Nephrotic Syndrome: From Pathophysiology to Novel Therapeutic Approaches
Abstract
1. Introduction
2. Edema Development: The Underfill and Overfill Hypotheses
2.1. The Underfill Hypothesis
2.1.1. Hypoalbuminemia
2.1.2. Hypovolemia
2.1.3. Renin-Angiotensin-Aldosterone System
- To our knowledge, there are no large randomized trials of renin suppression with direct inhibitors or beta-blockers. Meltzer et al. showed no increase in diuresis or natriuresis after propranolol treatment [29];
- The role of angiotensin II in the proximal tubular uptake of sodium via angiotensin II receptor type 1 (AT1) in nephrotic syndrome is controversial. Instead, angiotensin II increases sodium reabsorption in the cortical-collecting duct (CCD) system through epithelial sodium channel (ENaC) aldosterone-independent stimulation [30]. Nevertheless, angiotensin-converting enzyme-inhibitors (ACE-inhibitors), particularly captopril, failed to increase sodium and water excretion, although marked diuresis was noted in healthy subjects [31];
- Aldosterone antagonists have been shown to cause little or no improvement in the natriuretic effect in nephrotic patients or murine models [32,33,34]. Shapiro et al. demonstrated that spironolactone caused significant increase in sodium excretion in nephrotic patients compared to a placebo [24]. However, it is essential to consider that this was a small-scale study involving only five patients with nephrotic syndrome [35]. Moreover, in rats with unilateral puromycin aminonucleoside-induced (PAN-induced) nephrotic syndrome, sodium retention occurred only in the affected kidney, suggesting a localized mechanism to explain edema rather than a systemic factor such as hyperaldosteronism [36]. Additionally, in rats with PAN-induced nephrotic syndrome, ENaC activity was correlated with increased aldosterone levels, while adrenalectomized rats or corticosteroid-clamped rats maintained their sodium and water retention independent of hyperaldosteronism [37,38].
2.1.4. Arginine Vasopressin
2.1.5. Sympathetic Nervous System (SNS)
2.1.6. Atrial Natriuretic Peptide (ANP)
2.2. The Overfill Hypothesis
2.2.1. Serin Proteases
2.2.2. Plasmin
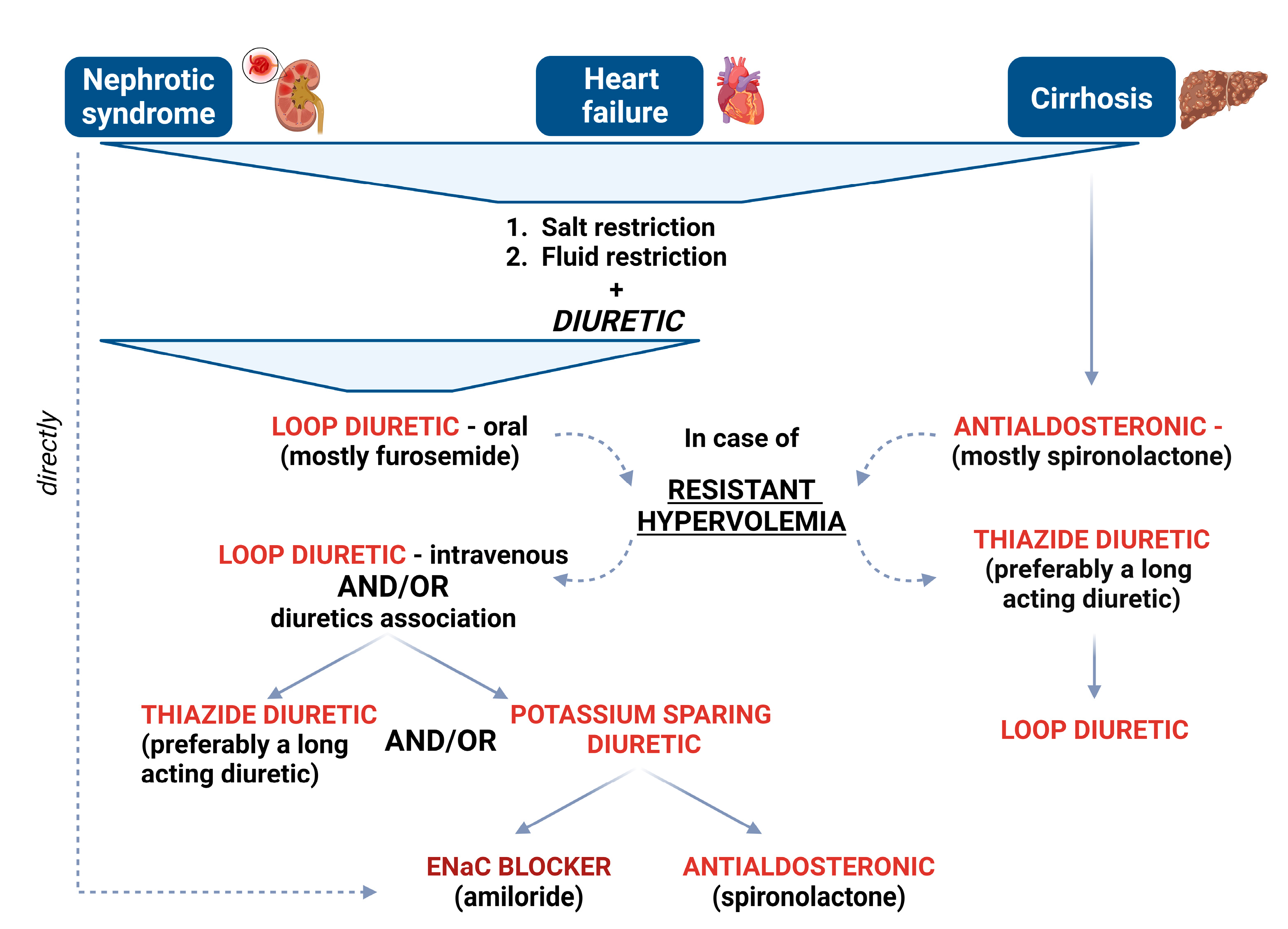
3. Diuretic-Resistant Hypervolemia
4. Diuretic Treatment
- Sulfonamide-loop diuretics, thiazide diuretics, and carbonic anhydrase inhibitors (CA inhibitors);
- Potassium-sparing diuretic-ENaC antagonists and aldosterone antagonists;
- Vasopressin-receptor antagonists (vaptans);
- Osmotic diuretics.
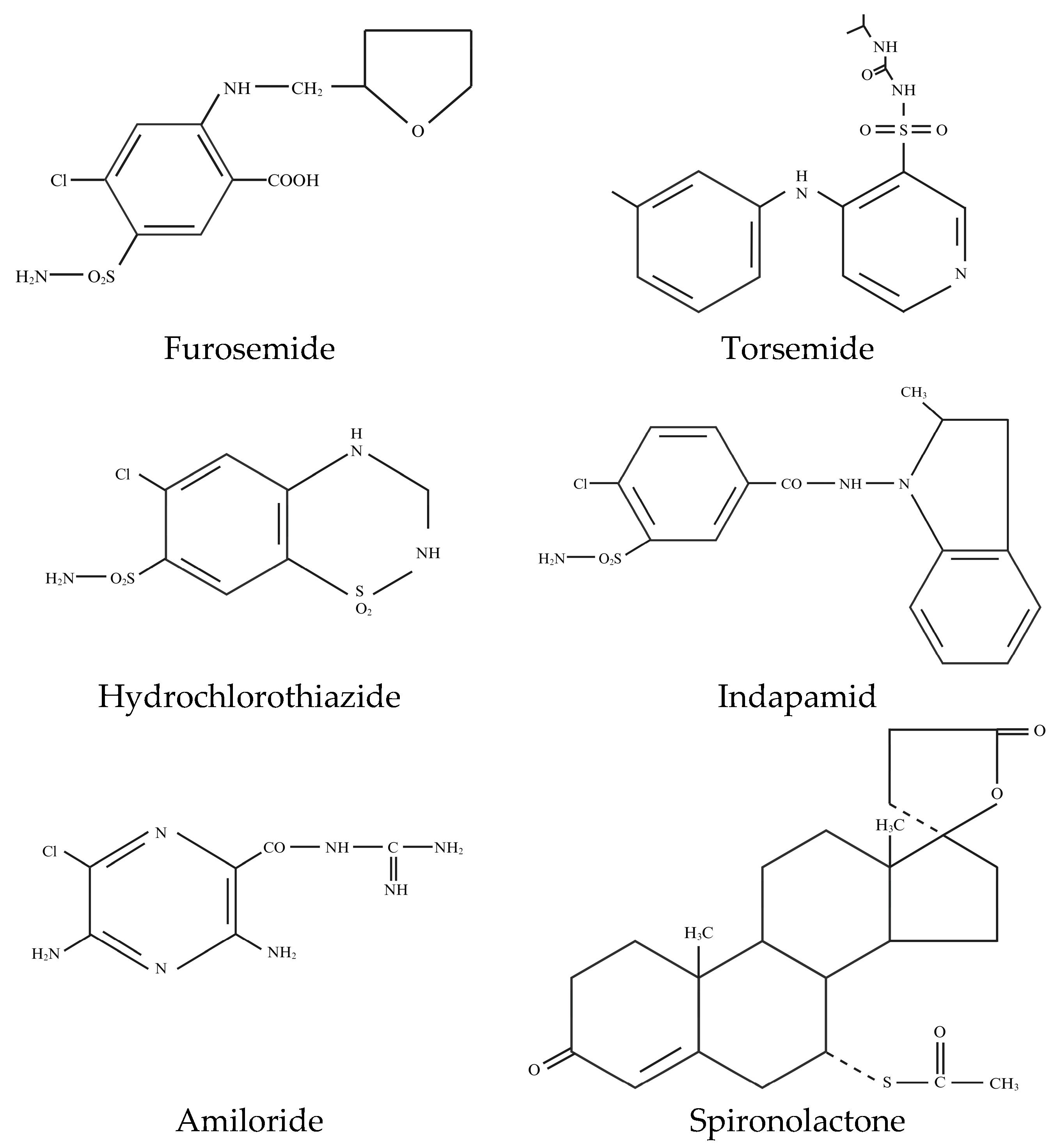
4.1. Loop Diuretics
4.2. Thiazide Diuretics
4.3. Potassium-Sparing Diuretics
4.4. Carbonic Anhydrase (CA) Inhibitors
4.5. Vasopressin Receptor Antagonists (Vaptans)
4.6. Osmotic Diuretics
4.7. Sodium–Glucose Cotransporter 2 (SGLT2)-Inhibitors
4.7.1. Managing Volume Overload in Certain Conditions
Heart Failure
End-Stage Liver Disease
Nephrotic Syndrome
4.7.2. New Pharmacological Targets
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, S.; Pepper, R.J.; Ashman, N.; Walsh, S.B. Nephrotic Syndrome: Oedema Formation and Its Treatment with Diuretics. Front. Physiol. 2019, 9, 1868. [Google Scholar] [CrossRef] [PubMed]
- Qavi, A.H.; Kamal, R.; Schrier, R.W. Clinical Use of Diuretics in Heart Failure, Cirrhosis, and Nephrotic Syndrome. Int. J. Nephrol. 2015, 2015, 975934. [Google Scholar] [CrossRef]
- Ellis, D. Pathophysiology, evaluation, and management of edema in childhood nephrotic syndrome. Front. Pediatr. 2016, 3, 111. [Google Scholar] [CrossRef]
- Humphreys, M.H. Mechanisms and management of nephrotic edema. Kidney Int. 1994, 45, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Meena, J.; Bagga, A. Current Perspectives in Management of Edema in Nephrotic Syndrome. Indian J. Pediatr. 2020, 87, 633–640. [Google Scholar] [CrossRef]
- Starling, E.H. On the Absorption of Fluids from the Connective Tissue Spaces. J. Physiol. 1896, 19, 312–326. [Google Scholar] [CrossRef]
- Taylor, A.E. Capillary fluid filtration. Starling forces and lymph flow. Circ. Res. 1981, 49, 557–575. [Google Scholar] [CrossRef] [PubMed]
- Siddall, E.C.; Radhakrishnan, J. The pathophysiology of edema formation in the nephrotic syndrome. Kidney Int. 2012, 82, 635–642. [Google Scholar] [CrossRef]
- Walle, J.V.; Donckerwolcke, R.; Boer, P.; Van Isselt, H.W.; Koomans, H.A.; Joles, J.A. Blood volume, colloid osmotic pressure and F-cell ratio in children with the nephrotic syndrome. Kidney Int. 1996, 49, 1471–1477. [Google Scholar] [CrossRef][Green Version]
- Rostoker, G.; Behar, A.; Lagrue, G. Vascular hyperpermeability in nephrotic edema. Nephron 2000, 85, 194–200. [Google Scholar] [CrossRef]
- Walle, J.G.J.V.; Donckerwolcke, R.A.; Koomans, H.A. Pathophysiology of edema formation in children with nephrotic syndrome not due to minimal change disease. J. Am. Soc. Nephrol. 1999, 10, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Juncos, L.I. Intrarenal mechanisms of salt and water retention in the nephritic syndrome. Kidney Int. 2002, 61, 1182–1195. [Google Scholar] [CrossRef]
- Vande Walle, J.G.; Donckerwolcke, R.A.; van Isselt, J.W.; Derkx, F.H.; Joles, J.A.; Koomans, H.A. Volume regulation in children with early relapse of minimal-change nephrosis with or without hypovolaemic symptoms. Lancet 1995, 346, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Koomans, H.A.; Geers, A.B.; Dorhout Mees, E.J.; Kortlandt, W. Lowered tissue-fluid oncotic pressure protects the blood volume in the nephrotic syndrome. Nephron 1986, 42, 317–322. [Google Scholar] [CrossRef]
- Aukland, K.; Nicolaysen, G. Interstitial fluid volume: Local regulatory mechanisms. Physiol. Rev. 1981, 61, 556–643. [Google Scholar] [CrossRef]
- Fauchald, P.; Noddeland, H.; Norseth, J. Interstitial fluid volume, plasma volume and colloid osmotic pressure in patients with nephrotic syndrome. Scand. J. Clin. Lab. Investig. 1984, 44, 661–667. [Google Scholar] [CrossRef]
- Lecomte, J.; Juchmès, J. [So-called absence of edema in analbuminemia]. Rev. Med. Liege 1978, 33, 766–770. [Google Scholar]
- Koot, B.G.P.; Houwen, R.; Pot, D.-J.; Nauta, J. Congenital analbuminaemia: Biochemical and clinical implications. A case report and literature review. Eur. J. Pediatr. 2004, 163, 664–670. [Google Scholar] [CrossRef]
- Oliver, W.J. Physiologic Responses Associated with Steroid-Induced Diuresis in the Nephrotic Syndrome. J. Lab. Clin. Med. 1963, 62, 449–464. [Google Scholar]
- Duffy, M.; Jain, S.; Harrell, N.; Kothari, N.; Reddi, A.S. Albumin and Furosemide Combination for Management of Edema in Nephrotic Syndrome: A Review of Clinical Studies. Cells 2015, 4, 622–630. [Google Scholar] [CrossRef]
- Ho, J.J.; Adnan, A.S.; Kueh, Y.C.; Ambak, N.J.; Van Rostenberghe, H.; Jummaat, F. Human albumin infusion for treating oedema in people with nephrotic syndrome. Cochrane Database Syst. Rev. 2019, 2019, CD009692. [Google Scholar] [CrossRef]
- Fliser, D.; Zurbrüggen, I.; Mutschler, E.; Bischoff, I.; Nussberger, J.; Franek, E.; Ritz, E. Coadministration of albumin and furosemide in patients with the nephrotic syndrome. Kidney Int. 1999, 55, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Na, K.Y.; Han, J.S.; Kim, Y.S.; Ahn, C.; Kim, S.; Lee, J.S.; Bae, K.S.; Jang, I.J.; Shin, S.G.; Huh, W.; et al. Does albumin preinfusion potentiate diuretic action of furosemide in patients with nephrotic syndrome? J. Korean Med. Sci. 2001, 16, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, A.; Mehdizadeh, A.; Alavi-Darazam, I.; Rahimi, E.; Kargar, C.; Sepehrvand, N. Co-administration of albumin-furosemide in patients with the nephrotic syndrome. Saudi J. Kidney Dis. Transpl. 2011, 22, 471–475. [Google Scholar] [PubMed]
- Dharmaraj, R.; Hari, P.; Bagga, A. Randomized cross-over trial comparing albumin and frusemide infusions in nephrotic syndrome. Pediatr. Nephrol. 2009, 24, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Akcicek, F.; Yalniz, T.; Basci, A.; Ok, E.; Mees, E.J. Diuretic effect of frusemide in patients with nephrotic syndrome: Is it potentiated by intravenous albumin? BMJ 1995, 310, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Geers, A.B.; Koomans, H.A.; Roos, J.C.; Boer, P.; Dorhout Mees, E.J. Functional relationships in the nephrotic syndrome. Kidney Int. 1984, 26, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Bojestig, M.; Nystrom, F.H.; Arnqvist, H.J.; Ludvigsson, J.; Karlberg, B.E. The renin-angiotensin-aldosterone system is suppressed in adults with Type 1 diabetes. J. Renin. Angiotensin. Aldosterone. Syst. 2000, 1, 353–356. [Google Scholar] [CrossRef]
- Meltzer, J.I.; Keim, H.J.; Laragh, J.H.; Sealey, J.E.; Jan, K.M.; Chien, S. Nephrotic syndrome: Vasoconstriction and hypervolemic types indicated by renin-sodium profiling. Ann. Intern. Med. 1979, 91, 688–696. [Google Scholar] [CrossRef]
- Peti-Peterdi, J.; Warnock, D.G.; Bell, P.D. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J. Am. Soc. Nephrol. 2002, 13, 1131–1135. [Google Scholar] [CrossRef]
- Brown, E.A.; Markandu, N.D.; Sagnella, G.A.; Jones, B.E.; MacGregor, G.A. Lack of effect of captopril on the sodium retention of the nephrotic syndrome. Nephron 1984, 37, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Deschênes, G.; Wittner, M.; Stefano, A.; Jounier, S.; Doucet, A. Collecting duct is a site of sodium retention in PAN nephrosis: A rationale for amiloride therapy. J. Am. Soc. Nephrol. 2001, 12, 598–601. [Google Scholar]
- Russo, R.; Schena, F.P.; Colombo Pirola, L. [Controlled clinical study on 2 antialdosterone diuretics in the nephrotic syndrome]. Clin. Ter. 1984, 109, 23–29. [Google Scholar]
- Usberti, M.; Gazzotti, R.M. Hyporeninemic hypoaldosteronism in patients with nephrotic syndrome. Am. J. Nephrol. 1998, 18, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.D.; Hasbargen, J.; Hensen, J.; Schrier, R.W. Role of aldosterone in the sodium retention of patients with nephrotic syndrome. Am. J. Nephrol. 1990, 10, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, I.; Rennke, H.G.; Hoyer, J.R.; Badr, K.F.; Schor, N.; Troy, J.L.; Lechene, C.P.; Brenner, B.M. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J. Clin. Investig. 1983, 71, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Lourdel, S.; Loffing, J.; Favre, G.; Paulais, M.; Nissant, A.; Fakitsas, P.; Créminon, C.; Féraille, E.; Verrey, F.; Teulon, J.; et al. Hyperaldosteronemia and activation of the epithelial sodium channel are not required for sodium retention in puromycin-induced nephrosis. J. Am. Soc. Nephrol. 2005, 16, 3642–3650. [Google Scholar] [CrossRef]
- de Seigneux, S.; Kim, S.W.; Hemmingsen, S.C.; Frøkiaer, J.; Nielsen, S. Increased expression but not targeting of ENaC in adrenalectomized rats with PAN-induced nephrotic syndrome. Am. J. Physiol. Renal Physiol. 2006, 291, F208–F217. [Google Scholar] [CrossRef][Green Version]
- Reddy, P.; Mooradian, A.D. Diuretics: An update on the pharmacology and clinical uses. Am. J. Ther. 2009, 16, 74–85. [Google Scholar] [CrossRef]
- Schrier, R.W.; Howard, R.L. Pathophysiology of vasopressin in edematous disorders. Nihon Naibunpi Gakkai Zasshi 1989, 65, 1311–1327. [Google Scholar] [CrossRef][Green Version]
- Bockenhauer, D. Draining the edema: A new role for aquaretics? Pediatr. Nephrol. 2014, 29, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, E.B.; Danielsen, H.; Sørensen, S.S.; Jespersen, B. Renal water excretion before and after remission of nephrotic syndrome: Relationship between free water clearance and kidney function, arginine vasopressin, angiotensin II and aldosterone in plasma before and after oral water loading. Clin. Sci. 1986, 71, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Meena, J.; Sinha, A.; Hari, P.; Bagga, A. Therapy with the Combination of Tolvaptan and Furosemide for Refractory Edema in Nephrotic Syndrome. Indian J. Nephrol. 2020, 30, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.E.; Ellison, D.H. Diuretics in States of Volume Overload: Core Curriculum 2022. Am. J. Kidney Dis. 2022, 80, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.N.; Abraham, W.T.; Van Putten, V.J.; Hasbargen, J.A.; Schrier, R.W. Increased norepinephrine secretion in patients with the nephrotic syndrome and normal glomerular filtration rates: Evidence for primary sympathetic activation. Am. J. Nephrol. 1993, 13, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.R.J. Neural control of renal tubular sodium reabsorption. Nephron 1979, 23, 116–118. [Google Scholar] [CrossRef]
- Neahring, J.C.; Jones, S.Y.; DiBona, G.F. Cardiopulmonary baroreflex function in nephrotic rats. J. Am. Soc. Nephrol. 1995, 5, 2082–2086. [Google Scholar] [CrossRef]
- Perico, N.; Remuzzi, G. Renal handling of sodium in the nephrotic syndrome. Am. J. Nephrol. 1993, 13, 413–421. [Google Scholar] [CrossRef]
- Valentin, J.P.; Ying, W.Z.; Sechi, L.A.; Ling, K.T.; Qiu, C.; Couser, W.G.; Humphreys, M.H. Phosphodiesterase inhibitors correct resistance to natriuretic peptides in rats with Heymann Nephritis. J. Am. Soc. Nephrol. 1996, 7, 582–593. [Google Scholar] [CrossRef]
- Orisio, S.; Perico, N.; Benatti, L.; Longaretti, L.; Amuchastegui, S.; Remuzzi, G. Renal cyclophilin-like protein gene expression parallels changes in sodium excretion in experimental nephrosis and is positively modulated by atrial natriuretic peptide. J. Am. Soc. Nephrol. 1993, 3, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Inagami, T. Molecular cloning of a complementary DNA to rat cyclophilin-like protein mRNA. Kidney Int. 1990, 37, 1460–1465. [Google Scholar] [CrossRef]
- Klein, J.D. Corin: An ANP protease that may regulate sodium reabsorption in nephrotic syndrome. Kidney Int. 2010, 78, 635–637. [Google Scholar] [CrossRef]
- Polzin, D.; Kaminski, H.J.; Kastner, C.; Wang, W.; Krämer, S.; Gambaryan, S.; Russwurm, M.; Peters, H.; Wu, Q.; Vandewalle, A.; et al. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010, 78, 650–659. [Google Scholar] [CrossRef]
- Besse-Eschmann, V.; Klisic, J.; Nief, V.; Le Hir, M.; Kaissling, B.; Ambühl, P.M. Regulation of the proximal tubular sodium/proton exchanger NHE3 in rats with puromycin aminonucleoside (PAN)-induced nephrotic syndrome. J. Am. Soc. Nephrol. 2002, 13, 2199–2206. [Google Scholar] [CrossRef]
- Klisic, J.; Zhang, J.; Nief, V.; Reyes, L.; Moe, O.W.; Ambühl, P.M. Albumin regulates the Na+/H+ exchanger 3 in OKP cells. J. Am. Soc. Nephrol. 2003, 14, 3008–3016. [Google Scholar] [CrossRef][Green Version]
- Orce, G.G.; Castillo, G.A.; Margolius, H.S. Inhibition of short-circuit current in toad urinary bladder by inhibitors of glandular kallikrein. Am. J. Physiol. 1980, 239, F459–F465. [Google Scholar] [CrossRef] [PubMed]
- Vallet, V.; Chraibi, A.; Gaeggeler, H.P.; Horisberger, J.D.; Rossier, B.C. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 1997, 389, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Durieux, M.E.; Salafranca, M.N.; Lynch, K.R. Trypsin induces Ca(2+)-activated Cl- currents in X. laevis oocytes. FEBS Lett. 1994, 337, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Chraïbi, A.; Vallet, V.; Firsov, D.; Hess, S.K.; Horisberger, J.D. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J. Gen. Physiol. 1998, 111, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Hughey, R.P.; Mueller, G.M.; Bruns, J.B.; Kinlough, C.L.; Poland, P.A.; Harkleroad, K.L.; Carattino, M.D.; Kleyman, T.R. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J. Biol. Chem. 2003, 278, 37073–37082. [Google Scholar] [CrossRef] [PubMed]
- Hughey, R.P.; Bruns, J.B.; Kinlough, C.L.; Harkleroad, K.L.; Tong, Q.; Carattino, M.D.; Johnson, J.P.; Stockand, J.D.; Kleyman, T.R. Epithelial sodium channels are activated by furin-dependent proteolysis. J. Biol. Chem. 2004, 279, 18111–18114. [Google Scholar] [CrossRef]
- Bruns, J.B.; Carattino, M.D.; Sheng, S.; Maarouf, A.B.; Weisz, O.A.; Pilewski, J.M.; Hughey, R.P.; Kleyman, T.R. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J. Biol. Chem. 2007, 282, 6153–6160. [Google Scholar] [CrossRef] [PubMed]
- Ray, E.C.; Rondon-Berrios, H.; Boyd, C.R.; Kleyman, T.R. Sodium retention and volume expansion in nephrotic syndrome: Implications for hypertension. Adv. Chronic Kidney Dis. 2015, 22, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kakizoe, Y.; Kitamura, K.; Ko, T.; Wakida, N.; Maekawa, A.; Miyoshi, T.; Shiraishi, N.; Adachi, M.; Zhang, Z.; Masilamani, S.; et al. Aberrant ENaC activation in Dahl salt-sensitive rats. J. Hypertens. 2009, 27, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Zachar, R.M.; Skjødt, K.; Marcussen, N.; Walter, S.; Toft, A.; Nielsen, M.R.; Jensen, B.L.; Svenningsen, P. The epithelial sodium channel γ-subunit is processed proteolytically in human kidney. J. Am. Soc. Nephrol. 2015, 26, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, K.; Kakizoe, Y.; Onoue, T.; Hayata, M.; Morinaga, J.; Yamazoe, R.; Ueda, M.; Mizumoto, T.; Adachi, M.; Miyoshi, T.; et al. In vivo contribution of serine proteases to the proteolytic activation of γENaC in aldosterone-infused rats. Am. J. Physiol. Renal Physiol. 2012, 303, F939–F943. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, P.; Bistrup, C.; Friis, U.G.; Bertog, M.; Haerteis, S.; Krueger, B.; Stubbe, J.; Jensen, O.N.; Thiesson, H.C.; Uhrenholt, T.R.; et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J. Am. Soc. Nephrol. 2009, 20, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, G.R.; Weyer, K.; Friis, U.G.; Svenningsen, P.; Lund, I.K.; Nielsen, R.; Mollet, G.; Antignac, C.; Bistrup, C.; Jensen, B.L.; et al. Urokinase-type plasminogen activator contributes to amiloride-sensitive sodium retention in nephrotic range glomerular proteinuria in mice. Acta Physiol. 2019, 227, e13362. [Google Scholar] [CrossRef]
- Bohnert, B.N.; Daiminger, S.; Wörn, M.; Sure, F.; Staudner, T.; Ilyaskin, A.V.; Batbouta, F.; Janessa, A.; Schneider, J.C.; Essigke, D.; et al. Urokinase-type plasminogen activator (uPA) is not essential for epithelial sodium channel (ENaC)-mediated sodium retention in experimental nephrotic syndrome. Acta Physiol. 2019, 227, e13286. [Google Scholar] [CrossRef]
- Svenningsen, P.; Andersen, H.; Nielsen, L.H.; Jensen, B.L. Urinary serine proteases and activation of ENaC in kidney--implications for physiological renal salt handling and hypertensive disorders with albuminuria. Pflugers Arch. 2015, 467, 531–542. [Google Scholar] [CrossRef]
- Fila, M.; Sassi, A.; Brideau, G.; Cheval, L.; Morla, L.; Houillier, P.; Walter, C.; Gennaoui, M.; Collignon, L.; Keck, M.; et al. A variant of ASIC2 mediates sodium retention in nephrotic syndrome. JCI Insight 2021, 6, e148588. [Google Scholar] [CrossRef]
- Cadnapaphornchai, M.A.; Tkachenko, O.; Shchekochikhin, D.; Schrier, R.W. The nephrotic syndrome: Pathogenesis and treatment of edema formation and secondary complications. Pediatr. Nephrol. 2014, 29, 1159–1167. [Google Scholar] [CrossRef]
- Knauf, H.; Mutschler, E. Sequential nephron blockade breaks resistance to diuretics in edematous states. J. Cardiovasc. Pharmacol. 1997, 29, 367–372. [Google Scholar] [CrossRef]
- Brater, D.C. Update in diuretic therapy: Clinical pharmacology. Semin. Nephrol. 2011, 31, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Brater, D.C. Resistance to loop diuretics. Why it happens and what to do about it. Drugs 1985, 30, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Brater, D.C. Diuretic resistance: Mechanisms and therapeutic strategies. Cardiology 1994, 84 (Suppl. S2), 57–67. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Fu, B.; Liu, Y.; Hao, N.; Ji, Y.; Yang, H. Diuretic resistance in patients with kidney disease: Challenges and opportunities. Biomed. Pharmacother. 2023, 157, 114058. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Onbe, T.; Kumagai, I.; Murakami, K.; Ota, Z. A proteinase inhibitor reduces proteinuria in nephrotic syndrome. Am. J. Nephrol. 1991, 11, 164–165. [Google Scholar] [CrossRef]
- Ellison, D.H.; Felker, G.M. Diuretic Treatment in Heart Failure. N. Engl. J. Med. 2017, 377, 1964–1975. [Google Scholar] [CrossRef]
- Agarwal, R.; Sinha, A.D.; Cramer, A.E.; Balmes-Fenwick, M.; Dickinson, J.H.; Ouyang, F.; Tu, W. Chlorthalidone for Hypertension in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2021, 385, 2507–2519. [Google Scholar] [CrossRef]
- Roush, G.C.; Kaur, R.; Ernst, M.E. Diuretics: A review and update. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 5–13. [Google Scholar] [CrossRef]
- Lupușoru, G.; Ailincăi, I.; Frățilă, G.; Ungureanu, O.; Andronesi, A.; Lupușoru, M.; Banu, M.; Văcăroiu, I.; Dina, C.; Sinescu, I. Tumor Lysis Syndrome: An Endless Challenge in Onco-Nephrology. Biomedicines 2022, 10, 1012. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Kehrenberg, M.C.A.; Bachmann, H.S. Diuretics: A contemporary pharmacological classification? Naunyn. Schmiedebergs. Arch. Pharmacol. 2022, 395, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Fallahzadeh, M.A.; Dormanesh, B.; Fallahzadeh, M.K.; Roozbeh, J.; Fallahzadeh, M.H.; Sagheb, M.M. Acetazolamide and Hydrochlorothiazide Followed by Furosemide Versus Furosemide and Hydrochlorothiazide Followed by Furosemide for the Treatment of Adults With Nephrotic Edema: A Randomized Trial. Am. J. Kidney Dis. 2017, 69, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.L.; Ellison, D.H. Diuretics and salt transport along the nephron. Semin. Nephrol. 2011, 31, 475–482. [Google Scholar] [CrossRef]
- Ansary, T.M.; Nakano, D.; Nishiyama, A. Diuretic Effects of Sodium Glucose Cotransporter 2 Inhibitors and Their Influence on the Renin-Angiotensin System. Int. J. Mol. Sci. 2019, 20, 629. [Google Scholar] [CrossRef]
- Masuda, T.; Muto, S.; Fukuda, K.; Watanabe, M.; Ohara, K.; Koepsell, H.; Vallon, V.; Nagata, D. Osmotic diuresis by SGLT2 inhibition stimulates vasopressin-induced water reabsorption to maintain body fluid volume. Physiol. Rep. 2020, 8, e14360. [Google Scholar] [CrossRef]
- Delanaye, P.; Scheen, A.J. The diuretic effects of SGLT2 inhibitors: A comprehensive review of their specificities and their role in renal protection. Diabetes Metab. 2021, 47, 101285. [Google Scholar] [CrossRef]
- Yasui, A.; Lee, G.; Hirase, T.; Kaneko, T.; Kaspers, S.; von Eynatten, M.; Okamura, T. Empagliflozin Induces Transient Diuresis Without Changing Long-Term Overall Fluid Balance in Japanese Patients With Type 2 Diabetes. Diabetes Ther. 2018, 9, 863–871. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; de Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 853–862. [Google Scholar] [CrossRef]
- Griffin, M.; Rao, V.S.; Ivey-Miranda, J.; Fleming, J.; Mahoney, D.; Maulion, C.; Suda, N.; Siwakoti, K.; Ahmad, T.; Jacoby, D.; et al. Empagliflozin in Heart Failure: Diuretic and Cardiorenal Effects. Circulation 2020, 142, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Ohara, K.; Murakami, T.; Imai, T.; Nakagawa, S.; Okada, M.; Miki, A.; Myoga, A.; Onishi, A.; Sekiguchi, C.; et al. Sodium-Glucose cotransporter 2 Inhibition with Dapagliflozin Ameliorates Extracellular Volume Expansion in Diabetic Kidney Disease Patients. POJ Diabetes Obes. Manag. 2017, 1, 1–8. [Google Scholar]
- Ohara, K.; Masuda, T.; Murakami, T.; Imai, T.; Yoshizawa, H.; Nakagawa, S.; Okada, M.; Miki, A.; Myoga, A.; Sugase, T.; et al. Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on fluid distribution: A comparison study with furosemide and tolvaptan. Nephrology 2019, 24, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Schork, A.; Saynisch, J.; Vosseler, A.; Jaghutriz, B.A.; Heyne, N.; Peter, A.; Häring, H.-U.; Stefan, N.; Fritsche, A.; Artunc, F. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: A prospective study using bioimpedance spectroscopy. Cardiovasc. Diabetol. 2019, 18, 46. [Google Scholar] [CrossRef]
- Schulze, P.C.; Bogoviku, J.; Westphal, J.; Aftanski, P.; Haertel, F.; Grund, S.; von Haehling, S.; Schumacher, U.; Möbius-Winkler, S.; Busch, M. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients With Acute Decompensated Heart Failure (EMPAG-HF). Circulation 2022, 146, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) W. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2022, 388, 117–127. [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Dharia, A.; Khan, A.; Sridhar, V.S.; Cherney, D.Z.I. SGLT2 Inhibitors: The Sweet Success for Kidneys. Annu. Rev. Med. 2023, 74, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, C.; Zhang, P.; Luo, T.; Huang, Y.; Yang, X. A profile of SGLT-2 inhibitors in hyponatremia: The evidence to date. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2023, 184, 106415. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ye, L.; Yan, Q.; Zhang, X.; Wang, L. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Water and Sodium Metabolism. Front. Pharmacol. 2022, 13, 800490. [Google Scholar] [CrossRef]
- Pelletier, R.; Ng, K.; Alkabbani, W.; Labib, Y.; Mourad, N.; Gamble, J.-M. Adverse events associated with sodium glucose co-transporter 2 inhibitors: An overview of quantitative systematic reviews. Ther. Adv. drug Saf. 2021, 12, 2042098621989134. [Google Scholar] [CrossRef]
- Wile, D. Diuretics: A review. Ann. Clin. Biochem. 2012, 49, 419–431. [Google Scholar] [CrossRef]
- Felker, G.M.; Mentz, R.J.; Cole, R.T.; Adams, K.F.; Egnaczyk, G.F.; Fiuzat, M.; Patel, C.B.; Echols, M.; Khouri, M.G.; Tauras, J.M.; et al. Efficacy and Safety of Tolvaptan in Patients Hospitalized With Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1399–1406. [Google Scholar] [CrossRef]
- Refardt, J.; Imber, C.; Sailer, C.O.; Jeanloz, N.; Potasso, L.; Kutz, A.; Widmer, A.; Urwyler, S.A.; Ebrahimi, F.; Vogt, D.R.; et al. A Randomized Trial of Empagliflozin to Increase Plasma Sodium Levels in Patients with the Syndrome of Inappropriate Antidiuresis. J. Am. Soc. Nephrol. 2020, 31, 615–624. [Google Scholar] [CrossRef]
- Refardt, J.; Imber, C.; Nobbenhuis, R.; Sailer, C.O.; Haslbauer, A.; Monnerat, S.; Bathelt, C.; Vogt, D.R.; Berres, M.; Winzeler, B.; et al. Treatment Effect of the SGLT2 Inhibitor Empagliflozin on Chronic Syndrome of Inappropriate Antidiuresis: Results of a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J. Am. Soc. Nephrol. 2023, 34, 322–332. [Google Scholar] [CrossRef]
- Morgan, D.B.; Davidson, C. Hypokalaemia and diuretics: An analysis of publications. Br. Med. J. 1980, 280, 905–908. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Testani, J.M.; Pitt, B. Pathophysiology of Diuretic Resistance and Its Implications for the Management of Chronic Heart Failure. Hypertension 2020, 76, 1045–1054. [Google Scholar] [CrossRef]
- Felker, G.M.; Lee, K.L.; Bull, D.A.; Redfield, M.M.; Stevenson, L.W.; Goldsmith, S.R.; LeWinter, M.M.; Deswal, A.; Rouleau, J.L.; Ofili, E.O.; et al. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 364, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef] [PubMed]
- Bart, B.A.; Goldsmith, S.R.; Lee, K.L.; Givertz, M.M.; O’Connor, C.M.; Bull, D.A.; Redfield, M.M.; Deswal, A.; Rouleau, J.L.; LeWinter, M.M.; et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N. Engl. J. Med. 2012, 367, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Brater, D.C. Diuretic Therapy. N. Engl. J. Med. 1998, 339, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.H. Clinical Pharmacology in Diuretic Use. Clin. J. Am. Soc. Nephrol. 2019, 14, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Kimura, G. Diuretic Action of Sodium-Glucose Cotransporter 2 Inhibitors and Its Importance in the Management of Heart Failure. Circ. J. 2016, 80, 2277–2281. [Google Scholar] [CrossRef]
- Kravtsova, O.; Levchenko, V.; Klemens, C.A.; Rieg, T.; Liu, R.; Staruschenko, A. Effect of SGLT2 inhibition on salt-induced hypertension in female Dahl SS rats. Sci. Rep. 2023, 13, 19231. [Google Scholar] [CrossRef]
- List, J.F.; Whaley, J.M. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int. 2011, 79, S20–S27. [Google Scholar] [CrossRef]
- Amin, A.A.; Alabsawy, E.I.; Jalan, R.; Davenport, A. Epidemiology, Pathophysiology, and Management of Hepatorenal Syndrome. Semin. Nephrol. 2019, 39, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Servadei, D.; Trevisani, F.; Rusticali, A.G.; Gasbarrini, G. Importance of plasma aldosterone concentration on the natriuretic effect of spironolactone in patients with liver cirrhosis and ascites. Digestion 1985, 31, 189–193. [Google Scholar] [CrossRef] [PubMed]
- EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [CrossRef]
- Artunc, F.; Wörn, M.; Schork, A.; Bohnert, B.N. Proteasuria—The impact of active urinary proteases on sodium retention in nephrotic syndrome. Acta Physiol. 2019, 225, e13249. [Google Scholar] [CrossRef]
- Shen, W.; Alshehri, M.; Desale, S.; Wilcox, C. The Effect of Amiloride on Proteinuria in Patients with Proteinuric Kidney Disease. Am. J. Nephrol. 2021, 52, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xie, Z.; Zhang, L.; Huang, Y.; Ma, J.; Dong, W.; Li, Z.; Chen, Y.; Liang, H.; Wu, Y.; et al. The effect of amiloride in decreasing albuminuria in patients with diabetic kidney diseases: A prospective, crossover, open-label study. Ren. Fail. 2021, 43, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Liern, M.; Colazo, A.; Vallejo, G.; Zotta, E. Antiproteinuric action of amiloride in paediatric patient with corticoresistant nephrotic syndrome. Nefrologia 2021, 41, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, J.D.; Belin, D. Amiloride selectively inhibits the urokinase-type plasminogen activator. FEBS Lett. 1987, 214, 187–191. [Google Scholar] [CrossRef]
- Hinrichs, G.R.; Mortensen, L.A.; Jensen, B.L.; Bistrup, C. Amiloride resolves resistant edema and hypertension in a patient with nephrotic syndrome; a case report. Physiol. Rep. 2018, 6, e13743. [Google Scholar] [CrossRef] [PubMed]
- Hoorn, E.J.; Ellison, D.H. Diuretic Resistance. Am. J. Kidney Dis. 2017, 69, 136–142. [Google Scholar] [CrossRef]
- Singh, B.N.; Richmond, D.E.; Wilson, J.D.; Simmonds, H.A.; North, J.D. Evaluation of MK-870: A new potassium-sparing diuretic. Br. Med. J. 1967, 1, 143–146. [Google Scholar] [CrossRef][Green Version]
- Lant, A.F.; Smith, A.J.; Wilson, G.M. Clinical evaluation of amiloride, a potassium-sparing diuretic. Clin. Pharmacol. Ther. 1969, 10, 50–63. [Google Scholar] [CrossRef]
- Schapel, G.J.; Edwards, D.G.; Robinson, J. Potassium-sparing effect of amiloride in a diuretic factorial study in man. Clin. Exp. Pharmacol. Physiol. 1975, 2, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Williams, B.; Morant, S.V.; Webb, D.J.; Caulfield, M.J.; Cruickshank, J.K.; Ford, I.; McInnes, G.; Sever, P.; Salsbury, J.; et al. Effect of amiloride, or amiloride plus hydrochlorothiazide, versus hydrochlorothiazide on glucose tolerance and blood pressure (PATHWAY-3): A parallel-group, double-blind randomised phase 4 trial. Lancet Diabetes Endocrinol. 2016, 4, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.F.C.F.D.; Scala, L.C.N.N.; Vilela-Martin, J.F.; Whelton, P.K.; Poli-de-Figueiredo, C.E.; Pereira E Silva, R.; Gus, M.; Bortolotto, L.A.; Consolim-Colombo, F.M.; Schlatter, R.P.; et al. Effectiveness of chlorthalidone/amiloride versus losartan in patients with stage I hypertension and diabetes mellitus: Results from the PREVER-treatment randomized controlled trial. Acta Diabetol. 2021, 58, 215–220. [Google Scholar] [CrossRef]
- Sun, Q.; Sever, P. Amiloride: A review. J. Renin. Angiotensin. Aldosterone. Syst. 2020, 21, 1470320320975893. [Google Scholar] [CrossRef] [PubMed]
- Unruh, M.L.; Pankratz, V.S.; Demko, J.E.; Ray, E.C.; Hughey, R.P.; Kleyman, T.R. Trial of Amiloride in Type 2 Diabetes with Proteinuria. Kidney Int. Rep. 2017, 2, 893–904. [Google Scholar] [CrossRef]
- Andersen, H.; Hansen, P.B.L.; Bistrup, C.; Nielsen, F.; Henriksen, J.E.; Jensen, B.L. Significant natriuretic and antihypertensive action of the epithelial sodium channel blocker amiloride in diabetic patients with and without nephropathy. J. Hypertens. 2016, 34, 1621–1629. [Google Scholar] [CrossRef]
- Vidt, D.G. Mechanism of action, pharmacokinetics, adverse effects, and therapeutic uses of amiloride hydrochloride, a new potassium-sparing diuretic. Pharmacotherapy 1981, 1, 179–187. [Google Scholar] [CrossRef]
- Almajid, A.N.; Cassagnol, M. Amiloride; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Frățilă, G.; Sorohan, B.M.; Achim, C.; Andronesi, A.; Obrișcă, B.; Lupușoru, G.; Zilișteanu, D.; Jurubiță, R.; Bobeică, R.; Bălănică, S.; et al. Oral Furosemide and Hydrochlorothiazide/Amiloride versus Intravenous Furosemide for the Treatment of Resistant Nephrotic Syndrome. J. Clin. Med. 2023, 12. [Google Scholar] [CrossRef]
- Trimarchi, H.; Forrester, M.; Lombi, F.; Pomeranz, V.; Raña, M.S.; Karl, A.; Andrews, J. Amiloride as an Alternate Adjuvant Antiproteinuric Agent in Fabry Disease: The Potential Roles of Plasmin and uPAR. Case Rep. Nephrol. 2014, 2014, 854521. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, S.; Shi, W.; Yang, Y. Amiloride off-target effect inhibits podocyte urokinase receptor expression and reduces proteinuria. Nephrol. Dial. Transplant. 2012, 27, 1746–1755. [Google Scholar] [CrossRef]
- Xu, L.B.; Chi, N.; Shi, W. Amiloride, a urokinase-type plasminogen activator receptor (uTPA) inhibitor, reduces proteinurea in podocytes. Genet. Mol. Res. 2015, 14, 9518–9529. [Google Scholar] [CrossRef]
- Stæhr, M.; Buhl, K.B.; Andersen, R.F.; Svenningsen, P.; Nielsen, F.; Hinrichs, G.R.; Bistrup, C.; Jensen, B.L. Aberrant glomerular filtration of urokinase-type plasminogen activator in nephrotic syndrome leads to amiloride-sensitive plasminogen activation in urine. Am. J. Physiol. Renal Physiol. 2015, 309, F235–F241. [Google Scholar] [CrossRef]
- Wei, C.; Möller, C.C.; Altintas, M.M.; Li, J.; Schwarz, K.; Zacchigna, S.; Xie, L.; Henger, A.; Schmid, H.; Rastaldi, M.P.; et al. Modification of kidney barrier function by the urokinase receptor. Nat. Med. 2008, 14, 55–63. [Google Scholar] [CrossRef]
- Lupușoru, G.; Ailincăi, I.; Sorohan, B.M.; Andronesi, A.; Achim, C.; Micu, G.; Caragheorgheopol, A.; Manda, D.; Lupușoru, M.; Ismail, G. Serum soluble urokinase plasminogen activator receptor as a potential biomarker of renal impairment severity in diabetic nephropathy. Diabetes Res. Clin. Pract. 2021, 182, 109116. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, G.; Zhang, Y.; Cui, Z.; Wang, F.; Liu, X.; Chu, R.; Zhao, M. Urinary soluble urokinase receptor levels are elevated and pathogenic in patients with primary focal segmental glomerulosclerosis. BMC Med. 2014, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Patel-Chamberlin, M.; Varasteh Kia, M.; Xu, J.; Barone, S.; Zahedi, K.; Soleimani, M. The Role of Epithelial Sodium Channel ENaC and the Apical Cl−/HCO3− Exchanger Pendrin in Compensatory Salt Reabsorption in the Setting of Na-Cl Cotransporter (NCC) Inactivation. PLoS ONE 2016, 11, e0150918. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Marenzi, G.; Lauri, G.; Perego, G.; Schianni, M.; Sganzerla, P.; Guazzi, M.D. Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency: Failure of furosemide to provide the same result. Am. J. Med. 1994, 96, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Titko, T.; Perekhoda, L.; Drapak, I.; Tsapko, Y. Modern trends in diuretics development. Eur. J. Med. Chem. 2020, 208, 112855. [Google Scholar] [CrossRef]
- Vardanyan, R.; Hruby, V. Chapter 21—Diuretics. In Synthesis of Best-Seller Drugs; Vardanyan, R., Hruby, V., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 317–327. ISBN 978-0-12-411492-0. [Google Scholar]
- Eknoyan, G. Chapter I—A History of Diuretics; Seldin, D., Giebisch, G.B.T.-D.A., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 3–28. ISBN 978-0-12-635690-8. [Google Scholar]
- Sica, D.A.; Gehr, T.W. Triamterene and the kidney. Nephron 1989, 51, 454–461. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frățilă, V.-G.; Lupușoru, G.; Sorohan, B.M.; Obrișcă, B.; Mocanu, V.; Lupușoru, M.; Ismail, G. Nephrotic Syndrome: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2024, 12, 569. https://doi.org/10.3390/biomedicines12030569
Frățilă V-G, Lupușoru G, Sorohan BM, Obrișcă B, Mocanu V, Lupușoru M, Ismail G. Nephrotic Syndrome: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines. 2024; 12(3):569. https://doi.org/10.3390/biomedicines12030569
Chicago/Turabian StyleFrățilă, Valentina-Georgiana, Gabriela Lupușoru, Bogdan Marian Sorohan, Bogdan Obrișcă, Valentin Mocanu, Mircea Lupușoru, and Gener Ismail. 2024. "Nephrotic Syndrome: From Pathophysiology to Novel Therapeutic Approaches" Biomedicines 12, no. 3: 569. https://doi.org/10.3390/biomedicines12030569
APA StyleFrățilă, V.-G., Lupușoru, G., Sorohan, B. M., Obrișcă, B., Mocanu, V., Lupușoru, M., & Ismail, G. (2024). Nephrotic Syndrome: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines, 12(3), 569. https://doi.org/10.3390/biomedicines12030569






