MSR405: Inhibiting Neuroinflammation after Spinal Cord Injury in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. MSR405 Synthesis
2.2. Animals and Grouping
2.3. SCI Model Establishment and Drug Administration
2.4. Behavioral Tests
2.5. Tissue Collection and Processing
2.6. Hematoxylin-Eosin (HE) and Nissl Staining
2.7. Immunofluorescence Staining
2.8. Cell Culture and Drug Treatment
2.9. Cell Viability Assay
2.10. Nitic Oxide (NO) Assay
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
3.1. Structural Analysis of Compounds
3.2. MSR405 Treatment Promoted the Recovery of Motor Function in SCI Rats
3.3. MSR405 Attenuated Neurological Damage
3.4. MSR405 Inhibited M1 Microglia Polarization in SCI Rats
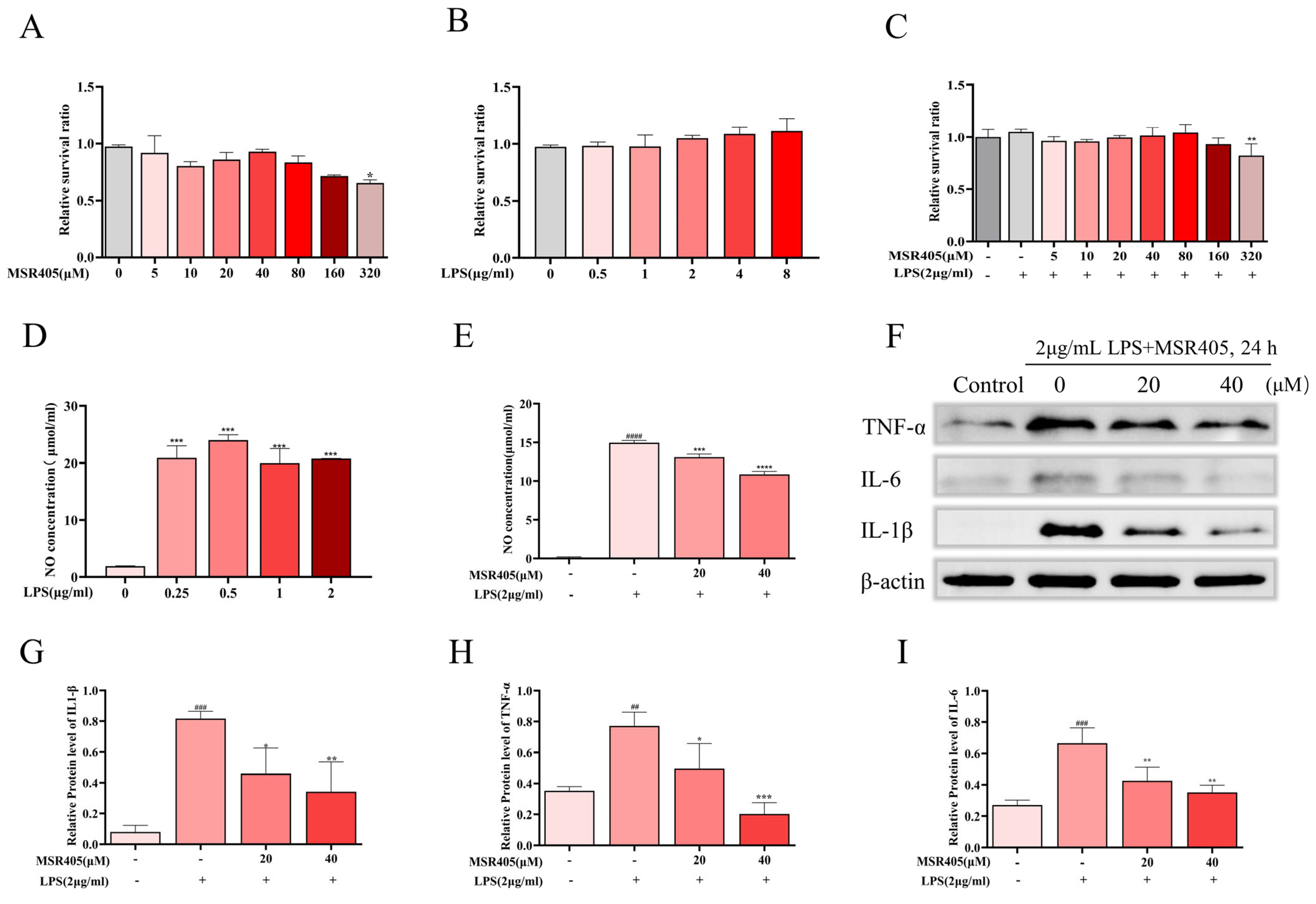
3.5. Effects of MSR405 on BV-2 Cell Viability
3.6. MSR405 Suppressed LPS-Induced NO Production and Inflammatory Response in BV2 Cells
3.7. Effects of MSR405 on LPS-Induced Polarization of BV-2 Cells
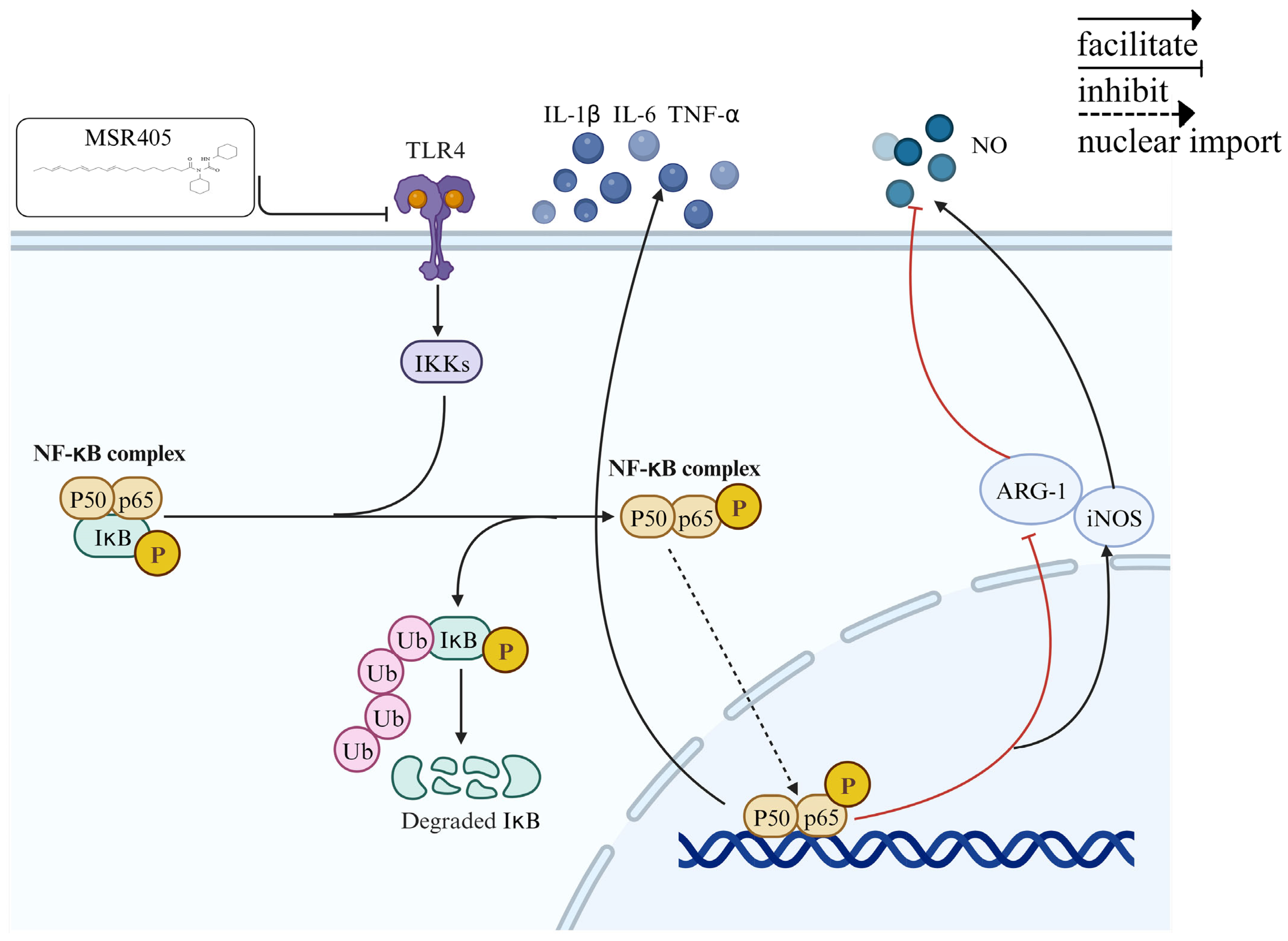
3.8. MSR405 Reduces Microglia Activation via the TLR4/NF-κB Signaling Pathway
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clifford, T.; Finkel, Z.; Rodriguez, B.; Joseph, A.; Cai, L. Current Advancements in Spinal Cord Injury Research—Glial Scar Formation and Neural Regeneration. Cells 2023, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Bohara, R.; Krishna Kanala, V.; McMahon, S.; Pandit, A. Models and approaches to comprehend and address glial inflammation following spinal cord injury. Drug Discov. Today 2023, 28, 103722. [Google Scholar] [CrossRef] [PubMed]
- Zeenat, A.; Alka, S.; Saloni, R.; Shah, W.; Rajeshwar Nath, S. Spinal Cord Injury Prevalence and Treatment Modalities. In Spinal Cord Injury; Luca, R., Giorgio, L., Andrea, P., Sokol, T., Eds.; Chapter 2; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Hu, Y.; Li, L.; Hong, B.; Xie, Y.; Li, T.; Feng, C.; Yang, F.; Wang, Y.; Zhang, J.; Yu, Y.; et al. Epidemiological features of traumatic spinal cord injury in China: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1131791. [Google Scholar] [CrossRef] [PubMed]
- Guízar-Sahagún, G.; Grijalva, I.; Franco-Bourland, R.E.; Madrazo, I. Aging with spinal cord injury: A narrative review of consequences and challenges. Ageing Res. Rev. 2023, 90, 102020. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after spinal cord injury: A review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflamm. 2021, 18, 284. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of Secondary Spinal Cord Injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Decker, J.T.; Margul, D.J.; Smith, D.R.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Local Immunomodulation with Anti-inflammatory Cytokine-Encoding Lentivirus Enhances Functional Recovery after Spinal Cord Injury. Mol. Ther. 2018, 26, 1756–1770. [Google Scholar] [CrossRef]
- Li, Q.S.; Jia, Y.J. Ferroptosis: A critical player and potential therapeutic target in traumatic brain injury and spinal cord injury. Neural Regen. Res. 2023, 18, 506–512. [Google Scholar] [CrossRef]
- Jin, Y.; Song, Y.; Lin, J.; Liu, T.; Li, G.; Lai, B.; Gu, Y.; Chen, G.; Xing, L. Role of inflammation in neurological damage and regeneration following spinal cord injury and its therapeutic implications. Burn. Trauma 2023, 11, tkac054. [Google Scholar] [CrossRef]
- Ni, H.; Jin, W.; Zhu, T.; Wang, J.; Yuan, B.; Jiang, J.; Liang, W.; Ma, Z. Curcumin modulates TLR4/NF-kappaB inflammatory signaling pathway following traumatic spinal cord injury in rats. J. Spinal Cord. Med. 2015, 38, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, J.; Jiang, J.; Song, J.; Zhu, W.; Zhang, F.; Shao, M.; Xu, H.; Ma, X.; Lyu, F. TLR4 promotes microglial pyroptosis via lncRNA-F630028O10Rik by activating PI3K/AKT pathway after spinal cord injury. Cell Death Dis. 2020, 11, 693. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Yu, Z.; Qi, J. Anti-neuroinflammatory effects of tannic acid against lipopolysaccharide-induced BV2 microglial cells via inhibition of NF-kappaB activation. Drug Dev. Res. 2019, 80, 262–268. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, X.-Y. Research Progress of Chinese Herbal MedicineRadix isatidis (Banlangen). Am. J. Chin. Med. 2013, 41, 743–764. [Google Scholar] [CrossRef]
- Peng, J.; Li, X.; Zheng, L.; Duan, L.; Gao, Z.; Hu, D.; Li, J.; Li, X.; Shen, X.; Xiao, H. Ban-Lan-Gen Granule Alleviates Dextran Sulfate Sodium-Induced Chronic Relapsing Colitis in Mice via Regulating Gut Microbiota and Restoring Gut SCFA Derived-GLP-1 Production. J. Inflamm. Res. 2022, 15, 1457–1470. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Q.; Chen, J.; Kuang, X.; Xia, J.; Xie, B.; Wang, F.; Liang, H.; Qi, Z. Synergistic effects of Isatis tinctoria L. and tacrolimus in the prevention of acute heart rejection in mice. Transplant. Immunol. 2009, 22, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.; Emond, V.; Chen, C.T.; Julien, C.; Bourasset, F.; Oddo, S.; LaFerla, F.; Bazinet, R.P.; Calon, F. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood–brain barrier: An in situ cerebral perfusion study. Neurochem. Int. 2009, 55, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Freund Levi, Y.; Vedin, I.; Cederholm, T.; Basun, H.; Faxén Irving, G.; Eriksdotter, M.; Hjorth, E.; Schultzberg, M.; Vessby, B.; Wahlund, L.O.; et al. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer’s disease: The OmegAD study. J. Intern. Med. 2014, 275, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Sabouri, S.; Kiani, A.; Farzaei, M.H.; Rashidi, K.; Mohammadi-Farani, A.; Mohammadi-Noori, E.; Abbaszadeh, F. Intrathecal administration of naringenin improves motor dysfunction and neuropathic pain following compression spinal cord injury in rats: Relevance to its antioxidant and anti-inflammatory activities. Korean J. Pain. 2022, 35, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef]
- Moon, L.; Bunge, M.B. From Animal Models to Humans. J. Neurol. Phys. Ther. 2005, 29, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.; Xia, K.; Wang, J.; Cheng, F.; Shi, K.; Ying, L.; Yu, C.; Xu, H.; Xiao, S.; et al. A bioactive injectable self-healing anti-inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury. Bioact. Mater. 2021, 6, 2523–2534. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Q. The NF-kappaB Pathway: A Focus on Inflammatory Responses in Spinal Cord Injury. Mol. Neurobiol. 2023, 60, 5292–5308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Mei, X.; Yang, D.; Tu, G. Resveratrol inhibits inflammation after spinal cord injury via SIRT-1/NF-κB signaling pathway. Neurosci. Lett. 2021, 762, 136151. [Google Scholar] [CrossRef]
- Marques, S.A.; Garcez, V.F.; Del Bel, E.A.; Martinez, A.M. A simple, inexpensive and easily reproducible model of spinal cord injury in mice: Morphological and functional assessment. J. Neurosci. Methods 2009, 177, 183–193. [Google Scholar] [CrossRef]
- Tang, W.; Liu, L.; Yan, Y.; Xia, Y. Sodium houttuyfonate exerts its neuroprotection effect by inhibiting the M1 microglia polarization in a TLR4/NF-kappaB signal pathway. Brain Res. 2023, 1809, 148358. [Google Scholar] [CrossRef]
- Dyck, S.; Kataria, H.; Alizadeh, A.; Santhosh, K.T.; Lang, B.; Silver, J.; Karimi-Abdolrezaee, S. Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPσ receptors promotes a beneficial inflammatory response following spinal cord injury. J. Neuroinflamm. 2018, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, R.; Wang, H.; Hou, Y.; Li, Y.; Zhu, J.; Xu, F.; Fu, C. Biomaterials delivery strategies to repair spinal cord injury by modulating macrophage phenotypes. J. Tissue Eng. 2022, 13, 20417314221143059. [Google Scholar] [CrossRef]
- Chen, X.; Xue, J.; Zou, J.; Zhao, X.; Li, L.; Jia, R.; Zou, Y.; Wan, H.; Chen, Y.; Zhou, X.; et al. Resveratrol alleviated neuroinflammation induced by pseudorabies virus infection through regulating microglial M1/M2 polarization. Biomed. Pharmacother. 2023, 160, 114271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, D.; Fan, X.; Yuan, Y.; Wang, H.; Wang, D.; Mei, X. Gold nanoclusters conjugated berberine reduce inflammation and alleviate neuronal apoptosis by mediating M2 polarization for spinal cord injury repair. Regen. Biomater. 2022, 9, rbab072. [Google Scholar] [CrossRef]
- Bi, Y.; Duan, W.; Chen, J.; You, T.; Li, S.; Jiang, W.; Li, M.; Wang, G.; Pan, X.; Wu, J.; et al. Neutrophil Decoys with Anti-Inflammatory and Anti-Oxidative Properties Reduce Secondary Spinal Cord Injury and Improve Neurological Functional Recovery. Adv. Funct. Mater. 2021, 31, 2102912. [Google Scholar] [CrossRef]
- Karova, K.; Wainwright, J.V.; Machova-Urdzikova, L.; Pisal, R.V.; Schmidt, M.; Jendelova, P.; Jhanwar-Uniyal, M. Transplantation of neural precursors generated from spinal progenitor cells reduces inflammation in spinal cord injury via NF-κB pathway inhibition. J. Neuroinflamm. 2019, 16, 12. [Google Scholar] [CrossRef]
- Wang, L.; Song, Z.; Zou, H.; Chen, H.; Hu, Y.; Li, X.; Liu, J. CircRNA3616 knockdown attenuates inflammation and apoptosis in spinal cord injury by inhibiting TLR4/NF-kappaB activity via sponging miR-137. Mol. Cell Biochem. 2023, 478, 329–341. [Google Scholar] [CrossRef]
- He, Z.; Zhou, Y.; Lin, L.; Wang, Q.; Khor, S.; Mao, Y.; Li, J.; Zhen, Z.; Chen, J.; Gao, Z.; et al. Dl-3-n-butylphthalide attenuates acute inflammatory activation in rats with spinal cord injury by inhibiting microglial TLR4/NF-κB signalling. J. Cell. Mol. Med. 2017, 21, 3010–3022. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ye, J.; Chen, X.; Shi, J.; Wu, W.; Lin, W.; Lin, W.; Li, Y.; Fu, H.; Li, S. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J. Neuroinflamm. 2018, 15, 150. [Google Scholar] [CrossRef]
- Fei, M.; Li, Z.; Cao, Y.; Jiang, C.; Lin, H.; Chen, Z. MicroRNA-182 improves spinal cord injury in mice by modulating apoptosis and the inflammatory response via IKKβ/NF-κB. Lab. Investig. 2021, 101, 1238–1253. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, C.; Yang, Q.; Xu, H.; Lu, J.; Xu, K. Rea regulates microglial polarization and attenuates neuronal apoptosis via inhibition of the NF-κB and MAPK signalings for spinal cord injury repair. J. Cell. Mol. Med. 2020, 25, 1371–1382. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.; Gao, T.; Chen, Y.; Yang, Q.; Fu, C.; Zhu, Y.; Wang, F.; Liao, W. Isatidis Radix and Isatidis Folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 283, 114648. [Google Scholar] [CrossRef]
- Ma, P.-F.; Jiang, J.; Gao, C.; Cheng, P.-P.; Li, J.-L.; Huang, X.; Lin, Y.-Y.; Li, Q.; Peng, Y.-Z.; Cai, M.-C.; et al. Immunosuppressive Effect of Compound K on Islet Transplantation in an STZ-Induced Diabetic Mouse Model. Diabetes 2014, 63, 3458–3469. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D.; Wolenski, F.S. NF-κB: Where did it come from and why? Immunol. Rev. 2012, 246, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Christian, F.; Smith, E.; Carmody, R. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- An, K.; Qin, Q.; Yu, S.N.; Xue, M.J.; Wang, Z.Z.; Lin, Q.R.; Ma, Y.H.; Yan, G.L.; Mo, S.R.; Chen, Y.Y.; et al. Combination of N, N′-dicyclohexyl-N-arachidonic acylurea and tacrolimus prolongs cardiac allograft survival in mice. Immunol. Cell Biol. 2020, 98, 382–396. [Google Scholar] [CrossRef]
- Kim, K.B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.M. alpha-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xiao, Y.; Gao, J.; Gao, J.; Li, R.; Qi, Z.; Liu, X. MSR405: Inhibiting Neuroinflammation after Spinal Cord Injury in Rats. Biomedicines 2024, 12, 614. https://doi.org/10.3390/biomedicines12030614
Liu Y, Xiao Y, Gao J, Gao J, Li R, Qi Z, Liu X. MSR405: Inhibiting Neuroinflammation after Spinal Cord Injury in Rats. Biomedicines. 2024; 12(3):614. https://doi.org/10.3390/biomedicines12030614
Chicago/Turabian StyleLiu, Yu, Yu Xiao, Jimeng Gao, Jiaxin Gao, Ruicheng Li, Zhongquan Qi, and Xiaocun Liu. 2024. "MSR405: Inhibiting Neuroinflammation after Spinal Cord Injury in Rats" Biomedicines 12, no. 3: 614. https://doi.org/10.3390/biomedicines12030614
APA StyleLiu, Y., Xiao, Y., Gao, J., Gao, J., Li, R., Qi, Z., & Liu, X. (2024). MSR405: Inhibiting Neuroinflammation after Spinal Cord Injury in Rats. Biomedicines, 12(3), 614. https://doi.org/10.3390/biomedicines12030614




