and morphology using a novel multichannel device. Integr. Biol. 2015, 7, 693–712. [Google Scholar] [CrossRef]
- Feng, J.-F.; Liu, J.; Zhang, X.-Z.; Zhang, L.; Jiang, J.-Y.; Nolta, J.; Zhao, M. Guided Migration of Neural Stem Cells Derived from Human Embryonic Stem Cells by an Electric Field. Stem Cells 2012, 30, 349–355. [Google Scholar] [CrossRef]
- Maji, S.; Yan, I.K.; Parasramka, M.; Mohankumar, S.; Matsuda, A.; Patel, T. In vitro toxicology studies of extracellular vesicles. J. Appl. Toxicol. 2017, 37, 310–318. [Google Scholar] [CrossRef]
- Lin, S.; Zhu, B.; Huang, G.; Zeng, Q.; Wang, C. Microvesicles derived from human bone marrow mesenchymal stem cells promote U2OS cell growth under hypoxia: The role of PI3K/AKT and HIF-1α. Hum. Cell 2019, 32, 64–74. [Google Scholar] [CrossRef]
- Lee, J.-K.; Park, S.-R.; Jung, B.-K.; Jeon, Y.-K.; Lee, Y.-S.; Kim, M.-K.; Kim, Y.-G.; Jang, J.-Y.; Kim, C.-W. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef]
- Rodini, C.O.; da Silva, P.B.G.; Assoni, A.F.; Carvalho, V.M.; Okamoto, O.K. Mesenchymal stem cells enhance tumorigenic properties of human glioblastoma through independent cell-cell communication mechanisms. Oncotarget 2018, 9, 24766–24777. [Google Scholar] [CrossRef]
- Tan, T.T.; Lai, R.C.; Padmanabhan, J.; Sim, W.K.; Choo, A.B.H.; Lim, S.K. Assessment of Tumorigenic Potential in Mesenchymal-Stem/Stromal-Cell-Derived Small Extracellular Vesicles (MSC-sEV). Pharmaceuticals 2021, 14, 345. [Google Scholar] [CrossRef]
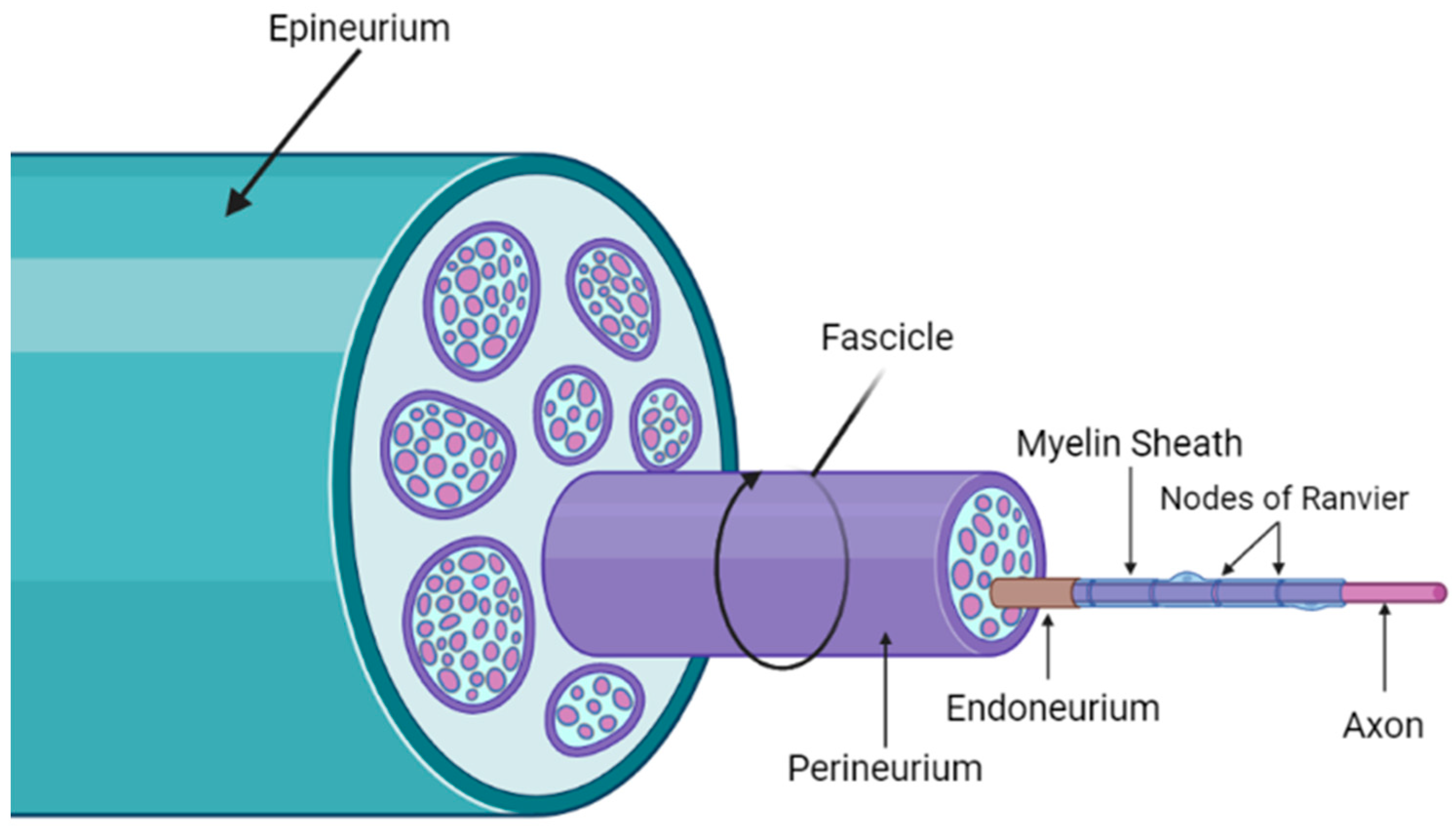
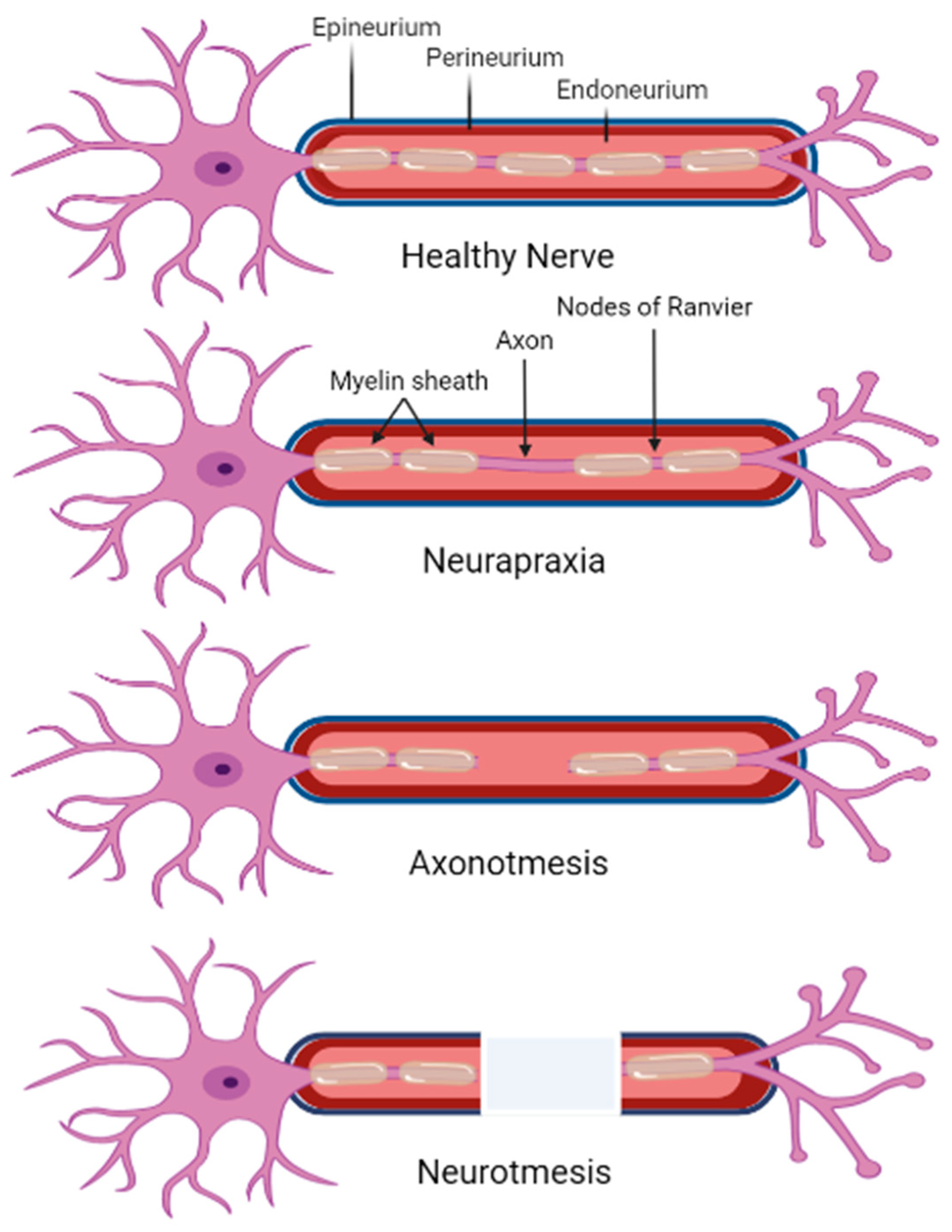
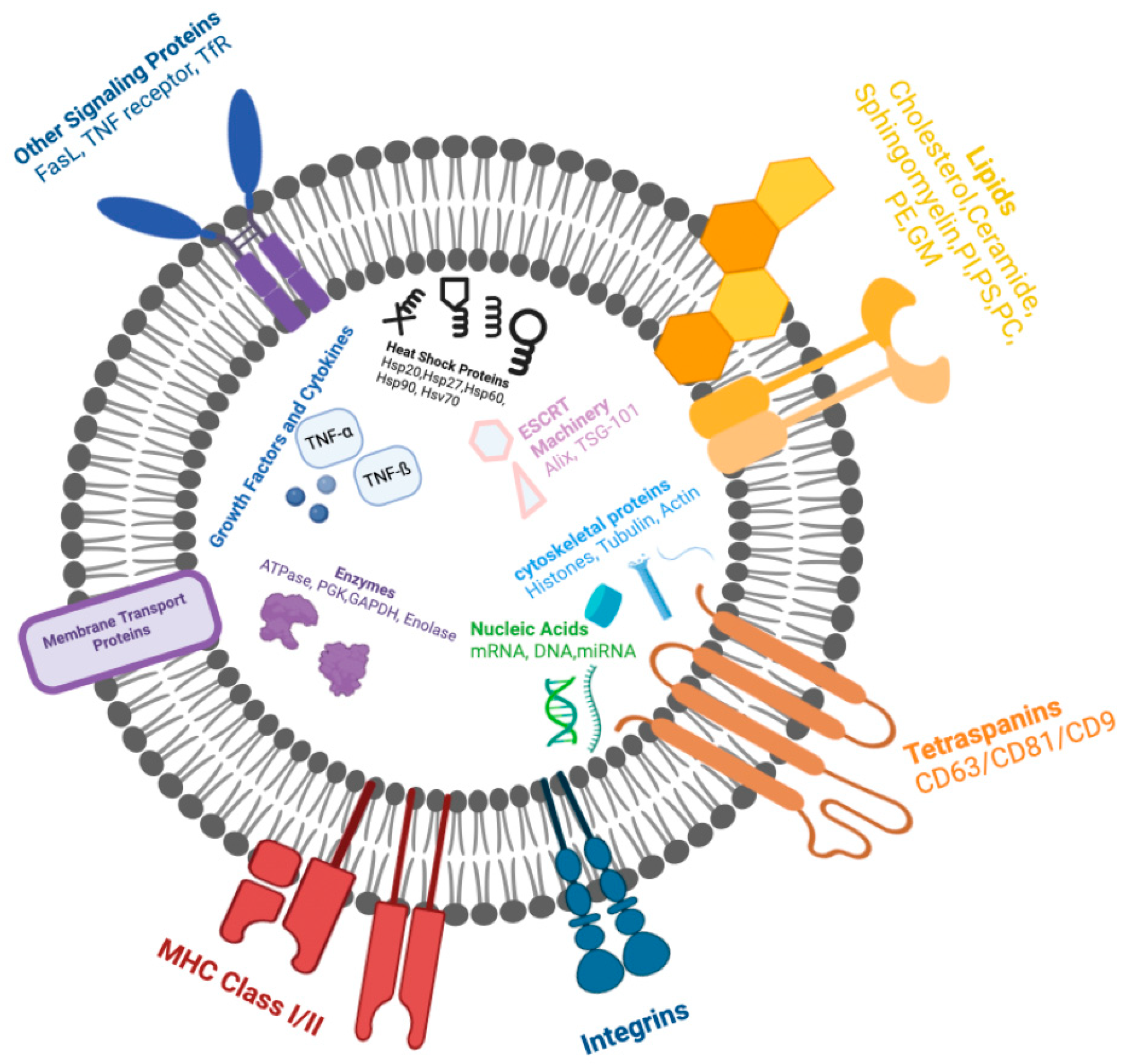

| Form of DPN | Description | Reference |
|---|---|---|
| Focal | Affecting 1 or a singular group of nerves (i.e., carpal tunnel). | [39] |
| Multifocal Peripheral Neuropathy | Length-dependent motor/sensory neuropathy. | [24] |
| Autonomic | Loss of involuntary bodily function. | [40] |
| Diabetic Amyotrophy (Proximal Neuropathy) | Unilateral or bilateral pain and sensory loss and muscular atrophy in quadriceps, hips, and gluteus maximus. | [24] |
| Idiopathic Neuropathy | Undetermined etiology of neuropathy. | [41] |
| Drug | Treated Condition | CIPN Pathogenesis |
|---|---|---|
| Platinum Compounds | Tumors in cranium, digestive, urinary, respiratory, and reproductive systems. | Mitochondrial dysfunction. Increased oxidative stress. Voltage-gated K+ and Na+ hyperactivity. |
| Taxanes | Tumors in breast, ovaries, prostate, lungs, and bladder. | Mitochondrial dysfunction. Increased oxidative stress. Voltage-gated K+ and Na+ hyperactivity. Altered functionality of skin-based receptors (Aβ, C, and Aδ nerve fibers). |
| Vinca Alkaloids | Tumors in kidneys, liver, lungs, breast, and brain. Hematological malignancies, testicular, and non-small cell lung cancer. | Mitochondrial dysfunction. Microtubule function inhibition. |
| Immunomodulators | Example: thalidomide. MM, glioblastoma, breast, and prostate cancer. | Inhibition of growth factors (VEGF, TNF-α, NF-kB, b-FGF). ROS activation. Induced hypoxia and ischemia. |
| Proteosome Inhibitors | Example: bortezomib. Progressive, relapsed, or refractory MM. | Mitochondrial dysfunction. Increased oxidative stress. Increased apoptosis via release of Ca2+ in endoplasmic reticulum. |
| Protein | Properties | Benefit to Neural Regeneration | Reference |
|---|---|---|---|
| Elastin | Highly elastic, water-soluble, hydrophobic. | Promotes cellular adhesion, proliferation, stem cell differentiation, the release of growth factors, drug delivery. | [169] |
| Fibrinogen | Produces fibrin network, composed of polypeptide chains. | Facilitate stem cell proliferation, adhesion, and differentiation. | [170] |
| Laminin | Abundant in native ECM. | Basement membrane. Facilitate cellular attachment, differentiation, and neurite outgrowth. | [171,172] |
| Silk | Naturally occurring in ECM. | Promotes oxygen and permeability. Biodegradable. Supports SC and neuron growth and attachment. | [173,174] |
| Collagen | Abundant in native ECM. | Fibroblast proliferation, angiogenesis, regulation of pro- and anti-inflammatory response. | [175,176] |
| Hyaluronic Acid | Abundant in embryonic tissue and ECM. | Maintains ECM, regulates binding proteins in cellular adhesion, proliferation, pro/anti-inflammatory response depending on molecular weight. | [177] |
| MSC Source | Neuropathy Treated | Title of Study | Reference |
|---|---|---|---|
| hUC-MSC | DPN | Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. | [216] |
| BM-MSC | DPN | The bone marrow-derived mesenchymal stem cells (BMSCs) alleviate diabetic peripheral neuropathy induced by STZ via activating GSK-3β/β-catenin signaling pathway. | [217] |
| ASC | DPN | Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. | [218] |
| hMSC | CIPN | Nasal administration of mesenchymal stem cells prevents accelerated age-related tauopathy after chemotherapy in mice. | [219] |
| BM-MSC | CIPN | Bone marrow-derived mesenchymal stem cells alleviate paclitaxel-induced mechanical allodynia in rats. | [220] |
| MSC | CIPN | Nasal administration of mesenchymal stem cells reverses chemotherapy-induced peripheral neuropathy in mice. | [221] |
| ASC | CIPN | Adipose-derived stem cells decrease pain in rat model of oxaliplatin-induced neuropathy: Role of VEGF-A modulation. | [222] |
| hASC and hUC-MSC | Neuropathic symptoms via partial sciatic nerve ligation | Intravenous administration of human mesenchymal stem cells derived from adipose tissue and umbilical cord improves NP via suppression of neuronal damage and anti-inflammatory actions in rats. | [223] |
| ASC | Peripheral nerve injury repair for NP relief | Role of adipose tissue grafting and adipose-derived stem cells in peripheral nerve surgery. | [194] |
| Exosome Source | Neuropathy Treated | Title of Study | Reference |
|---|---|---|---|
| hMSC | DPN | Treatment of diabetic peripheral neuropathy with engineered mesenchymal stromal cell-derived exosomes enriched with microRNA-146a provide amplified therapeutic efficacy. | [237] |
| hBM-MSC | DPN | Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. | [240] |
| hBM-MSC | DPN | Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. | [241] |
| SC-EV | DPN | Exosomes derived from Schwann cells ameliorate peripheral neuropathy in type 2 diabetic mice. | [247] |
| CEC-sEV | CIPN | Small extracellular vesicles ameliorate peripheral neuropathy and enhance chemotherapy of oxaliplatin on ovarian cancer. | [238] |
| hBM-MSC-EVs | CIPN | Bone marrow mesenchymal stem cells and their derived exosomes resole doxorubicin-induced chemobrain: Critical role of their miRNA cargo. | [248] |
| hUC-MSC | Microglial activation of NP | Huc-MSCs-derived exosomes attenuate NP by inhibiting activation of the TLR2/MyD88/NF-kB signaling pathway in the spinal microglia by targeting Rasad2. | [249] |
| MSC | Microglial activation of NP | Mesenchymal stem cell-derived extracellular vesicles carrying miR-99b-3p restrain microglial activation and NP by stimulating autophagy. | [250] |
| BM-MSC | NP via sciatic nerve chronic constriction injury | Exosomes carried miR-181c-5p alleviates NP in CCI rat models. | [251] |
| MSC | NP via spinal neuroinflammation | Extracellular vesicles derived from mesenchymal stem cells alleviate neuroinflammation and mechanical allodynia in interstitial cystitis rats by inhibiting NLRP3 inflammasome activation. | [252] |
| hUC-MSC | Alleviate inflammatory pain | Huc-MSCs-derived exosomes attenuate inflammatory pain by regulating microglia pyroptosis and autophagy via the miR-146a-5p/TRAF6 axis. | [253] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berry, D.; Ene, J.; Nathani, A.; Singh, M.; Li, Y.; Zeng, C. Effects of Physical Cues on Stem Cell-Derived Extracellular Vesicles toward Neuropathy Applications. Biomedicines 2024, 12, 489. https://doi.org/10.3390/biomedicines12030489
Berry D, Ene J, Nathani A, Singh M, Li Y, Zeng C. Effects of Physical Cues on Stem Cell-Derived Extracellular Vesicles toward Neuropathy Applications. Biomedicines. 2024; 12(3):489. https://doi.org/10.3390/biomedicines12030489
Chicago/Turabian StyleBerry, Danyale, Justice Ene, Aakash Nathani, Mandip Singh, Yan Li, and Changchun Zeng. 2024. "Effects of Physical Cues on Stem Cell-Derived Extracellular Vesicles toward Neuropathy Applications" Biomedicines 12, no. 3: 489. https://doi.org/10.3390/biomedicines12030489
APA StyleBerry, D., Ene, J., Nathani, A., Singh, M., Li, Y., & Zeng, C. (2024). Effects of Physical Cues on Stem Cell-Derived Extracellular Vesicles toward Neuropathy Applications. Biomedicines, 12(3), 489. https://doi.org/10.3390/biomedicines12030489






