Targeted Delivery of Abaloparatide to Spinal Fusion Site Accelerates Fusion Process in Rats
Abstract
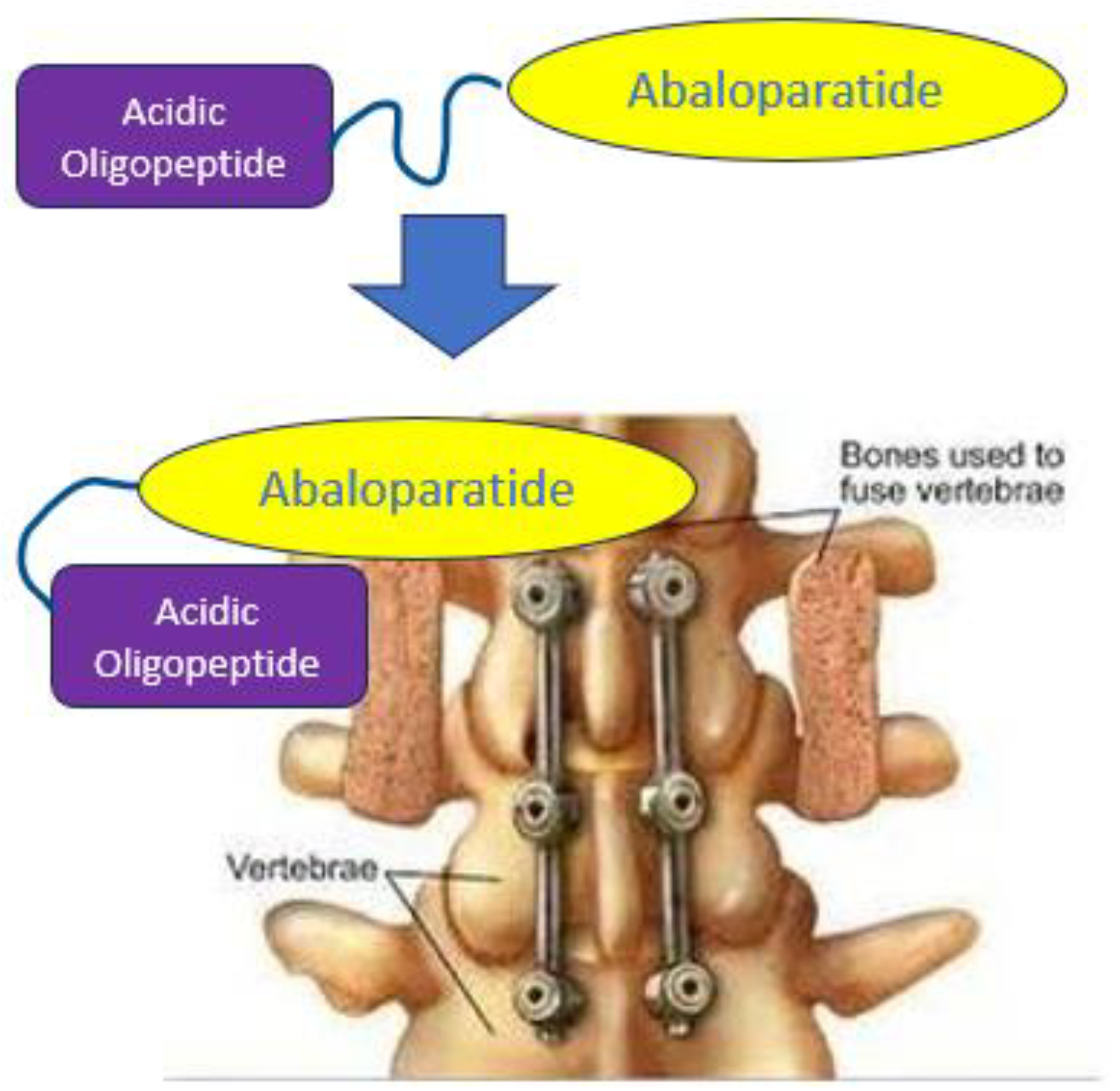
1. Introduction
2. Methods
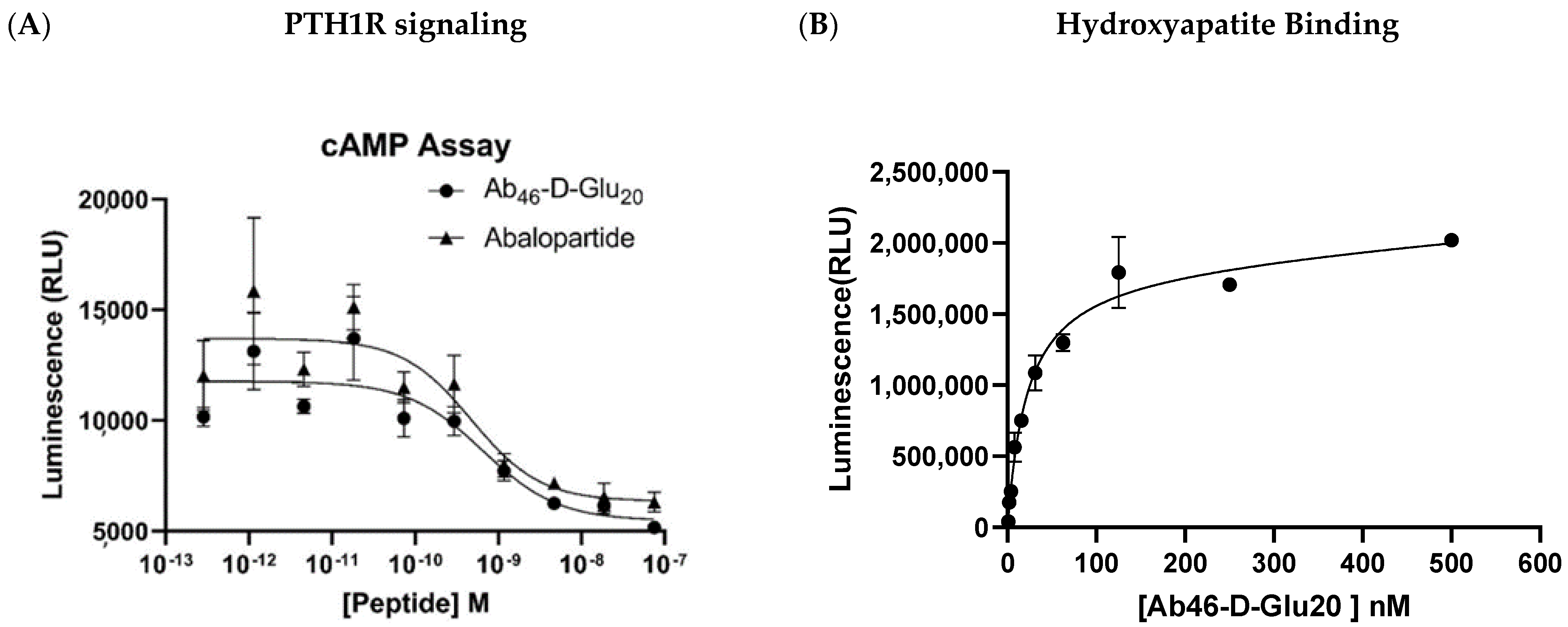
2.1. PTH1R Signaling Assay
2.2. Hydroxyapatite Binding Study
2.3. Biodistribution Study
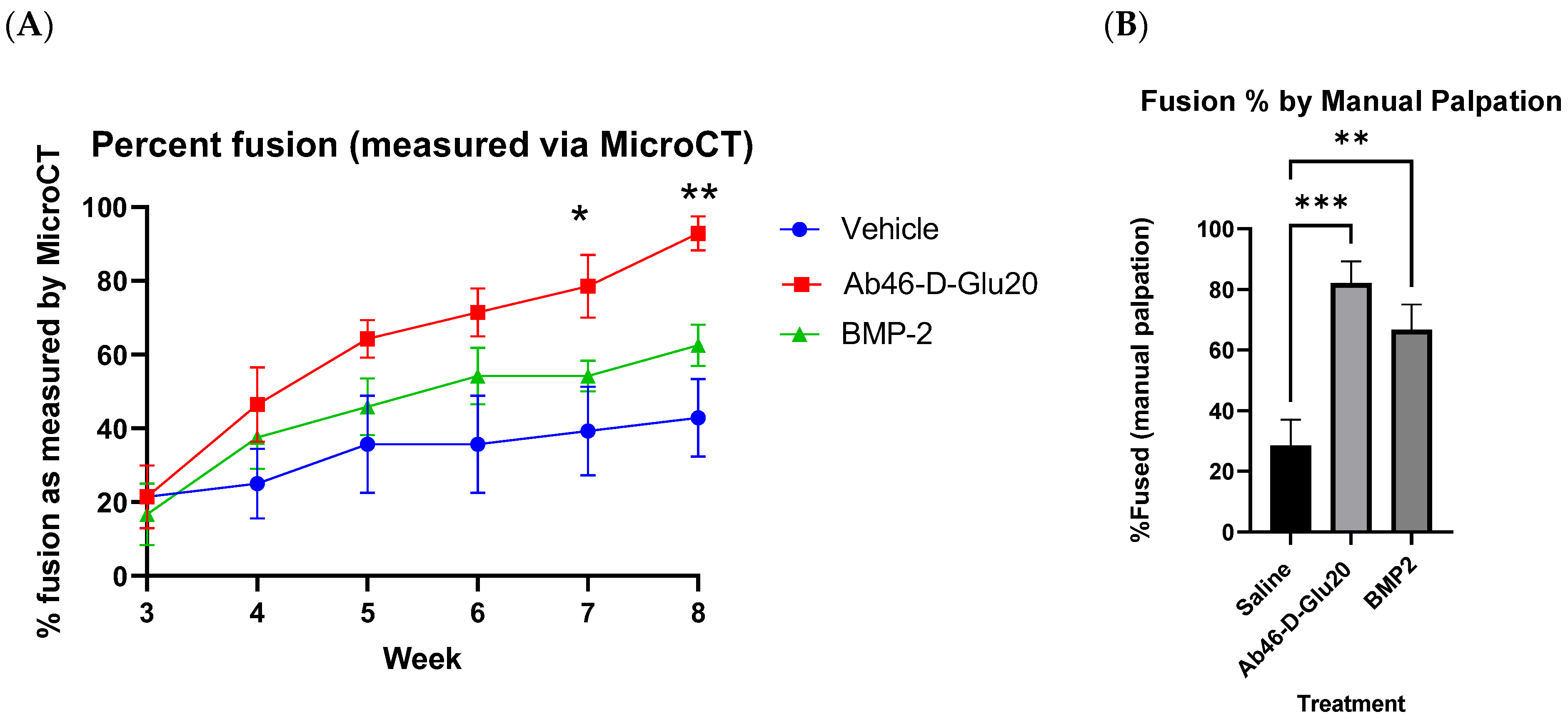
2.4. Efficacy Study
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, B.I.; Mirza, S.K.; Spina, N.; Spiker, W.R.; Lawrence, B.; Brodke, D.S. Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States, 2004 to 2015. Spine 2019, 44, 369–376. Available online: http://insights.ovid.com/crossref?an=00007632-201903010-00014 (accessed on 1 November 2023). [CrossRef]
- Guglielmi, G.N.; Seibly, J.M. Return to Work Guidelines Following Neurosurgical Procedures. Cureus 2020, 12, e11982. [Google Scholar] [CrossRef]
- Rajaee, S.S.; Kanim, L.E.A.; Bae, H.W.; Rajaee, S.S.; Kanim, L.E.A.; Research, C.; Bae, H.W. National trends in revision spinal fusion in the USA patient characteristics and complications. Bone Jt. J. 2014, 96, 807–816. Available online: www.hcupus.ahrq.gov/ (accessed on 1 November 2023). [CrossRef]
- Irmola, T.M.; Häkkinen, A.; Järvenpää, S.; Marttinen, I.; Vihtonen, K.; Neva, M. Reoperation Rates Following Instrumented Lumbar Spine Fusion. Spine (Phila Pa 1976). Lippincott Williams Wilkins 2018, 43, 295–301. [Google Scholar]
- Hunter, D.J. Spondylolysis and spondylolisthesis: Prevalence and association with low back pain in the adult community-based population. Spine 2013, 34, 199–205. [Google Scholar]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qiu, Y.; Sheng, F.; Yuan, X.; Xu, L.; Bao, H.; Dai, J.; Zhu, Z. An effective delivery vehicle of demineralized bone matrix incorporated with engineered collagen-binding human bone morphogenetic protein-2 to accelerate spinal fusion at low dose. J. Mater. Sci. Mater. Med. 2018, 29, 2. [Google Scholar] [CrossRef]
- Salamon, M.L.; Althausen, P.L.; Gupta, M.C.; Laubach, J. The effects of BMP-7 in a rat posterolateral intertransverse process fusion model. J. Spinal Disord. Tech. 2003, 16, 90–95. Available online: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L36184260 (accessed on 1 November 2023). [CrossRef] [PubMed]
- Lv, Q.B.; Gao, X.; Pan, X.X.; Jin, H.M.; Lou, X.T.; Li, S.M.; Yan, Y.Z.; Wu, C.C.; Lin, Y.; Ni, W.F.; et al. Biomechanical properties of novel transpedicular transdiscal screw fixation with interbody arthrodesis technique in lumbar spine: A finite element study. J. Orthop. Translat. 2018, 15, 50–58. [Google Scholar] [CrossRef]
- Reisener, M.J.; Pumberger, M.; Shue, J.; Girardi, F.P.; Hughes, A.P. Trends in lumbar spinal fusion—A literature review. J. Spine Surg. 2020, 6, 752–776. [Google Scholar] [CrossRef]
- Patel, D.V.; Yoo, J.S.; Karmarkar, S.S.; Lamoutte, E.H.; Singh, K. Interbody options in lumbar fusion. J. Spine Surg. 2019, 5, S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Plantz, M.A.; Hsu, W.K. Recent Research Advances in Biologic Bone Graft Materials for Spine Surgery. Curr. Rev. Musculoskelet. Med. 2020, 13, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Petscavage-Thomas, J.; Ouyang, T.; Bible, J. Spine fixation hardware: An update. Am. J. Roentgenol. 2020, 215, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Y.; Zou, D.; Yuan, B.; Ke, H.Z.; Li, W. Therapeutics for enhancement of spinal fusion: A mini review. J. Orthop. Transl. 2021, 31, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cottrill, E.; Kyle, A.; Rowland, A.; Stirton, J. SYSTEMATIC REVIEW 176 Investigational growth factors utilized in animal models of spinal fusion: Systematic review CASE REPORT 206 Fracture of allograft interbody spacer resulting in post-operative radiculopathy: A case report 212 Recalcitrant distal humeral non-union following previous Leiomyosarcoma excision treated with retainment of a radiated non-angiogenic segment augmented with 20 cm free fibula composite graft: A case report. World J. Orthop. 2019, 10, 176–218. [Google Scholar] [PubMed]
- James, A.W.; Shen, J.; Tsuei, R.; Nguyen, A.; Khadarian, K.; Meyers, C.A.; Pan, H.C.; Li, W.; Kwak, J.H.; Asatrian, G.; et al. NELL-1 induces Sca-1+ mesenchymal progenitor cell expansion in models of bone maintenance and repair. J. Clin. Investig. 2017, 2, 92573. [Google Scholar] [CrossRef] [PubMed]
- White, A.P.; Vaccaro, A.R.; Hall, J.A.; Whang, P.G.; Friel, B.C.; McKee, M.D. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int. Orthop. 2007, 31, 735–741. [Google Scholar] [CrossRef]
- Nielsen, J.J.; Low, S.A.; Ramseier, N.T.; Hadap, R.V.; Young, N.A.; Wang, M.; Low, P.S. Analysis of the bone fracture targeting properties of osteotropic ligands. J. Control. Release 2021, 329, 570–584. [Google Scholar] [CrossRef]
- Nielsen, J.J. Targeted Delivery of Bone Anabolics to Bone Fractures. Ph.D. Thesis, Purdue University Graduate School, West Lafayette, IN, USA, 2020. [Google Scholar]
- Low, S.A.; Nielsen, J.J.; Coakley, C.M.; Thomas, M.; Mbachu, E.U.; Chen, C.L.; Jones-Hall, Y.; Tremblay, M.I.; Hicks, J.R.; Low, P.S. An engineered dual function peptide to repair fractured bones. J. Control Release 2022, 350, 688–697. [Google Scholar] [CrossRef]
- Low, S.A.; Galliford, C.V.; Yang, J.; Low, P.S.; City, S.L.; Chemistry, P.; City, S.L.; Lafayette, W. Biodistribution of fracture-targeted GSK3β inhibitor-loaded micelles for improved fracture healing. Biomacromolecules 2015, 16, 3145–3153. [Google Scholar] [CrossRef]
- Wang, M.; Park, S.; Nam, Y.; Nielsen, J.; Low, S.A.; Srinivasarao, M.; Low, P.S. Bone-Fracture-Targeted Dasatinib-Oligoaspartic Acid Conjugate Potently Accelerates Fracture Repair. Bioconjug. Chem. 2018, 29, 3800–3809. [Google Scholar] [CrossRef]
- Low, S.A.; Galliford, C.V.; Jones-Hall, Y.L.; Roy, J.; Yang, J.; Low, P.S.; Kopeček, J. Healing efficacy of fracture-targeted GSK3β inhibitor-loaded micelles for improved fracture repair. Nanomedicine 2017, 12, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, N.; Zamudio, J.R.; Jong, R.M.; Soukup, D.; Resnick, R.; Sarma, K.; Ward, A.J.; Raj, A.; Lee, J.; Sharp, P.A.; et al. Acceleration of Spinal Fusion Using Syngeneic and Allogeneic Adult Adipose Derived Stem Cells in a Rat Model. PLoS ONE 2017, 32, 736–740. [Google Scholar]
- Drespe, I.H.; Polzhofer, G.K.; Turner, A.S.; Grauer, J.N. Animal models for spinal fusion. Spine J. 2005, 5 (Suppl. S6), S209–S216. [Google Scholar] [CrossRef]
- Sandhu, H.S.; Khan, S.N. Animal Models for Preclinical Assessment of Bone Morphogenetic Proteins in the Spine. Spine 2002, 27, S32–S38. [Google Scholar] [CrossRef]
- Foley, J.P.; Fred, E.J.; Minardi, S.; Yamaguchi, J.T.; Greene, A.C.; Furman, A.A.; Lyons, J.G.; Paul, J.T.; Nandurkar, T.S.; Blank, K.R.; et al. Sex-based Difference in Response to Recombinant Human Bone Morphogenetic Protein-2 in a Rat Posterolateral Fusion Model. Spine 2022, 47, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Dimar, J.; Ante, W.; Zhang, P.; Glassman, S. The Effects of Non-Steroidal Anti-Inflammatory Drugs on Posterior Spinal Fusions in the Rat. Spine 1996, 21, 1870–1876. [Google Scholar] [CrossRef]
- Hattersley, G.; Dean, T.; Corbin, B.A.; Bahar, H.; Gardella, T.J. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology 2016, 157, 141–149. [Google Scholar] [CrossRef]
- Sahbani, K.; Cardozo, C.P.; Bauman, W.A.; Tawfeek, H.A. Abaloparatide exhibits greater osteoanabolic response and higher cAMP stimulation and β-arrestin recruitment than teriparatide. Physiol. Rep. 2019, 7, e14225. [Google Scholar] [CrossRef]
- Dean, T.; Vilardaga, P.; Potts, J.T.; Gardella, T.J. Altered Selectivity of Parathyroid Hormone (PTH) and PTH-Related Protein (PTHrP) for Distinct Conformations of the PTH/PTHrP Receptor. Mol. Endocrinol. 2008, 22, 156–166. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2194631/?report=printable (accessed on 1 November 2023). [CrossRef]
- Grauer, J.N.; Bomback, D.A.; Lugo, R.; Troiano, N.W.; Patel, T.C.; Friedlaender, G.E. Posterolateral lumbar fusions in athymic rats: Characteristization of a model. Spine J. 2004, 4, 281–286. [Google Scholar] [CrossRef]
- Han, X.; Zhang, W.; Gu, J.; Zhao, H.; Ni, L.; Han, J.; Zhou, Y.; Gu, Y.; Zhu, X.; Sun, J.; et al. Accelerated postero-lateral spinal fusion by collagen scaffolds modified with engineered collagen-binding human bone morphogenetic protein-2 in rats. PLoS ONE 2014, 9, e98480. [Google Scholar] [CrossRef]
- Alsaleh, K.A.M.; Tougas, C.A.; Roffey, D.M.; Wai, E.K. Osteoconductive bone graft extenders in posterolateral thoracolumbar spinal fusion: A systematic review. Spine 2012, 37, 993–1000. [Google Scholar] [CrossRef]
- Liang, B.; Huang, J.; Xu, J.; Li, X.; Li, J. Local delivery of a novel PTHrP: Via mesoporous bioactive glass scaffolds to improve bone regeneration in a rat posterolateral spinal fusion model. RSC Adv. 2018, 8, 12484–12493. [Google Scholar] [CrossRef]
- Lin, S.; Cui, L.; Chen, G.; Huang, J.; Yang, Y.; Zou, K.; Lai, Y.; Wang, X.; Zou, L.; Wu, T.; et al. PLGA/β-TCP composite scaffold incorporating salvianolic acid B promotes bone fusion by angiogenesis and osteogenesis in a rat spinal fusion model. Biomaterials 2019, 196, 109–121. [Google Scholar] [CrossRef]
- Chandler, H.; Lanske, B.; Varela, A.; Guillot, M.; Boyer, M.; Brown, J.; Pierce, A.; Ominsky, M.; Mitlak, B.; Baron, R.; et al. Abaloparatide, a novel osteoanabolic PTHrP analog, increases cortical and trabecular bone mass and architecture in orchiectomized rats by increasing bone formation without increasing bone resorption. Bone 2019, 120, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Hattersley, G.; Fitzpatrick, L.A.; Harris, A.G.; Shevroja, E.; Banks, K.; Leder, B.Z.; Zanchetta, J.R.; Hans, D. Abaloparatide-SC improves trabecular microarchitecture as assessed by trabecular bone score (TBS): A 24-week randomized clinical trial. Osteoporos. Int. 2018, 29, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Öhman-Mägi, C.; Holub, O.; Wu, D.; Hall, R.M.; Persson, C. Density and mechanical properties of vertebral trabecular bone—A review. JOR Spine 2021, 4, e1176. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, J.J.; Low, S.A.; Chen, C.; Li, X.; Mbachu, E.; Trigg, L.; Sun, S.; Tremby, M.; Hadap, R.; Low, P.S. Targeted Delivery of Abaloparatide to Spinal Fusion Site Accelerates Fusion Process in Rats. Biomedicines 2024, 12, 612. https://doi.org/10.3390/biomedicines12030612
Nielsen JJ, Low SA, Chen C, Li X, Mbachu E, Trigg L, Sun S, Tremby M, Hadap R, Low PS. Targeted Delivery of Abaloparatide to Spinal Fusion Site Accelerates Fusion Process in Rats. Biomedicines. 2024; 12(3):612. https://doi.org/10.3390/biomedicines12030612
Chicago/Turabian StyleNielsen, Jeffery J., Stewart A. Low, Christopher Chen, Xinlan Li, Ephraim Mbachu, Lina Trigg, Siyuan Sun, Madeline Tremby, Rahul Hadap, and Philip S. Low. 2024. "Targeted Delivery of Abaloparatide to Spinal Fusion Site Accelerates Fusion Process in Rats" Biomedicines 12, no. 3: 612. https://doi.org/10.3390/biomedicines12030612
APA StyleNielsen, J. J., Low, S. A., Chen, C., Li, X., Mbachu, E., Trigg, L., Sun, S., Tremby, M., Hadap, R., & Low, P. S. (2024). Targeted Delivery of Abaloparatide to Spinal Fusion Site Accelerates Fusion Process in Rats. Biomedicines, 12(3), 612. https://doi.org/10.3390/biomedicines12030612






