The Assessment of a Novel Endoscopic Ultrasound-Compatible Cryocatheter to Ablate Pancreatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
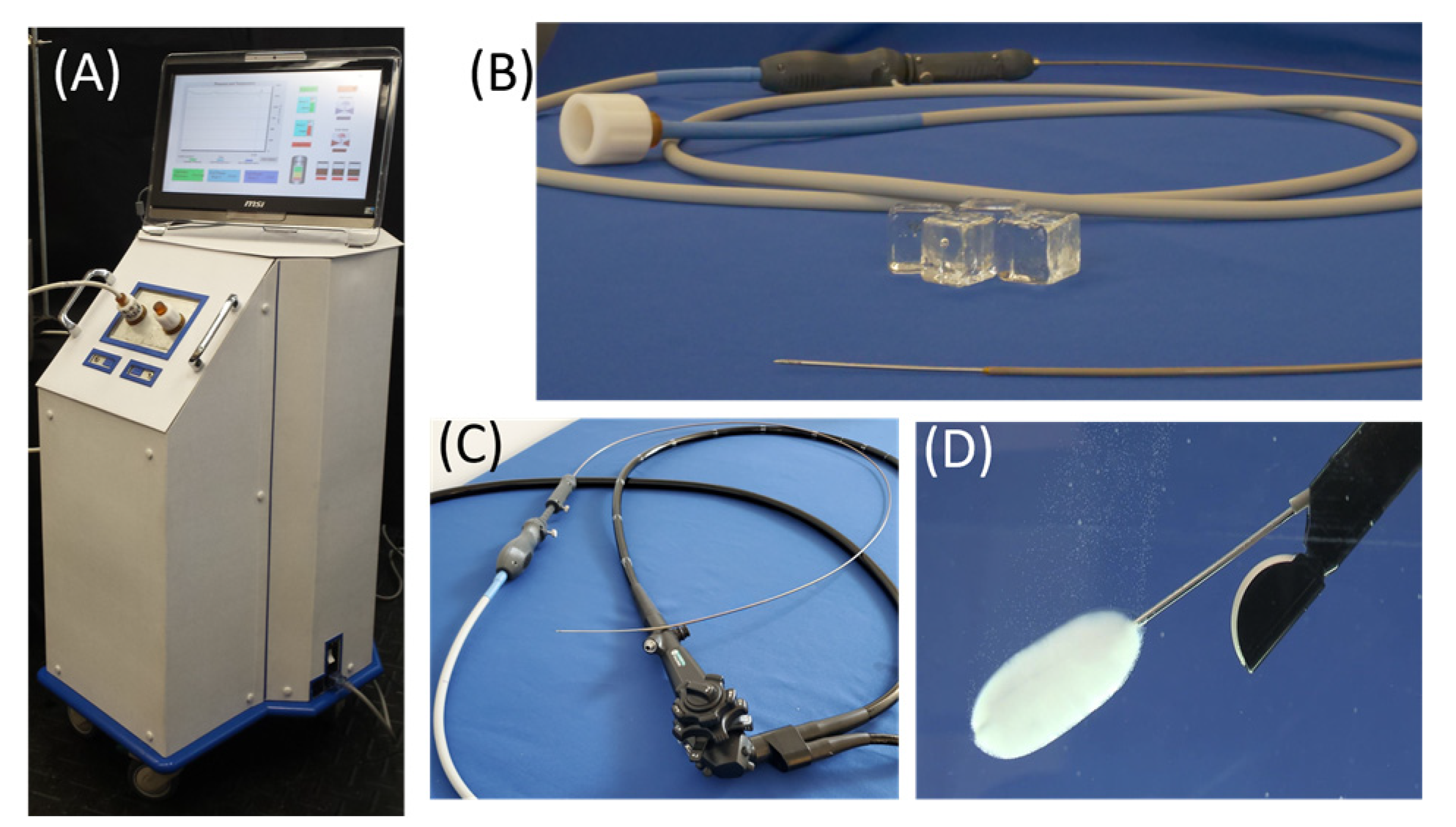
2.1. FrostBite and the Pressurized Sub-Cooled Nitrogen (PSN) System
2.2. Isotherm and Calorimetry Testing
2.3. Cell and 3D TEM Culture
2.4. Freeze Procedure
2.5. Cell Viability Assessment
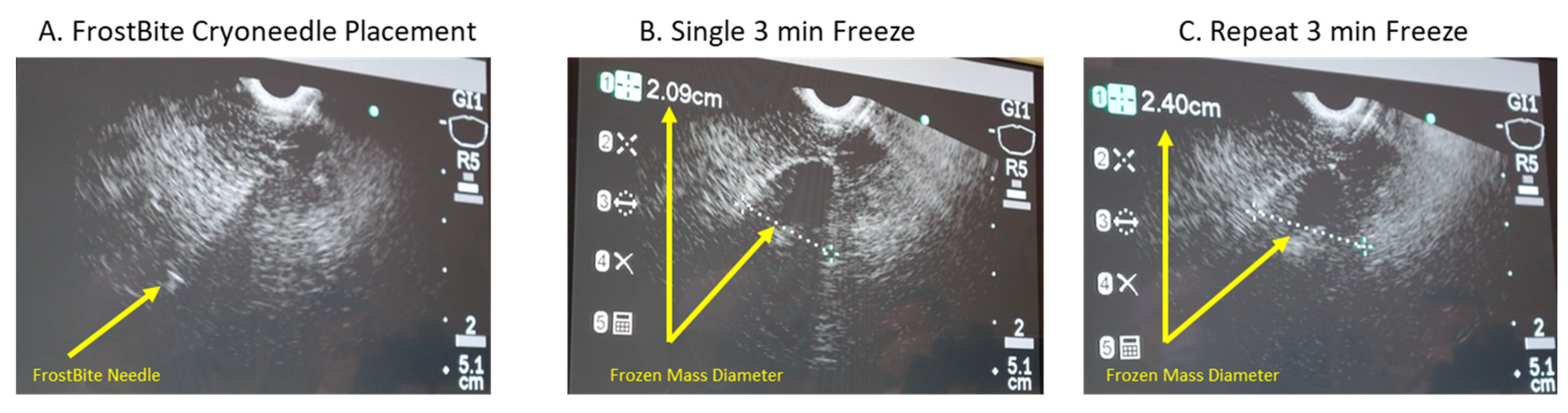
2.6. Pilot In Vivo Porcine Study
2.7. Data Analysis
3. Results
3.1. Isotherm Distribution
3.2. Calorimetry Testing
3.3. Tissue Engineered Model Freezing
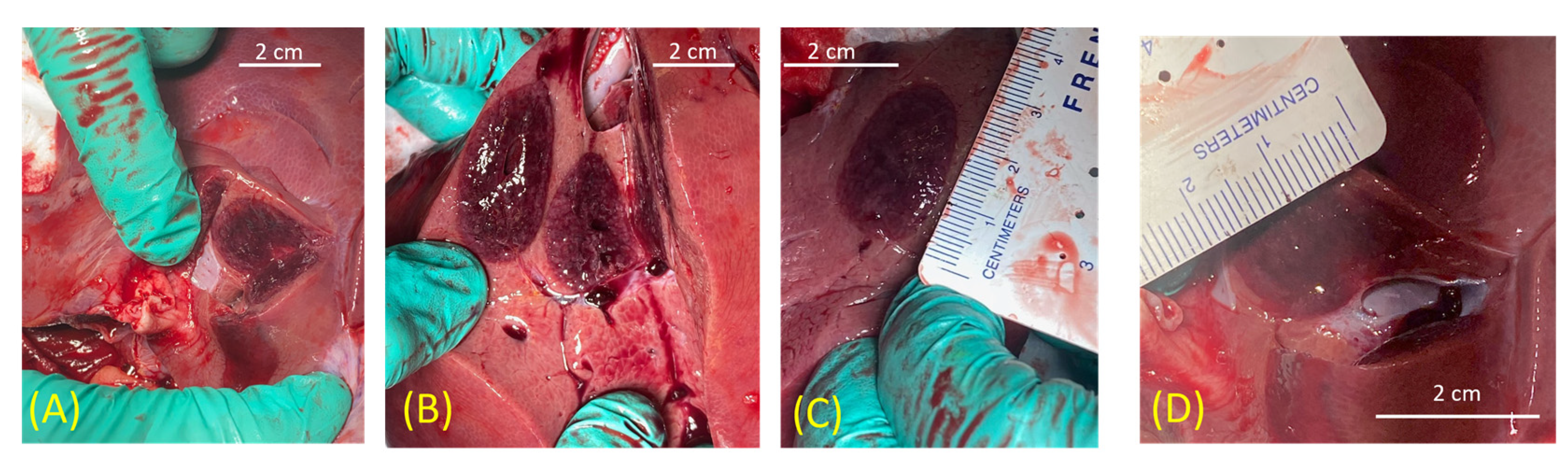
3.4. In Vivo Porcine Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pancreas Cancer: Statistics. Available online: http://cancer.net (accessed on 5 November 2023).
- Survival Rates for Pancreas Cancer. Available online: http://cancer.org (accessed on 5 November 2023).
- World Cancer Research Fund: Pancreatic Cancer Statistics. Available online: https://www.wcrf.org (accessed on 5 November 2023).
- Chiu, D.; Niu, L.; Mu, F.; Peng, X.; Zhou, L.; Li, H.; Li, R.; Ni, J.; Jiang, N.; Hu, Y.; et al. The experimental study for efficacy and safety of pancreatic cryosurgery. Cryobiology 2010, 60, 281–286. [Google Scholar] [CrossRef]
- Kovach, S.J.; Hendrickson, R.J.; Cappadona, C.R.; Schmidt, C.M.; Groen, K.; Koniaris, L.G.; Sitzmann, J.V. Cryoablation of unresectable pancreatic cancer. Surgery 2002, 131, 463–464. [Google Scholar] [CrossRef]
- Fegrachi, S.; Molenaar, I.Q.; Klaessens, J.H.; Besselink, M.G.; Offerhaus, J.A.; van Hillegersberg, R. Radiofrequency ablation of the pancreas: Two-week follow-up in a porcine model. Eur. J. Surg. Oncol. 2014, 40, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Fegrachi, S.; Molenaar, I.Q.; Klaessens, J.H.; Besselink, M.G.; Offerhaus, J.A.; van Hillegersberg, R. Radiofrequency ablation of the pancreas with and without intraluminal duodenal cooling in a porcine model. J. Surg. Res. 2013, 184, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Pancreatic Cancer. Available online: https://www.cancer.gov/types/pancreatic/patient/pancreatic-treatment-pdq (accessed on 15 November 2021).
- Vivaldi, C.; Fornaro, L.; Cappelli, C.; Pecora, I.; Catanese, S.; Salani, F.; Cacciato Insilla, A.; Kauffmann, E.; Donati, F.; Pasquini, G.; et al. Early Tumor Shrinkage and Depth of Response Evaluation in Metastatic Pancreatic Cancer Treated with First Line Chemotherapy: An Observational Retrospective Cohort Study. Cancers 2019, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Kaga, Y.; Sunakawa, Y.; Kubota, Y.; Tagawa, T.; Yamamoto, T.; Ikusue, T.; Uto, Y.; Miyashita, K.; Toshima, H.; Kobayashi, K.; et al. Early tumor shrinkage as a predictor of favorable outcomes in patients with advanced pancreatic cancer treated with FOLFIRINOX. Oncotarget 2016, 7, 67314–67320. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Ohtsuka, T.; Kimura, R.; Matsuda, R.; Mori, Y.; Nakata, K.; Kakihara, D.; Fujimori, N.; Ohno, T.; Oda, Y.; et al. Neoadjuvant Chemotherapy with Gemcitabine Plus Nab-Paclitaxel for Borderline Resectable Pancreatic Cancer Potentially Improves Survival and Facilitates Surgery. Ann. Surg. Oncol. 2019, 26, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Oba, A.; Ho, F.; Bao, Q.R.; Al-Musawi, M.H.; Schulick, R.D.; Del Chiaro, M. Neoadjuvant Treatment in Pancreatic Cancer. Front. Oncol. 2020, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Shridhar, R.; Huston, J.; Meredith, K. Correlation of tumor size and survival in pancreatic cancer. J. Gastrointest. Oncol. 2018, 9, 910–921. [Google Scholar] [CrossRef]
- Takano, N.; Yamada, S.; Sonohara, F.; Inokawa, Y.; Takami, H.; Hayashi, M.; Koike, M.; Fujii, T.; Kodera, Y. The impact of early tumor shrinkage on conversion surgery and the survival in patients with unresectable locally advanced pancreatic cancer. Surg. Today 2021, 51, 1099–1107. [Google Scholar] [CrossRef]
- Girelli, R.; Frigerio, I.; Salvia, R.; Barbi, E.; Tinazzi Martini, P.; Bassi, C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br. J. Surg. 2010, 97, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Brugge, W.R. EUS-guided tumor ablation with heat, cold, microwave, or radiofrequency: Will there be a winner? Gastrointest. Endosc. 2009, 69, S212–S216. [Google Scholar] [CrossRef] [PubMed]
- Chennat, J. Current status of endoscopic ultrasound guided ablation techniques. Gastroenterology 2011, 140, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Trevino, J.M.; Varadarajulu, S. Endoscopic ultrasonography-guided ablation therapy. J. Hepatobiliary Pancreat. Sci. 2011, 18, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.M.; Van Dam, J. Endoscopic ultrasound-guided intratumoural therapy for pancreatic cancer. Can. J. Gastroenterol. 2008, 22, 405–410. [Google Scholar] [CrossRef]
- Zhou, Y.F. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8–27. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Booth, C.M. Treatment of metastatic pancreatic cancer: 25 years of innovation with little progress for patients. Lancet Oncol. 2024, 25, 167–170. [Google Scholar] [CrossRef]
- Babaian, R.J.; Donnelly, B.; Bahn, D.; Baust, J.G.; Dineen, M.; Ellis, D.; Katz, A.; Pisters, L.; Rukstalis, D.; Shinohara, K.; et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J. Urol. 2008, 180, 1993–2004. [Google Scholar] [CrossRef]
- Baust, J.G.; Gage, A.A.; Robilottto, A.T.; Baust, J.M. The pathophysiology of thermoablation: Optimizing cryoablation. Curr. Opin. Urol. 2009, 19, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Gage, A.A.; Baust, J.M.; Baust, J.G. Experimental cryosurgery investigations in vivo. Cryobiology 2009, 59, 229–243. [Google Scholar] [CrossRef]
- Kuhne, M.; Suter, Y.; Altmann, D.; Ammann, P.; Schaer, B.; Osswald, S.; Sticherling, C. Cryoballoon versus radiofrequency catheter ablation of paroxysmal atrial fibrillation: Biomarkers of myocardial injury, recurrence rates, and pulmonary vein reconnection patterns. Heart Rhythm. 2010, 7, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Kojodjojo, P.; O’Neill, M.D.; Lim, P.B.; Malcolm-Lawes, L.; Whinnett, Z.I.; Salukhe, T.V.; Linton, N.W.; Lefroy, D.; Mason, A.; Wright, I.; et al. Pulmonary venous isolation by antral ablation with a large cryoballoon for treatment of paroxysmal and persistent atrial fibrillation: Medium-term outcomes and non-randomised comparison with pulmonary venous isolation by radiofrequency ablation. Heart 2010, 96, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.Y.; Choy, C.C.; Lau, C.L.; Lo, Y.K.; Chu, P.S.; Yuen, H.C.; Choi, Y.C.; Lau, S.T. Cryoablation versus radiofrequency ablation for atrioventricular nodal reentrant tachycardia: Patient pain perception and operator stress. Pacing Clin. Electrophysiol. 2011, 34, 2–7. [Google Scholar] [CrossRef]
- Correas, J.M.; Delavaud, C.; Gregory, J.; Le Guilchet, T.; Lamhaut, L.; Timsit, M.O.; Mejean, A.; Helenon, O. Ablative Therapies for Renal Tumors: Patient Selection, Treatment Planning, and Follow-Up. Semin. Ultrasound CT MR 2017, 38, 78–95. [Google Scholar] [CrossRef]
- Gage, A.A.; Baust, J.G. Cryosurgery for tumors–A clinical overview. Technol. Cancer Res. Treat. 2004, 3, 187–199. [Google Scholar] [CrossRef]
- Cohen, J.K.; Miller, R.J.J.; Ahmed, S.; Lotz, M.J.; Baust, J. Ten-year biochemical disease control for patients with prostate cancer treated with cryosurgery as primary therapy. Urology 2008, 71, 515–518. [Google Scholar] [CrossRef]
- Defaye, P.; Kane, A.; Jacon, P.; Mondesert, B. Cryoballoon for pulmonary vein isolation: Is it better tolerated than radiofrequency? Retrospective study comparing the use of analgesia and sedation in both ablation techniques. Arch. Cardiovasc. Dis. 2010, 103, 388–393. [Google Scholar] [CrossRef]
- Ritch, C.R.; Katz, A.E. Update on cryotherapy for localized prostate cancer. Curr. Urol. Rep. 2009, 10, 206–211. [Google Scholar] [CrossRef]
- Young, J.L.; Kolla, S.B.; Pick, D.L.; Sountoulides, P.; Kaufmann, O.G.; Ortiz-Vanderdys, C.G.; Huynh, V.B.; Kaplan, A.G.; Andrade, L.A.; Osann, K.E.; et al. In vitro, ex vivo and in vivo isotherms for renal cryotherapy. J. Urol. 2010, 183, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, L.; Lai, K.; Li, Z. Prostate cryoablation: A mini review. Frigid Zone Med. 2023, 3, 253–256. [Google Scholar] [CrossRef]
- Sabel, M.S.; Su, G.; Griffith, K.A.; Chang, A.E. Rate of freeze alters the immunologic response after cryoablation of breast cancer. Ann. Surg. Oncol. 2010, 17, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.M.; Rabin, Y.; Polascik, T.J.; Santucci, K.L.; Snyder, K.K.; Buskirk, R.G.V.; Baust, J.G. Defeating Cancers’ Adaptive Defensive Strategies Using Thermal Therapies: Examining Cancer’s Therapeutic Resistance, Ablative, and Computational Modeling Strategies as a means for Improving Therapeutic Outcome. Technol. Cancer Res. Treat. 2018, 17, 1533033818762207. [Google Scholar] [CrossRef] [PubMed]
- Aarts, B.M.; Klompenhouwer, E.G.; Rice, S.L.; Imani, F.; Baetens, T.; Bex, A.; Horenblas, S.; Kok, M.; Haanen, J.; Beets-Tan, R.G.H.; et al. Cryoablation and immunotherapy: An overview of evidence on its synergy. Insights Imaging 2019, 10, 53. [Google Scholar] [CrossRef]
- Li, L.Y.; Yang, M.; Gao, X.; Zhang, H.B.; Li, J.F.; Xu, W.F.; Lin, Z.; Zhou, X.L. Prospective comparison of five mediators of the systemic response after high-intensity focused ultrasound and targeted cryoablation for localized prostate cancer. BJU Int. 2009, 104, 1063–1067. [Google Scholar] [CrossRef]
- Korpan, N.N.; Goltsev, A.N.; Dronov, O.I.; Bondarovych, M.O. Cryoimmunology: Opportunities and challenges in biomedical science and practice. Cryobiology 2021, 100, 1–11. [Google Scholar] [CrossRef]
- Niu, L.; Chen, J.; He, L.; Liao, M.; Yuan, Y.; Zeng, J.; Li, J.; Zuo, J.; Xu, K. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas 2013, 42, 1143–1149. [Google Scholar] [CrossRef]
- Liu, E.; Shehata, M.; Liu, T.; Amorn, A.; Cingolani, E.; Kannarkat, V.; Chugh, S.S.; Wang, X. Prevention of esophageal thermal injury during radiofrequency ablation for atrial fibrillation. J. Interv. Card. Electrophysiol. 2012, 35, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Snyder, K.K.; Baust, J.G.; Baust, J.M.; Gage, A.A. Mechanisms of Cryoablation. In Cryoablation of Cardiac Arrhythmias; Bredikis, A.J., Wilber, D.J., Eds.; W.B. Saunders: St. Louis, MI, USA, 2011; pp. 13–21. [Google Scholar]
- Canto, M.I.; Dunbar, K.; Wang, J.; Montgomery, E.; Okolo, P. Low Flow CO2-cryotherapy for primary and “rescue” endoscopic therapy of Barrett’s esophagus with high grade dysplasia or early adenocarcinoma. Available online: http://www.gi-supply.com/wp-content/uploads/2014/06/canto-cryo-poster.pdf (accessed on 5 November 2023).
- Baust, J.G.; Snyder, K.K.; Santucci, K.L.; Robilotto, A.T.; Van Buskirk, R.G.; Baust, J.M. Cryoablation: Physical and molecular basis with putative immunological consequences. Int. J. Hyperthermia 2019, 36, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Abdo, J.; Cornell, D.L.; Mittal, S.K.; Agrawal, D.K. Immunotherapy Plus Cryotherapy: Potential Augmented Abscopal Effect for Advanced Cancers. Front. Oncol. 2018, 8, 85. [Google Scholar] [CrossRef]
- Baust, J.M.; Robilotto, A.T.; Gage, A.A.; Baust, J.G. Enhanced Cryoablative Methodologies. In Multiscale Technologies for Cryomedicine; Bischof, J.C., Xi, Y., Eds.; World Scientific Publishing Co.: Singapore, 2016; p. 388. [Google Scholar]
- Gessl, I.; Waldmann, E.; Penz, D.; Majcher, B.; Dokladanska, A.; Hinterberger, A.; Szymanska, A.; Trauner, M.; Ferlitsch, M. Resection rates and safety profile of cold vs. hot snare polypectomy in polyps sized 5-10mm and 11-20mm. Dig. Liver Dis. 2019, 51, 536–541. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Y.; Li, Q.; Yan, F.; Zhao, J.; Dong, X.; Zhang, Y. Cryoablation plus chemotherapy in colorectal cancer patients with liver metastases. Tumour Biol. 2014, 35, 10841–10848. [Google Scholar] [CrossRef]
- Scholvinck, D.W.; Kunzli, H.T.; Kestens, C.; Siersema, P.D.; Vleggaar, F.P.; Canto, M.I.; Cosby, H.; Abrams, J.A.; Lightdale, C.J.; Tejeda-Ramirez, E.; et al. Treatment of Barrett’s esophagus with a novel focal cryoablation device: A safety and feasibility study. Endoscopy 2015, 47, 1106–1112. [Google Scholar] [CrossRef]
- Polascik, T.J.; Mayes, J.M.; Mouraviev, V. Nerve-sparing focal cryoablation of prostate cancer. Curr. Opin. Urol. 2009, 19, 182–187. [Google Scholar] [CrossRef]
- Sabel, M.S. Cryoablation for breast cancer: No need to turn a cold shoulder. J. Surg. Oncol. 2008, 97, 485–486. [Google Scholar] [CrossRef]
- Callstrom, M.R.; Dupuy, D.E.; Solomon, S.B.; Beres, R.A.; Littrup, P.J.; Davis, K.W.; Paz-Fumagalli, R.; Hoffman, C.; Atwell, T.D.; Charboneau, J.W.; et al. Percutaneous image-guided cryoablation of painful metastases involving bone: Multicenter trial. Cancer 2013, 119, 1033–1041. [Google Scholar] [CrossRef]
- Ba, Y.F.; Li, X.D.; Zhang, X.; Ning, Z.H.; Zhang, H.; Liu, Y.N.; He, S.H.; Zhu, Y.; Li, C.S.; Wang, Q.H.; et al. Comparison of the analgesic effects of cryoanalgesia vs. parecoxib for lung cancer patients after lobectomy. Surg. Today 2015, 45, 1250–1254. [Google Scholar] [CrossRef]
- Cox, J.L.; Boineau, J.P.; Schuessler, R.B.; Jaquiss, R.D.; Lappas, D.G. Modification of the maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results. J. Thorac. Cardiovasc. Surg. 1995, 110, 473–484. [Google Scholar] [CrossRef]
- Schwagten, B.; Knops, P.; Janse, P.; Kimman, G.; Van Belle, Y.; Szili-Torok, T.; Jordaens, L. Long-term follow-up after catheter ablation for atrioventricular nodal reentrant tachycardia: A comparison of cryothermal and radiofrequency energy in a large series of patients. J. Interv. Card. Electrophysiol. 2011, 30, 55–61. [Google Scholar] [CrossRef]
- Yorgun, H.; Canpolat, U.; Oksul, M.; Sener, Y.Z.; Ates, A.H.; Crijns, H.; Aytemir, K. Long-term outcomes of cryoballoon-based left atrial appendage isolation in addition to pulmonary vein isolation in persistent atrial fibrillation. Europace 2019, 21, 1653–1662. [Google Scholar] [CrossRef]
- Friedman, P.L. Catheter cryoablation of cardiac arrhythmias. Curr. Opin. Cardiol. 2005, 20, 48–54. [Google Scholar] [CrossRef]
- Xu, K.C.; Niu, L.Z.; Hu, Y.Z.; He, W.B.; He, Y.S.; Zuo, J.S. Cryosurgery with combination of (125)iodine seed implantation for the treatment of locally advanced pancreatic cancer. J. Dig. Dis. 2008, 9, 32–40. [Google Scholar] [CrossRef]
- Xu, K.C.; Niu, L.Z.; Hu, Y.Z.; He, W.B.; He, Y.S.; Li, Y.F.; Zuo, J.S. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J. Gastroenterol. 2008, 14, 1603–1611. [Google Scholar] [CrossRef]
- Mahnken, A.H.; Konig, A.M.; Figiel, J.H. Current Technique and Application of Percutaneous Cryotherapy. Rofo 2018, 190, 836–846. [Google Scholar] [CrossRef]
- Luo, X.M.; Niu, L.Z.; Chen, J.B.; Xu, K.C. Advances in cryoablation for pancreatic cancer. World J. Gastroenterol. 2016, 22, 790–800. [Google Scholar] [CrossRef]
- Pai, M.; Habib, N.; Senturk, H.; Lakhtakia, S.; Reddy, N.; Cicinnati, V.R.; Kaba, I.; Beckebaum, S.; Drymousis, P.; Kahaleh, M.; et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J. Gastrointest. Surg. 2015, 7, 52–59. [Google Scholar] [CrossRef]
- Testoni, S.G.G.; Healey, A.J.; Dietrich, C.F.; Arcidiacono, P.G. Systematic review of endoscopy ultrasound-guided thermal ablation treatment for pancreatic cancer. Endosc. Ultrasound 2020, 9, 83–100. [Google Scholar] [CrossRef]
- Arcidiacono, P.G.; Carrara, S.; Reni, M.; Petrone, M.C.; Cappio, S.; Balzano, G.; Boemo, C.; Cereda, S.; Nicoletti, R.; Enderle, M.D.; et al. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest. Endosc. 2012, 76, 1142–1151. [Google Scholar] [CrossRef]
- Baust, J.M.; Robilotto, A.T.; Santucci, K.L.; Snyder, K.K.; Van Buskirk, R.G.; Katz, A.; Corcoran, A.; Baust, J.G. Evaluation of a Novel Cystoscopic Compatible Cryocatheter for the Treatment of Bladder Cancer. Bladder Cancer 2020, 6, 303–318. [Google Scholar] [CrossRef]
- Baust, J.M.; Santucci, K.L.; Van Buskirk, R.G.; Raijman, I.; Fisher, W.E.; Baust, J.G.; Snyder, K.K. An In Vitro Investigation into Cryoablation and Adjunctive Cryoablation/Chemotherapy Combination Therapy for the Treatment of Pancreatic Cancer Using the PANC-1 Cell Line. Biomedicines 2022, 10, 450. [Google Scholar] [CrossRef]
- Baust, J.M.; Robilotto, A.; Snyder, K.K.; Santucci, K.; Stewart, J.; Van Buskirk, R.; Baust, J.G. Assessment of Cryosurgical Device Performance Using a 3D Tissue-Engineered Cancer Model. Technol. Cancer Res. Treat. 2017, 16, 900–909. [Google Scholar] [CrossRef]
- Baumann, K.W.; Baust, J.M.; Snyder, K.K.; Baust, J.G.; Van Buskirk, R.G. Characterization of Pancreatic Cancer Cell Thermal Response to Heat Ablation or Cryoablation. Technol. Cancer Res. Treat. 2017, 16, 393–405. [Google Scholar] [CrossRef]
- Baumann, K.W.; Baust, J.M.; Snyder, K.K.; Baust, J.G.; Van Buskirk, R.G. Dual thermal ablation of pancreatic cancer cells as an improved combinatorial treatment stragegy. Liver Pancreat. Sci. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Robilotto, A.T.; Santucci, K.L.; Snyder, K.K.; Van Buskirk, R.G.; Baust, J.G.; Baust, J.M. Assessment of a novel supercritical nitrogen cryosurgical device using prostate and renal cancer tissue engineered models. Med. Devices Diagn. Eng. 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Kim, K.M.; Chung, S.; Kim, S.Y.; Kim, D.J.; Kim, J.S.; Lim, C.; Park, K.H. Comparison of Radiofrequency Ablation and Cryoablation for the Recovery of Atrial Contractility and Survival. Korean J. Thorac. Cardiovasc. Surg. 2018, 51, 266–272. [Google Scholar] [CrossRef]
- Attanasio, P.; Huemer, M.; Shokor Parwani, A.; Boldt, L.H.; Mugge, A.; Haverkamp, W.; Wutzler, A. Pain Reactions during Pulmonary Vein Isolation under Deep Sedation: Cryothermal versus Radiofrequency Ablation. Pacing Clin. Electrophysiol. 2016, 39, 452–457. [Google Scholar] [CrossRef]
- Lakhtakia, S.; Ramchandani, M.; Galasso, D.; Gupta, R.; Venugopal, S.; Kalpala, R.; Reddy, D.N. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest. Endosc. 2016, 83, 234–239. [Google Scholar] [CrossRef]
- Song, T.J.; Seo, D.W.; Lakhtakia, S.; Reddy, N.; Oh, D.W.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest. Endosc. 2016, 83, 440–443. [Google Scholar] [CrossRef]
- Crino, S.F.; D’Onofrio, M.; Bernardoni, L.; Frulloni, L.; Iannelli, M.; Malleo, G.; Paiella, S.; Larghi, A.; Gabbrielli, A. EUS-guided Radiofrequency Ablation (EUS-RFA) of Solid Pancreatic Neoplasm Using an 18-gauge Needle Electrode: Feasibility, Safety, and Technical Success. J. Gastrointestin Liver Dis. 2018, 27, 67–72. [Google Scholar] [CrossRef]
- Scopelliti, F.; Pea, A.; Conigliaro, R.; Butturini, G.; Frigerio, I.; Regi, P.; Giardino, A.; Bertani, H.; Paini, M.; Pederzoli, P.; et al. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg. Endosc. 2018, 32, 4022–4028. [Google Scholar] [CrossRef]
- Choi, J.H.; Seo, D.W.; Song, T.J.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy 2018, 50, 1099–1104. [Google Scholar] [CrossRef]
- Khoury, T.; Sbeit, W.; Fusaroli, P.; Campana, D.; Brighi, N.; Napoleon, B.; Lisotti, A. Safety and efficacy of endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine neoplasms: Systematic review and meta-analysis. Dig. Endosc. 2023. [Google Scholar] [CrossRef]
- Barthet, M.; Giovannini, M.; Gasmi, M.; Lesavre, N.; Boustiere, C.; Napoleon, B.; LaQuiere, A.; Koch, S.; Vanbiervliet, G.; Gonzalez, J.M. Long-term outcome after EUS-guided radiofrequency ablation: Prospective results in pancreatic neuroendocrine tumors and pancreatic cystic neoplasms. Endosc. Int. Open 2021, 9, E1178–E1185. [Google Scholar] [CrossRef]
- Barthet, M.; Giovannini, M.; Lesavre, N.; Boustiere, C.; Napoleon, B.; Koch, S.; Gasmi, M.; Vanbiervliet, G.; Gonzalez, J.M. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: A prospective multicenter study. Endoscopy 2019, 51, 836–842. [Google Scholar] [CrossRef]
- Littrup, P.J.; Jallad, B.; Vorugu, V.; Littrup, G.; Currier, B.; George, M.; Herring, D. Lethal isotherms of cryoablation in a phantom study: Effects of heat load, probe size, and number. J. Vasc. Interv. Radiol. 2009, 20, 1343–1351. [Google Scholar] [CrossRef]
- Shah, T.T.; Arbel, U.; Foss, S.; Zachman, A.; Rodney, S.; Ahmed, H.U.; Arya, M. Modeling Cryotherapy Ice Ball Dimensions and Isotherms in a Novel Gel-based Model to Determine Optimal Cryo-needle Configurations and Settings for Potential Use in Clinical Practice. Urology 2016, 91, 234–240. [Google Scholar] [CrossRef]
- Clarke, D.M.; Robilotto, A.T.; Rhee, E.; VanBuskirk, R.G.; Baust, J.G.; Gage, A.A.; Baust, J.M. Cryoablation of renal cancer: Variables involved in freezing-induced cell death. Technol. Cancer Res. Treat. 2007, 6, 69–79. [Google Scholar] [CrossRef]
- Baust, J.M.; Corcoran, A.; Robilotto, A.T.; Katz, A.; Santucci, K.L.; Van Buskirk, R.G.; Baust, J.G.; Snyder, K.K. A New Cystoscopic Cryocatheter and Method for the In Situ Destruction of Bladder Cancer: A Preliminary In Vivo Study. J. Endourol. 2023, Submitted. [Google Scholar] [CrossRef]
- Klossner, D.P.; Clarke, D.M.; Baust, J.M.; Van Buskirk, R.G.; Gage, A.A.; Baust, J.G. Thermal therapeutic options in the treatment of prostate cancer: Cellular responses to cryosurgery and hyperthermia. Cryobiology 2006, 53, 438. [Google Scholar] [CrossRef]
- Niu, L.; He, L.; Zhou, L.; Mu, F.; Wu, B.; Li, H.; Yang, Z.; Zuo, J.; Xu, K. Percutaneous ultrasonography and computed tomography guided pancreatic cryoablation: Feasibility and safety assessment. Cryobiology 2012, 65, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Niu, L.; Yang, D. Cryosurgery for pancreatic cancer. Gland. Surg. 2013, 2, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gu, Y.; Zhang, B.; Zhou, X.; Li, Y.; Qian, Z. Laparoscopic ultrasonography-guided cryoablation of locally advanced pancreatic cancer: A preliminary report. Jpn. J. Radiol. 2022, 40, 86–93. [Google Scholar] [CrossRef]
- Santucci, K.L.; Snyder, K.K.; Baust, J.G.; Van Buskirk, R.G.; Baust, J.M. Investigation of Liver Cancer Cell Response to Cryoablation and Adjunctive Based Cryo/Chemotherapy. Br. J. Cancer Res. 2020, 3, 407–414. [Google Scholar] [CrossRef]



| Freeze Protocol (mins) | Time to Ice (sec) | Iceball Size (mm (±SD)) | |
|---|---|---|---|
| Dia. | Length | ||
| Single 3 | 18.1 (±1.9) | 17.1 (±0.2) | 32.4 (±1.0) |
| Single 5 | 17.8 (±1.6) | 21.7 (±0.8) | 35.1 (±1.7) |
| Double 3/5/3 | 15.7 (±1.5) | 20.6 (±0.5) | 34.2 (±0.3) |
| Double 5/5/5 | 16.0 (±1.6) | 27.5 (±1.1) | 38.2 (±2.1) |
| Freeze Protocol (mins) | Single (First) Freeze Isotherm Dia (mm(±SD)) | Repeat (Second) Freeze Isotherm Dia (mm(±SD)) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 °C | −10 °C | −20 °C | −30 °C | −40 °C | 0 °C | −10 °C | −20 °C | −30 °C | −40 °C | |
| Single 3 | 16.4 (0.5) | 13.9 (0.4) | 11.6 (0.5) | 9.6 (0.3) | 8.5 (0.3) | |||||
| Single 5 | 21.2 (0.7) | 17.8 (0.4) | 14.8 (0.4) | 12.1 (0.4) | 9.7 (0.2) | |||||
| Double 3/5/3 | 16.8 (0.6) | 14.2 (0.5) | 12.0 (0.5) | 9.8 (0.4) | 8.6 (0.2) | 20.0 (0.4) | 17.0 (0.7) | 14.0 (0.6) | 11.6 (0.5) | 9.6 (0.4) |
| Double 5/5/5 | 22.2 (0.7) | 18.4 (0.6) | 15.2 (0.4) | 12.4 (0.5) | 9.8 (0.2) | 28.2 (1.0) | 23.2 (0.9) | 18.6 (0.6) | 15.0 (0.6) | 11.8 (0.4) |
| Time | Water Temperature (°C) | Cooling Power (W) | |||
|---|---|---|---|---|---|
| Start Temp | End Temp | ΔT | Total Watts (W) | W/mm2 | |
| 3 min | 33.6 (±0.3) | 28.7 (±0.4) | 4.9 (±0.8) | 51.2 (±6.3) | 1.09 (±0.04) |
| 3.5 min | 27.9 (±0.4) | 5.7 (±0.9) | 51.6 (±5.8) | 1.09 (±0.03) | |
| 4 min | 27.0 (±0.5) | 6.6 (±1.0) | 52.0 (±5.9) | 1.1 (±0.05) | |
| 4.5min | 26.1 (±0.5) | 7.4 (±1.1) | 52.2 (±5.5) | 1.11 (±0.04) | |
| 5 min | 25.1 (±0.6) | 8.4 (±1.2) | 53.3 (±5.3) | 1.13 (±0.2) | |
| Avg | 33.6 (±0.3) | 27.0 (±0.5) | 6.6 (±1.0) | 52.1 (±5.7) | 1.1 (±0.03) |
| Single 5 min Freeze | |||||
|---|---|---|---|---|---|
| 24 h Post-Thaw | 72 h Post-Thaw | ||||
| Freeze Zone | Necrotic Zone | Freeze Zone | Necrotic Zone | ||
| PANC-1 TEMs | Dia. (mm (±SD)) | 25.1 (0.4) | 20.1 (2.1) | 24.1 (2.0) | 20.6 (1.4) |
| Volume (cm3 (±SD)) | 13.0 (0.4) | 6.9 (0.8) | 12.1 (1.9) | 6.8 (0.6) | |
| % Lethality | 53.3 | 56.1 | |||
| Freeze # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Freeze Protocol | Single | Single | Single | Single | Single | Single | Double | Single | |
| Freeze Duration | 3 min | 3 min | 3 min | 5 min | 3 min | 3 min | 3/3/3 | 3 min | |
| Frozen Mass Size | EUS Diameter | N/A | 2 cm | 2 cm | 2.1 cm | 1.9 cm | N/A | 1st freeze: 2.1 cm 2nd freeze: 2.4 cm | N/A |
| Pathology Dia × Lgn | 2.1 cm × 3.2 cm | 2.1 cm × 3.3 cm | 2 cm × 3.1 cm | 2.2 cm × 3.4 cm | 2 cm × 3 cm | N/A * | 2.5 cm × 3.5 cm | N/A * | |
| % of Patients | |||||
|---|---|---|---|---|---|
| Author | # Patients | Average Survival (months) | % Survival @ 12 months | Pain Reduction | Incidence of Pancreatitis |
| Kovach et al. [5] | 9 | 5 | - | 66% | 0% (0/9) |
| Niu et al. [88] | 32 | 15.9 | 54% | >50% | 0% (0/32) |
| Xu et al. [60] | 49 | 16 | 63% | - | 12% (6/49) |
| Xu et al. [61] | 59 | 8.4 | 34% | - | 5% (3/59) |
| Wu et al. [90] | 10 | 3 month follow-up | - | 6.9 to 2.0 | 0% (0/10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baust, J.M.; Robilotto, A.; Raijman, I.; Santucci, K.L.; Van Buskirk, R.G.; Baust, J.G.; Snyder, K.K. The Assessment of a Novel Endoscopic Ultrasound-Compatible Cryocatheter to Ablate Pancreatic Cancer. Biomedicines 2024, 12, 507. https://doi.org/10.3390/biomedicines12030507
Baust JM, Robilotto A, Raijman I, Santucci KL, Van Buskirk RG, Baust JG, Snyder KK. The Assessment of a Novel Endoscopic Ultrasound-Compatible Cryocatheter to Ablate Pancreatic Cancer. Biomedicines. 2024; 12(3):507. https://doi.org/10.3390/biomedicines12030507
Chicago/Turabian StyleBaust, John M., Anthony Robilotto, Isaac Raijman, Kimberly L. Santucci, Robert G. Van Buskirk, John G. Baust, and Kristi K. Snyder. 2024. "The Assessment of a Novel Endoscopic Ultrasound-Compatible Cryocatheter to Ablate Pancreatic Cancer" Biomedicines 12, no. 3: 507. https://doi.org/10.3390/biomedicines12030507
APA StyleBaust, J. M., Robilotto, A., Raijman, I., Santucci, K. L., Van Buskirk, R. G., Baust, J. G., & Snyder, K. K. (2024). The Assessment of a Novel Endoscopic Ultrasound-Compatible Cryocatheter to Ablate Pancreatic Cancer. Biomedicines, 12(3), 507. https://doi.org/10.3390/biomedicines12030507






