Classical and Alternative Pathways of the Renin–Angiotensin–Aldosterone System in Regulating Blood Pressure in Hypertension and Obese Adolescents
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Angiotensin II
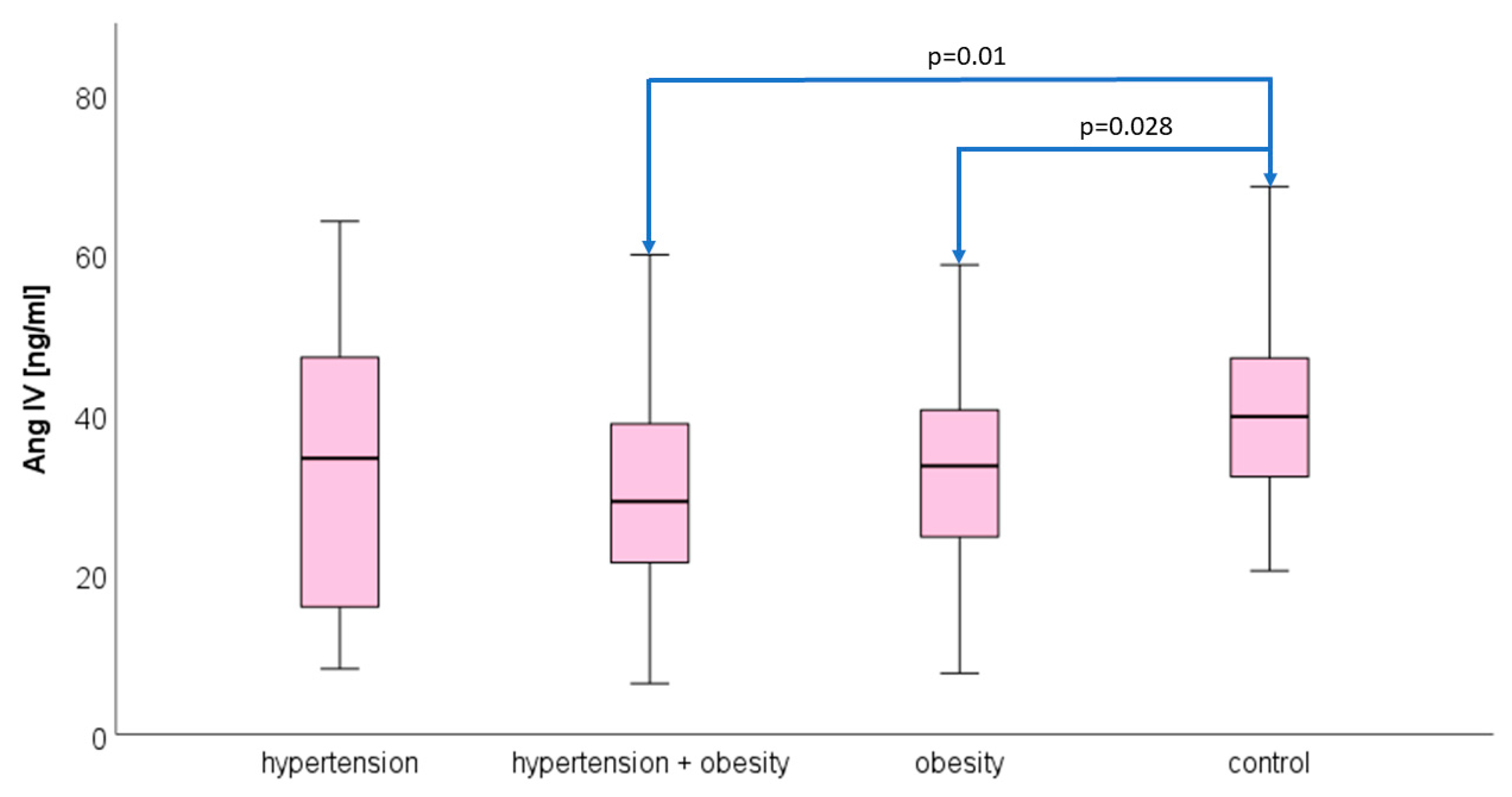
3.2. Angiotensin IV
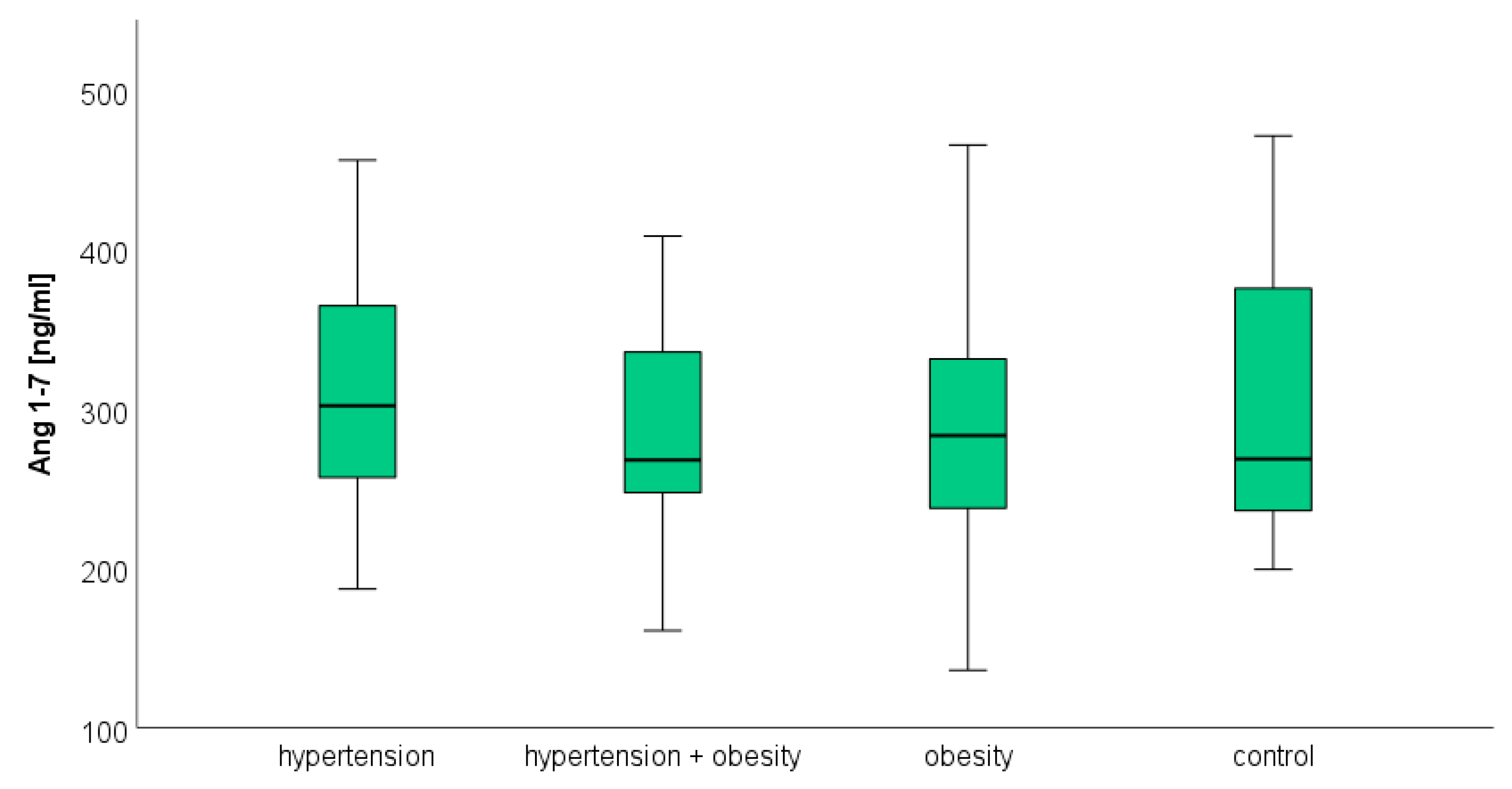
3.3. Angiotensin 1–7
3.4. Angiotensin 1–9
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases (accessed on 1 August 2023).
- Heart Disease Facts|Cdc.Gov. Available online: https://www.cdc.gov/heartdisease/facts.htm (accessed on 1 August 2023).
- Rao, G. Diagnosis, Epidemiology, and Management of Hypertension in Children. Pediatrics 2016, 138, e20153616. [Google Scholar] [CrossRef]
- Litwin, M.; Kułaga, Z. Obesity, Metabolic Syndrome, and Primary Hypertension. Pediatr. Nephrol. 2021, 36, 825–837. [Google Scholar] [CrossRef]
- Landsberg, L.; Aronne, L.J.; Beilin, L.J.; Burke, V.; Igel, L.I.; Lloyd-Jones, D.; Sowers, J. Obesity-Related Hypertension: Pathogenesis, Cardiovascular Risk, and Treatment. J. Clin. Hypertens. 2013, 15, 14–33. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-Angiotensin-Aldosterone (RAAS): The Ubiquitous System for Homeostasis and Pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef]
- Cruz-López, E.O.; Ye, D.; Wu, C.; Lu, H.S.; Uijl, E.; Mirabito Colafella, K.M.; Danser, A.H.J. Angiotensinogen Suppression: A New Tool to Treat Cardiovascular and Renal Disease. Hypertension 2022, 79, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Bharadwaj, D.; Prasad, G.; Grechko, A.V.; Sazonova, M.A.; Orekhov, A.N. Renin-Angiotensin System in Pathogenesis of Atherosclerosis and Treatment of CVD. Int. J. Mol. Sci. 2021, 22, 6702. [Google Scholar] [CrossRef]
- Vargas Vargas, R.A.; Varela Millán, J.M.; Fajardo Bonilla, E. Renin-Angiotensin System: Basic and Clinical Aspects—A General Perspective. Endocrinol. Diabetes Nutr. 2022, 69, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Tomasik, P.J. A New Perspective on the Renin-Angiotensin System. Diagnostics 2022, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension Guidelines for the Management of High Blood Pressure in Children and Adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef]
- Thomas, W.G.; Mendelsohn, F.A.O. Angiotensin Receptors: Form and Function and Distribution. Int. J. Biochem. Cell Biol. 2003, 35, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II Revisited: New Roles in Inflammation, Immunology and Aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef]
- e Silva, A.C.S.; Diniz, J.S.S.; Regueira Filho, A.; Santos, R.A.S. The Renin Angiotensin System in Childhood Hypertension: Selective Increase of Angiotensin-(1–7) in Essential Hypertension. J. Pediatr. 2004, 145, 93–98. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Draz, H.M. Relationship between Resistin and APAI-1 Levels with Insulin Resistance in Saudi Children. Pediatr. Int. 2010, 52, 551–556. [Google Scholar] [CrossRef]
- Chai, S.Y.; Fernando, R.; Peck, G.; Ye, S.-Y.; Mendelsohn, F.A.O.; Jenkins, T.A.; Albiston, A.L. The Angiotensin IV/AT4 Receptor. Cell. Mol. Life Sci. 2004, 61, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Harding, J.W. The Brain Angiotensin System and Extracellular Matrix Molecules in Neural Plasticity, Learning, and Memory. Prog. Neurobiol. 2004, 72, 263–293. [Google Scholar] [CrossRef]
- Molina-Van den Bosch, M.; Jacobs-Cachá, C.; Vergara, A.; Serón, D.; Soler, M.J. The renin-angiotensin system and the brain. Hipertens. Riesgo Vasc. 2021, 38, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.; Eldahshan, W.; Fagan, S.C.; Ergul, A. Within the Brain: The Renin Angiotensin System. Int. J. Mol. Sci. 2018, 19, 876. [Google Scholar] [CrossRef]
- Yang, R.; Smolders, I.; De Bundel, D.; Fouyn, R.; Halberg, M.; Demaegdt, H.; Vanderheyden, P.; Dupont, A.G. Brain and Peripheral Angiotensin II Type 1 Receptors Mediate Renal Vasoconstrictor and Blood Pressure Responses to Angiotensin IV in the Rat. J. Hypertens. 2008, 26, 998–1007. [Google Scholar] [CrossRef]
- Wong, Y.-C.; Sim, M.-K.; Lee, K.-O. Des-Aspartate-Angiotensin-I and Angiotensin IV Improve Glucose Tolerance and Insulin Signalling in Diet-Induced Hyperglycaemic Mice. Biochem. Pharmacol. 2011, 82, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Slamkova, M.; Zorad, S.; Krskova, K. Alternative Renin-Angiotensin System Pathways in Adipose Tissue and Their Role in the Pathogenesis of Obesity. Endocr. Regul. 2016, 50, 229–240. [Google Scholar] [CrossRef]
- Olkowicz, M.; Chlopicki, S.; Smolenski, R.T. Perspectives for Angiotensin Profiling with Liquid Chromatography/Mass Spectrometry to Evaluate ACE/ACE2 Balance in Endothelial Dysfunction and Vascular Pathologies. Pharmacol. Rep. 2015, 67, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.; Alenina, N.; Andrade-Navarro, M.A.; Santos, R.A. MAS and Its Related G Protein-Coupled Receptors, Mrgprs. Pharmacol. Rev. 2014, 66, 1080–1105. [Google Scholar] [CrossRef] [PubMed]
- Kohara, K.; Bridget^Brosnihan, K.; Ferrario, C.M. Angiotensin(1–7) in the Spontaneously Hypertensive Rat. Peptides 1993, 14, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.J.; Duncan, A.-M.; Kladis, A.; Harrap, S.B. Angiotensin Peptides in Spontaneously Hypertensive and Normotensive Donryu Rats. Hypertension 1995, 25, 928–934. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1–7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1–7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef]
- Moraes, P.L.; Kangussu, L.M.; da Silva, L.G.; Castro, C.H.; Santos, R.A.S.; Ferreira, A.J. Cardiovascular Effects of Small Peptides of the Renin Angiotensin System. Physiol. Rep. 2017, 5, e13505. [Google Scholar] [CrossRef]
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-Regulatory Renin-Angiotensin System in Cardiovascular Disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Moya, J.; Barrientos, V.; Alzamora, R.; Hevia, D.; Morales, C.; Pinto, M.; Escudero, N.; García, L.; Novoa, U.; et al. Angiotensin-(1–9) Reverses Experimental Hypertension and Cardiovascular Damage by Inhibition of the Angiotensin Converting Enzyme/Ang II Axis. J. Hypertens. 2014, 32, 771–783. [Google Scholar] [CrossRef]
- Ali, Q.; Wu, Y.; Hussain, T. Chronic AT2 Receptor Activation Increases Renal ACE2 Activity, Attenuates AT1 Receptor Function and Blood Pressure in Obese Zucker Rats. Kidney Int. 2013, 84, 931–939. [Google Scholar] [CrossRef]



| Number of Patients | Age | BMI | Systolic Pressure (SP) | Diastolic Pressure (DP) | |
|---|---|---|---|---|---|
| Hypertension | 28 | 15.05 ys ± 2.98 | 21.75 ± 3.44 kg/m2 | 132 ± 16 mmHg | 79 ± 11 mmHg |
| Hypertension + obesity | 17 | 13.95 ys ± 3.79 | 29.89 ± 4.64 kg/m2 | 138 ± 19 mmHg | 74 ± 14 mmHg |
| Obesity | 29 | 13.50 ys ± 3.39 | 28.40 ± 5.59 kg/m2 | 114 ± 10 mmHg | 68 ± 9 mmHg |
| Control | 52 | 12.95 ys ± 3.69 | 18.63 ± 3.9 kg/m2 | 112 ± 11 mmHg | 66 ± 10 mmHg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martyniak, A.; Drożdż, D.; Tomasik, P.J. Classical and Alternative Pathways of the Renin–Angiotensin–Aldosterone System in Regulating Blood Pressure in Hypertension and Obese Adolescents. Biomedicines 2024, 12, 620. https://doi.org/10.3390/biomedicines12030620
Martyniak A, Drożdż D, Tomasik PJ. Classical and Alternative Pathways of the Renin–Angiotensin–Aldosterone System in Regulating Blood Pressure in Hypertension and Obese Adolescents. Biomedicines. 2024; 12(3):620. https://doi.org/10.3390/biomedicines12030620
Chicago/Turabian StyleMartyniak, Adrian, Dorota Drożdż, and Przemysław J. Tomasik. 2024. "Classical and Alternative Pathways of the Renin–Angiotensin–Aldosterone System in Regulating Blood Pressure in Hypertension and Obese Adolescents" Biomedicines 12, no. 3: 620. https://doi.org/10.3390/biomedicines12030620
APA StyleMartyniak, A., Drożdż, D., & Tomasik, P. J. (2024). Classical and Alternative Pathways of the Renin–Angiotensin–Aldosterone System in Regulating Blood Pressure in Hypertension and Obese Adolescents. Biomedicines, 12(3), 620. https://doi.org/10.3390/biomedicines12030620







