Co-Culture Models: Key Players in In Vitro Neurotoxicity, Neurodegeneration and BBB Modeling Studies
Abstract
:1. Blood–Brain Barrier: Development and Characterization
1.1. Endothelial Cells
1.2. Pericytes
1.3. Neurons
1.4. Astrocytes
1.5. Microglia
1.6. Extracellular Matrix
1.7. ABC and SLC Transporters
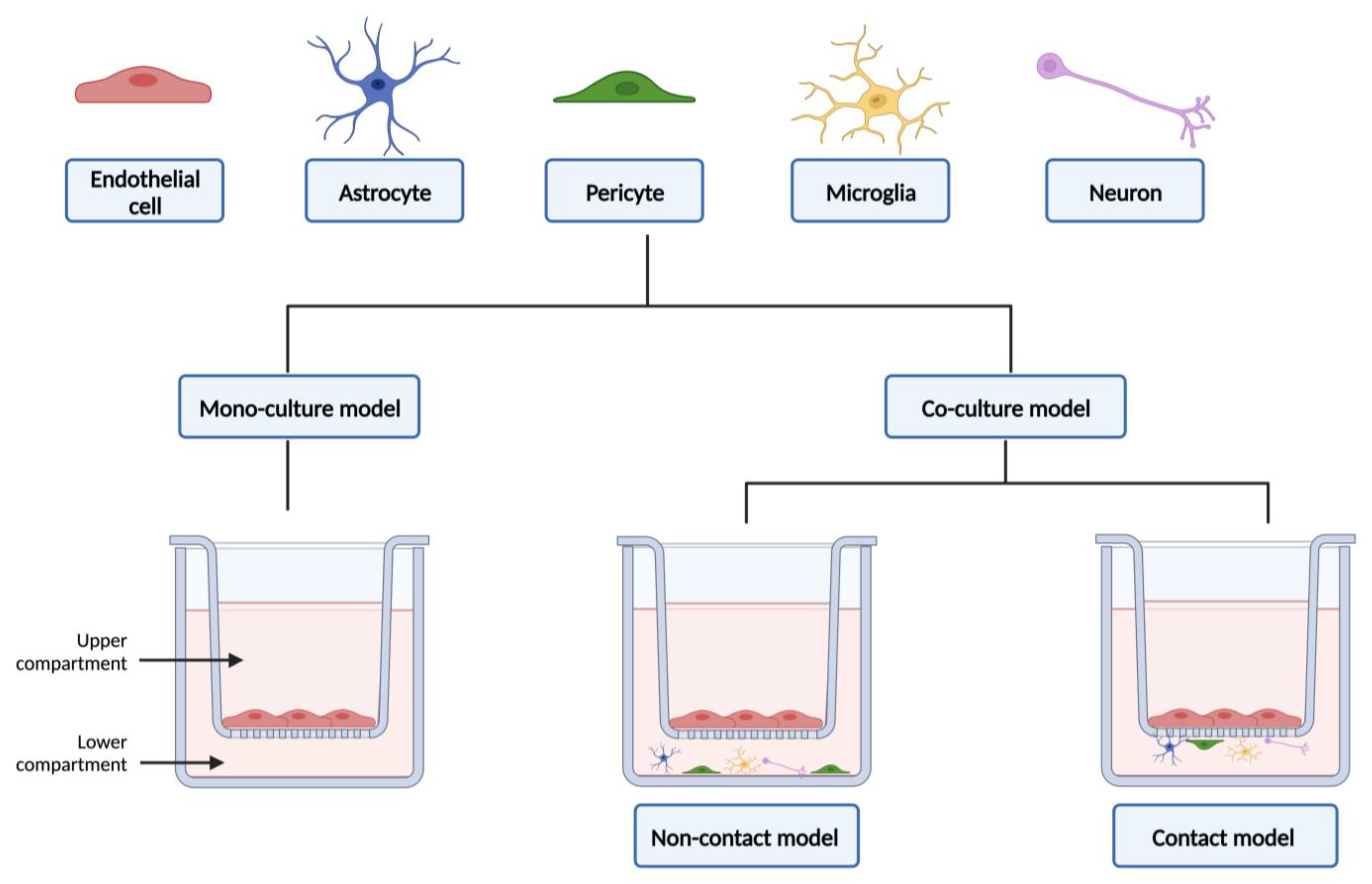
2. Cell Cultures as In Vitro BBB Models
2.1. Validation Markers for Cell Culture Models
2.2. Cell-Based Models’ Origins
2.3. In Vitro Models: Primary Cells
2.3.1. Bovine Brain Microvascular Endothelial Cells (BBMECs)
2.3.2. Porcine Brain Microvascular Endothelial Cells (PBMECs)
2.3.3. Rat Brain Microvascular Endothelial Cells (RBMECs)
2.3.4. Human Brain Microvascular Endothelial Cells (HBMECs)
2.4. In Vitro Models: Immortalized Cell Lines
2.4.1. BB19
2.4.2. RBE4
2.4.3. b.End3
2.4.4. b.End5
2.4.5. cEND
2.4.6. cerebEND
2.4.7. hCMEC/D3
2.4.8. TY08
2.4.9. TY09
2.4.10. TY10
2.4.11. HBEC-5i
2.4.12. HBMEC/cibeta (ciβ)
2.4.13. HBMEC/ci18
2.4.14. SH-SY5Y
2.4.15. Non-Brain Endothelial Cell Lines
Caco-2
Madin–Darby Canine Kidney (MDCK)
2.5. Future Perspectives: Microfluidic and Stem Cells
| Types of Stem Cells | Characteristics | References |
|---|---|---|
| Embryonic stem cells (ESCs) |
| [94,96] |
| Neural stem cells (NSCs) |
| [1,94] |
| Mesenchymal stem cells (MSCs) |
| [94,97] |
| Induced pluripotent stem cells (iPSCs) |
| [1,92] |
3. Co-Culture Models
3.1. Advantages and Limitations
3.2. From Static to Dynamic In Vitro Models of the Blood–Brain Barrier
3.3. A Compendium of the Applicability of Co-Culture Models in Permeability Studies
| Stem Cells | Co-Cultivated with | Advantages/Observations | References |
|---|---|---|---|
| iPSCs-derived brain endothelial cells |
|
| [92,93,130,131,132] |
|
| ||
|
| ||
|
|
3.4. Relevance of In Vitro Co-Culture Models in Neurotoxicity Assessments
3.5. The Use of In Vitro Co-Culture Models in the Study of Neurodegenerative Diseases
3.5.1. Alzheimer’s Disease
3.5.2. Amyotrophic Lateral Sclerosis
3.5.3. Parkinson’s Disease
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Lippmann, E.S.; Al-Ahmad, A.; Palecek, S.P.; Shusta, E.V. Modeling the Blood-Brain Barrier Using Stem Cell Sources. Fluids Barriers CNS 2013, 10, 2. [Google Scholar] [CrossRef]
- Barichello, T.; Collodel, A.; Hasbun, R.; Morales, R. An Overview of the Blood-Brain Barrier; Humana Press: New York, NY, USA, 2019; Volume 142, ISBN 9781493989454. [Google Scholar]
- Wilhelm, I.; Krizbai, I.A. In Vitro Models of the Blood-Brain Barrier for the Study of Drug Delivery to the Brain. Mol. Pharm. 2014, 11, 1949–1963. [Google Scholar] [CrossRef]
- Keaney, J.; Campbell, M. The Dynamic Blood-Brain Barrier. FEBS J. 2015, 282, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Vallon, M.; Chang, J.; Zhang, H.; Kuo, C.J. Developmental and Pathological Angiogenesis in the Central Nervous System. Cell. Mol. Life Sci. 2014, 71, 3489–3506. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Liebner, S. Novel Insights into the Development and Maintenance of the Blood-Brain Barrier. Cell Tissue Res. 2014, 355, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Goasdoué, K.; Miller, S.M.; Colditz, P.B.; Björkman, S.T. Review: The Blood-Brain Barrier; Protecting the Developing Fetal Brain. Placenta 2017, 54, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional Morphology of the Blood–Brain Barrier in Health and Disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef]
- Costea, L.; Mészáros; Bauer, H.; Bauer, H.C.; Traweger, A.; Wilhelm, I.; Farkas, A.E.; Krizbai, I.A. The Blood–Brain Barrier and Its Intercellular Junctions in Age-Related Brain Disorders. Int. J. Mol. Sci. 2019, 20, 5472. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The Blood-Brain Barrier: An Engineering Perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Erdo, F.; Krajcsi, P. Age-Related Functional and Expressional Changes in Efflux Pathways at the Blood–Brain Barrier. Front. Aging Neurosci. 2019, 10, 196. [Google Scholar] [CrossRef]
- Jackson, S.; Meeks, C.; Vézina, A.; Robey, R.W.; Tanner, K.; Gottesman, M.M. Model Systems for Studying the Blood-Brain Barrier: Applications and Challenges. Biomaterials 2019, 214, 119217. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The Blood-Brain Barrier: An Overview: Structure, Regulation, and Clinical Implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Tietz, S.; Engelhardt, B. Brain Barriers: Crosstalk between Complex Tight Junctions and Adherens Junctions. J. Cell Biol. 2015, 209, 493–506. [Google Scholar] [CrossRef]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between Tight Junctions & Adherens Junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Noumbissi, M.E.; Galasso, B.; Stins, M.F. Brain Vascular Heterogeneity: Implications for Disease Pathogenesis and Design of in Vitro Blood-Brain Barrier Models. Fluids Barriers CNS 2018, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Gastfriend, B.D.; Palecek, S.P.; Shusta, E.V. Modeling the Blood–Brain Barrier: Beyond the Endothelial Cells. Curr. Opin. Biomed. Eng. 2018, 5, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood-Brain Barrier: Structural Components and Function under Physiologic and Pathologic Conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yao, Y.; Tsirka, S.E.; Cao, Y. Cell-Culture Models of the Blood-Brain Barrier. Stroke 2014, 45, 2514–2526. [Google Scholar] [CrossRef] [PubMed]
- Kalvass, J.C.; Polli, J.W.; Bourdet, D.L.; Feng, B.; Huang, S.M.; Liu, X.; Smith, Q.R.; Zhang, L.K.; Zamek-Gliszczynski, M.J. Why Clinical Modulation of Efflux Transport at the Human Blood-Brain Barrier Is Unlikely: The ITC Evidence-Based Position. Clin. Pharmacol. Ther. 2013, 94, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Qosa, H.; Miller, D.S.; Pasinelli, P.; Trotti, D. Regulation of ABC Efflux Transporters at Blood-Brain Barrier in Health and Neurological Disorders. Brain Res. 2015, 1628, 298–316. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martins, E.; Barbosa, D.J.; Silva, V.; Remião, F.; Silva, R. Dysfunction of ABC Transporters at the Blood-Brain Barrier: Role in Neurological Disorders. Pharmacol. Ther. 2020, 213, 107554. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Vilas-Boas, V.; Carmo, H.; Dinis-Oliveira, R.J.; Carvalho, F.; De Lourdes Bastos, M.; Remião, F. Modulation of P-Glycoprotein Efflux Pump: Induction and Activation as a Therapeutic Strategy. Pharmacol. Ther. 2015, 149, 1–123. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M.D.L.; Remião, F. Cellular Models and in Vitro Assays for the Screening of Modulators of P-Gp, MRP1 and BCRP. Molecules 2017, 22, 600. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zepeda, D.; Taghi, M.; Scherrmann, J.M.; Decleves, X.; Menet, M.C. ABC Transporters at the Blood–Brain Interfaces, Their Study Models, and Drug Delivery Implications in Gliomas. Pharmaceutics 2020, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Nałęcz, K.A. Solute Carriers in the Blood–Brain Barier: Safety in Abundance. Neurochem. Res. 2017, 42, 795–809. [Google Scholar] [CrossRef]
- Kurosawa, Y.; Hamaoka, T. Creatine in the Brain. J. Phys. Fit. Sports Med. 2017, 6, 215–217. [Google Scholar] [CrossRef]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In Vitro Models of the Blood-Brain Barrier: An Overview of Commonly Used Brain Endothelial Cell Culture Models and Guidelines for Their Use. J. Cereb. Blood Flow. Metab. 2015, 36, 862–890. [Google Scholar] [CrossRef]
- Destefano, J.G.; Jamieson, J.J.; Linville, R.M.; Searson, P.C. Benchmarking in Vitro Tissue—Engineered Blood-Brain Barrier Models. Fluids Barriers CNS 2018, 15, 32. [Google Scholar] [CrossRef]
- Fujimoto, T.; Morofuji, Y.; Nakagawa, S.; Kovac, A.; Horie, N.; Izumo, T.; Niwa, M.; Matsuo, T.; Banks, W.A. Comparison of the Rate of Dedifferentiation with Increasing Passages among Cell Sources for an in Vitro Model of the Blood–Brain Barrier. J. Neural Transm. 2020, 127, 1117–1124. [Google Scholar] [CrossRef]
- Wilhelm, I.; Fazakas, C.; Krizbai, I.A. In Vitro Models of the Blood-Brain Barrier. Acta Neurobiol. Exp. 2011, 71, 113–128. [Google Scholar] [CrossRef]
- Design, D.; Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-Vitro Blood-Brain Barrier Models for Drug Screening and Permeation Studies: An Overview. Drug Des. Dev. Ther. 2019, 13, 3591–3605. [Google Scholar]
- Terasaki, T.; Ohtsuki, S.; Hori, S.; Takanaga, H.; Nakashima, E.; Hosoya, K. New Approaches to in Vitro Models of Blood-Brain Barrier Drug Transport. Drug Discov. Today 2003, 8, 944–954. [Google Scholar] [CrossRef]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative Study of Four Immortalized Human Brain Capillary Endothelial Cell Lines, HCMEC/D3, HBMEC, TY10, and BB19, and Optimization of Culture Conditions, for an in Vitro Blood-Brain Barrier Model for Drug Permeability Studies Comparative Stu. Fluids Barriers CNS 2013, 10, 33. [Google Scholar] [CrossRef]
- Méresse, S.; Dehouck, M.-P.; Delorme, P.; Bensaïd, M.; Tauber, J.-P.; Delbart, C.; Fruchart, J.-C.; Cecchelli, R. Bovine Brain Endothelial Cells Express Tight Junctions and Monoamine Oxidase Activity in Long-Term Culture. J. Neurochem. 1989, 53, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Psimadas, D.; Georgoulias, P.; Valotassiou, V.; Loudos, G. Molecular Nanomedicine Towards Cancer. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.K.; Scott, D.O.; Otis, K.W.; Lunte, S.M. Comparison of in Vitro BBMEC Permeability and in Vivo CNS Uptake by Microdialysis Sampling. J. Pharm. Biomed. Anal. 2002, 27, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Hersom, M.; Helms, H.C.; Schmalz, C.; Pedersen, T.; Buckley, S.T.; Brodin, B. The Insulin Receptor Is Expressed and Functional in Cultured Blood-Brain Barrier Endothelial Cells but Does Not Mediate Insulin Entry from Blood to Brain. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E531–E542. [Google Scholar] [CrossRef] [PubMed]
- van Bree, J.B.M.M.; Audus, K.L.; Borchardt, R.T. Carrier-Mediated Transport of Baclofen Across Monolayers of Bovine Brain Endothelial Cells in Primary Culture. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1988, 5, 369–371. [Google Scholar]
- Culot, M.; Lundquist, S.; Vanuxeem, D.; Nion, S.; Landry, C.; Delplace, Y.; Dehouck, M.P.; Berezowski, V.; Fenart, L.; Cecchelli, R. An in Vitro Blood-Brain Barrier Model for High Throughput (HTS) Toxicological Screening. Toxicol. Vitr. 2008, 22, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Freese, C.; Reinhardt, S.; Hefner, G.; Unger, R.E.; Kirkpatrick, C.J.; Endres, K. A Novel Blood-Brain Barrier Co-Culture System for Drug Targeting of Alzheimer’s Disease: Establishment by Using Acitretin as a Model Drug. PLoS ONE 2014, 9, e91003. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Omidi, Y.; Gumbleton, M. Primary Porcine Brain Microvascular Endothelial Cells: Biochemical and Functional Characterisation as a Model for Drug Transport and Targeting. J. Drug Target. 2007, 15, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Patabendige, A.; Skinner, R.A.; Morgan, L.; Abbott, N.J. A Detailed Method for Preparation of a Functional and Fl Exible Blood-Brain Barrier Model Using Porcine Brain Endothelial Cells. Brain Res. 2013, 1521, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Galla, H. Monocultures of Primary Porcine Brain Capillary Endothelial Cells: Still a Functional in Vitro Model for the Blood-Brain-Barrier. J. Control. Release 2018, 285, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Elbakary, B.; Badhan, R.K.S. A Dynamic Perfusion Based Blood-Brain Barrier Model for Cytotoxicity Testing and Drug Permeation. Sci. Rep. 2020, 10, 3788. [Google Scholar] [CrossRef] [PubMed]
- Regina, A.; Koman, A.; Piciotti, M.; El Hafny, B.; Center, M.S.; Bergmann, R.; Couraud, P.O.; Roux, F. Mrp1 Multidrug Resistance-Associated Protein and P-Glycoprotein Expression in Rat Brain Microvessel Endothelial Cells. J. Neurochem. 1998, 71, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, F.E.; Hacking, C. Pericyte Abundance Affects Sucrose Permeability in Cultures of Rat Brain Microvascular Endothelial Cells. Brain Res. 2005, 1049, 8–14. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Lu, S.; Wu, Y.; Sahi, J. Temporal Expression of Transporters and Receptors in a Rat Primary Co-Culture Blood-Brain Barrier Model. Xenobiotica 2014, 44, 941–951. [Google Scholar] [CrossRef]
- Kusuhara, H.; Sugiyama, Y. Efflux Transport Systems for Drugs at the Blood-Brain Barrier and Blood-Cerebrospinal Fluid Barrier (Part 1). Drug Discov. Today 2001, 6, 150–156. [Google Scholar] [CrossRef]
- Stone, N.L.; England, T.J.; Sullivan, S.E.O. A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells. Front. Cell. Neurosci. 2019, 13, 230. [Google Scholar] [CrossRef]
- Stins, M.F.; Badger, J.; Sik Kim, K. Bacterial Invasion and Transcytosis in Transfected Human Brain Microvascular Endothelial Cells. Microb. Pathog. 2001, 30, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Bernas, M.J.; Cardoso, F.L.; Daley, S.K.; Weinand, M.E.; Campos, A.R.; Ferreira, A.J.G.; Hoying, J.B.; Witte, M.H.; Brites, D.; Persidsky, Y.; et al. Establishment of Primary Cultures of Human Brain Microvascular Endothelial Cells to Provide an in Vitro Cellular Model of the Blood-Brain Barrier. Nat. Protoc. 2010, 5, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Kusch-Poddar, M.; Drewe, J.; Fux, I.; Gutmann, H. Evaluation of the Immortalized Human Brain Capillary Endothelial Cell Line BB19 as a Human Cell Culture Model for the Blood-Brain Barrier. Brain Res. 2005, 1064, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Veszelka, S.; Tóth, A.; Walter, F.R.; Tóth, A.E.; Gróf, I. Comparison of a Rat Primary Cell-Based Blood-Brain Barrier Model with Epithelial and Brain Endothelial Cell Lines: Gene Expression and Drug Transport. Front. Cell. Neurosci. 2018, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.; Couraud, P.O. Rat Brain Endothelial Cell Lines for the Study of Blood-Brain Barrier Permeability and Transport Functions. Cell Mol. Neurobiol. 2005, 25, 41–57. [Google Scholar] [CrossRef]
- Omidi, Y.; Campbell, L.; Barar, J.; Connell, D.; Akhtar, S.; Gumbleton, M. Evaluation of the Immortalised Mouse Brain Capillary Endothelial Cell Line, b.End3, as an in Vitro Blood-Brain Barrier Model for Drug Uptake and Transport Studies. Brain Res. 2003, 990, 95–112. [Google Scholar] [CrossRef]
- Brown, R.C.; Morris, A.P.; O’Neil, R.G. Tight Junction Protein Expression and Barrier Properties of Immortalized Mouse Brain Microvessel Endothelial Cells. Brain Res. 2007, 1130, 17–30. [Google Scholar] [CrossRef]
- Alamu, O.; Rado, M.; Ekpo, O.; Fisher, D. Differential Sensitivity of Two Endothelial Cell Lines to Hydrogen Peroxide Toxicity: Relevance for In Vitro Studies of the Blood-Brain Barrier. Cells 2020, 9, 403. [Google Scholar] [CrossRef]
- Steiner, O.; Coisne, C.; Engelhardt, B.; Lyck, R. Comparison of Immortalized BEnd5 and Primary Mouse Brain Microvascular Endothelial Cells as in Vitro Blood-ΚBrain Barrier Models for the Study of T Cell Extravasation. J. Cereb. Blood Flow. Metab. 2011, 31, 315–327. [Google Scholar] [CrossRef]
- Förster, C.; Silwedel, C.; Golenhofen, N.; Burek, M.; Kietz, S.; Mankertz, J.; Drenckhahn, D. Occludin as Direct Target for Glucocorticoid-Induced Improvement of Blood-Brain Barrier Properties in a Murine in Vitro System. J. Physiol. 2005, 565, 475–486. [Google Scholar] [CrossRef]
- Burek, M.; Salvador, E.; Förster, C.Y. Generation of an Immortalized Murine Brain Microvascular Endothelial Cell Line as an in Vitro Blood Brain Barrier Model. J. Vis. Exp. 2012, 66, e4022. [Google Scholar] [CrossRef]
- Blecharz, K.G.; Haghikia, A.; Stasiolek, M.; Kruse, N.; Drenckhahn, D.; Gold, R.; Roewer, N.; Chan, A.; Förster, C.Y. Glucocorticoid Effects on Endothelial Barrier Function in the Murine Brain Endothelial Cell Line CEND Incubated with Sera from Patients with Multiple Sclerosis. Mult. Scler. 2010, 16, 293–302. [Google Scholar] [CrossRef]
- Kaiser, M.; Burek, M.; Britz, S.; Lankamp, F.; Ketelhut, S.; Kemper, B.; Förster, C.; Gorzelanny, C.; Goycoolea, F.M. The Influence of Capsaicin on the Integrity of Microvascular Endothelial Cell Monolayers. Int. J. Mol. Sci. 2019, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Silwedel, C.; Förster, C. Differential Susceptibility of Cerebral and Cerebellar Murine Brain Microvascular Endothelial Cells to Loss of Barrier Properties in Response to Inflammatory Stimuli. J. Neuroimmunol. 2006, 179, 37–45. [Google Scholar] [CrossRef]
- Masuda, T.; Hoshiyama, T.; Uemura, T.; Hirayama-Kurogi, M.; Ogata, S.; Furukawa, A.; Couraud, P.O.; Furihata, T.; Ito, S.; Ohtsuki, S. Large-Scale Quantitative Comparison of Plasma Transmembrane Proteins between Two Human Blood-Brain Barrier Model Cell Lines, HCMEC/D3 and HBMEC/Ciβ. Mol. Pharm. 2019, 16, 2162–2171. [Google Scholar] [CrossRef]
- Weksler, B.; Romero, I.A.; Couraud, P.O. The HCMEC/D3 Cell Line as a Model of the Human Blood Brain Barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef]
- Daniels, B.P.; Cruz-orengo, L.; Jo, T.; Couraud, P.; Romero, I.A.; Weksler, B.; Cooper, J.A.; Doering, T.L.; Klein, R.S. Immortalized Human Cerebral Microvascular Endothelial Cells Maintain the Properties of Primary Cells in an in Vitro Model of Immune Migration across the Blood Brain Barrier. J. Neurosci. Methods 2013, 212, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, S.; Ikeda, C.; Uchida, Y.; Sakamoto, Y.; Miller, F.; Glacial, F.; Decleves, X.; Scherrmann, J.; Couraud, P.; Kubo, Y.; et al. Quantitative Targeted Absolute Proteomic Analysis of Transporters, Receptors and Junction Proteins for Validation of Human Cerebral Microvascular Endothelial Cell Line HCMEC/D3 as a Human Blood-Brain Barrier Model. Mol. Pharm. 2013, 10, 289–296. [Google Scholar] [CrossRef]
- Hinkel, S.; Mattern, K.; Dietzel, A.; Reichl, S.; Müller-goymann, C.C. Parametric Investigation of Static and Dynamic Cell Culture Conditions and Their Impact on HCMEC/D3 Barrier Properties. Int. J. Pharm. 2019, 566, 434–444. [Google Scholar] [CrossRef]
- Barrier, B.; De Waal, R.M.W.; Kuiperij, H.B.; Verbeek, M.M. Limitations of the HCMEC/D3 Cell Line as a Model for A b Clearance by the Human. J. Neurosci. Res. 2017, 1522, 1513–1522. [Google Scholar] [CrossRef]
- Sano, Y.; Shimizu, F.; Abe, M.; Maeda, T.; Kashiwamura, Y.; Ohtsuki, S.; Terasaki, T.; Obinata, M.; Kajiwara, K.; Fujii, M.; et al. Establishment of a New Conditionally Immortalized Human Brain Microvascular Endothelial Cell Line Retaining an in Vivo Blood-Brain Barrier Function. J. Cell Physiol. 2010, 225, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Kashiwamura, Y.; Abe, M.; Dieu, L.H.; Huwyler, J.; Shimizu, F.; Haruki, H.; Maeda, T.; Saito, K.; Tasaki, A.; et al. Stable Human Brain Microvascular Endothelial Cell Line Retaining Its Barrier-Specific Nature Independent of the Passage Number. Clin. Exp. Neuroimmunol. 2013, 4, 92–103. [Google Scholar] [CrossRef]
- Haruki, H.; Sano, Y.; Shimizu, F.; Omoto, M.; Tasaki, A.; Oishi, M.; Koga, M.; Saito, K.; Takahashi, T.; Nakada, T.; et al. NMO Sera Down-Regulate AQP4 in Human Astrocyte and Induce Cytotoxicity Independent of Complement. J. Neurol. Sci. 2013, 331, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Nishihara, H.; Kanda, T. Blood–Brain Barrier Dysfunction in Immuno-Mediated Neurological Diseases. Immunol. Med. 2018, 41, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Sano, Y.; Abe, M.; Shimizu, F.; Kashiwamura, Y.; Ohtsuki, S.; Terasaki, T.; Obinata, M.; Ueda, M.; Kanda, T. Establishment and Characterization of Spinal Cord Microvascular Endothelial Cell Lines. Clin. Exp. Neuroimmunol. 2013, 4, 326–338. [Google Scholar] [CrossRef]
- Sano, H.; Sano, Y.; Ishiguchi, E.; Shimizu, F.; Omoto, M.; Maeda, T.; Nishihara, H.; Takeshita, Y.; Takahashi, S.; Oishi, M.; et al. Establishment of a New Conditionally Immortalized Human Skeletal Muscle Microvascular Endothelial Cell Line. J. Cell Physiol. 2017, 232, 3286–3295. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Merlo, S.; Sano, Y.; Kanda, T.; Sortino, M.A. Astrocytes Contribute to Aβ-Induced Blood–Brain Barrier Damage through Activation of Endothelial MMP9. J. Neurochem. 2017, 142, 464–477. [Google Scholar] [CrossRef]
- Takahashi, S.; Maeda, T.; Sano, Y.; Nishihara, H.; Takeshita, Y.; Shimizu, F.; Kanda, T. Active Form of Vitamin D Directly Protects the Blood–Brain Barrier in Multiple Sclerosis. Clin. Exp. Neuroimmunol. 2017, 8, 244–254. [Google Scholar] [CrossRef]
- Wassmer, S.C.; Combes, V.; Candal, F.J.; Juhan-Vague, I.; Grau, G.E. Platelets Potentiate Brain Endothelial Alterations Induced by Plasmodium Falciparum. Infect. Immun. 2006, 74, 645–653. [Google Scholar] [CrossRef]
- Puech, C.; Hodin, S.; Forest, V.; He, Z.; Mismetti, P.; Delavenne, X.; Perek, N. Assessment of HBEC-5i Endothelial Cell Line Cultivated in Astrocyte Conditioned Medium as a Human Blood-Brain Barrier Model for ABC Drug Transport Studies. Int. J. Pharm. 2018, 551, 281–289. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, W.; Chen, Y.; Zou, M.; Peng, D.; Chen, D. HIV-1 Transactivator Protein Induces ZO-1 and Neprilysin Dysfunction in Brain Endothelial Cells via the Ras Signaling Pathway. Oxid. Med. Cell Longev. 2017, 2017, 3160360. [Google Scholar] [CrossRef] [PubMed]
- Kamiichi, A.; Furihata, T.; Kishida, S.; Ohta, Y.; Saito, K.; Kawamatsu, S.; Chiba, K. Establishment of a New Conditionally Immortalized Cell Line from Human Brain Microvascular Endothelial Cells: A Promising Tool for Human Blood-Brain Barrier Studies. Brain Res. 2012, 1488, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Furihata, T.; Kawamatsu, S.; Ito, R.; Saito, K.; Suzuki, S.; Kishida, S.; Saito, Y.; Kamiichi, A.; Chiba, K. Hydrocortisone Enhances the Barrier Properties of HBMEC/Ciβ, a Brain Microvascular Endothelial Cell Line, through Mesenchymal-to-Endothelial Transition-like Effects. Fluids Barriers CNS 2015, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Umehara, K.; Suzuki, S.; Kitamura, K.; Nunoya, K.I.; Yamaura, Y.; Imawaka, H.; Izumi, S.; Wakayama, N.; Komori, T.; et al. A Human Immortalized Cell-Based Blood-Brain Barrier Triculture Model: Development and Characterization as a Promising Tool for Drug-Brain Permeability Studies. Mol. Pharm. 2019, 16, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, A.; Donaghy, H.; Stoner, S.P.; Hudson, A.L.; Wheeler, H.R.; Diakos, C.I.; Howell, V.M.; Grau, G.E.; McKelvey, K.J. Are in Vitro Human Blood–Brain–Tumor-barriers Suitable Replacements for in Vivo Models of Brain Permeability for Novel Therapeutics? Cancers 2021, 13, 955. [Google Scholar] [CrossRef]
- Ferguson, R.; Subramanian, V. PA6 Stromal Cell Co-Culture Enhances SH- Differentiation to Mature Phenotypes. PLoS ONE 2016, 11, e0159051. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.K. Neuronal Cell Culture. Neuronal Cell Cult. Methods Protoc. 2013, 1078, 35–44. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y Cell Line in Parkinson’ s Disease Research: A Systematic Review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Barbosa, D.J.; Capela, J.P.; Silva, R.; Vilas-Boas, V.; Ferreira, L.M.; Branco, P.S.; Fernandes, E.; Bastos, M.D.L.; Carvalho, F. The Mixture of “Ecstasy” and Its Metabolites Is Toxic to Human SH-SY5Y Differentiated Cells at in Vivo Relevant Concentrations. Arch. Toxicol. 2014, 88, 455–473. [Google Scholar] [CrossRef]
- Dukes, J.D.; Whitley, P.; Chalmers, A.D. The MDCK Variety Pack: Choosing the Right Strain. BMC Cell Biol. 2011, 12, 2–5. [Google Scholar] [CrossRef]
- Aday, S.; Cecchelli, R.; Dehouck, M.P.; Ferreira, L. Stem Cell-Based Human Blood-Brain Barrier Models for Drug Discovery and Delivery. Trends Biotechnol. 2016, 34, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Minami, H.; Tashiro, K.; Okada, A.; Hirata, N. Generation of Brain Microvascular Endothelial-Like Cells from Human Induced Pluripotent Stem Cells by Co-Culture with C6 Glioma Cells. PLoS ONE 2015, 10, e0128890. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Cucullo, L. In-Vitro Blood-Brain Barrier Modeling: A Review of Modern and Fast-Advancing Technologies. J. Cereb. Blood Flow Metab. 2018, 38, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, E.K.; Bailey, A.K.; Potharazu, A.V.; Neely, M.D.; Bowman, A.B.; Lippmann, E.S. Accelerated Differentiation of Human Induced Pluripotent Stem Cells to Blood–Brain Barrier Endothelial Cells. Fluids Barriers CNS 2017, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Chiou, B.; Neal, E.H.; Bowman, A.B.; Lippmann, E.S.; Simpson, I.A.; Connor, J.R. Endothelial Cells Are Critical Regulators of Iron Transport in a Model of the Human Blood-Brain Barrier. J. Cereb. Blood Flow Metab. 2019, 39, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Ramiah Rajasekaran, P.; Chapin, A.A.; Quan, D.N.; Herberholz, J.; Bentley, W.E.; Ghodssi, R. 3D-Printed Electrochemical Sensor-Integrated Transwell Systems. Microsyst. Nanoeng. 2020, 6, 100. [Google Scholar] [CrossRef]
- Mondadori, C.; Crippa, M.; Moretti, M.; Candrian, C.; Lopa, S.; Arrigoni, C. Advanced Microfluidic Models of Cancer and Immune Cell Extravasation: A Systematic Review of the Literature. Front. Bioeng. Biotechnol. 2020, 8, 907. [Google Scholar] [CrossRef]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, Á.; Tanaka, K.; Niwa, M. A New Blood-Brain Barrier Model Using Primary Rat Brain Endothelial Cells, Pericytes and Astrocytes. Neurochem. Int. 2009, 54, 253–263. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Malina, K.C.; Cooper, I.; Teichberg, V.I. Closing the Gap between the In-Vivo and in-Vitro Blood-Brain Barrier Tightness. Brain Res. 2009, 1284, 12–21. [Google Scholar] [CrossRef]
- Roqué, P.J.; Costa, L.G. Co-Culture of Neurons and Microglia. Curr. Protoc. Toxicol. 2017, 74, 11.24.1–11.24.17. [Google Scholar] [CrossRef]
- Gomes, M.J.; Mendes, B.; Martins, S.; Sarmento, B. Cell-Based in Vitro Models for Studying Blood-Brain Barrier (BBB) Permeability. In Concepts and Models for Drug Permeability Studies: Cell and Tissue Based In Vitro Culture Models; Woodhead Publishing: Sawston, UK, 2016; pp. 169–188. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Q.; Liu, W.; He, Z.; Lin, J.M. Recent Advances in Microfluidic 3D Cellular Scaffolds for Drug Assays. TrAC Trends Anal. Chem. 2017, 87, 19–31. [Google Scholar] [CrossRef]
- Gupta, N.; Liu, J.R.; Patel, B.; Solomon, D.E.; Vaidya, B.; Gupta, V. Microfluidics-based 3D Cell Culture Models: Utility in Novel Drug Discovery and Delivery Research. Bioeng. Transl. Med. 2016, 1, 63–81. [Google Scholar] [CrossRef]
- Osaki, T.; Shin, Y.; Sivathanu, V.; Campisi, M.; Kamm, R.D. In Vitro Microfluidic Models for Neurodegenerative Disorders. Adv. Healthc. Mater. 2018, 7, 1700489. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Abdoli, A.; Rahmanian, M.; Bardania, H.; Bayandori, M.; Moosavi Basri, S.M.; Kalbasi, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Microfluidic Brain-on-a-Chip: Perspectives for Mimicking Neural System Disorders. Mol. Neurobiol. 2019, 56, 8489–8512. [Google Scholar] [CrossRef] [PubMed]
- Oddo, A.; Peng, B.; Tong, Z.; Wei, Y.; Tong, W.Y.; Thissen, H.; Voelcker, N.H. Advances in Microfluidic Blood–Brain Barrier (BBB) Models. Trends Biotechnol. 2019, 37, 1295–1314. [Google Scholar] [CrossRef] [PubMed]
- Garberg, P.; Ball, M.; Borg, N.; Cecchelli, R.; Fenart, L.; Hurst, R.D.; Lindmark, T.; Mabondzo, A.; Nilsson, J.E.; Raub, T.J.; et al. In Vitro Models for the Blood-Brain Barrier. Toxicol. Vitr. 2005, 19, 299–334. [Google Scholar] [CrossRef] [PubMed]
- Nakhlband, A.; Omidi, Y. Barrier Functionality of Porcine and Bovine Brain Capillary Endothelial Cells. BioImpacts 2011, 1, 153–159. [Google Scholar] [CrossRef]
- Cantrill, C.A.; Skinner, R.A.; Rothwell, N.J.; Penny, J.I. An Immortalised Astrocyte Cell Line Maintains the in Vivo Phenotype of a Primary Porcine in Vitro Blood-Brain Barrier Model. Brain Res. 2012, 1479, 17–30. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Burkhart, A.; Moos, T. A Triple Culture Model of the Blood-Brain Barrier Using Porcine Brain Endothelial Cells, Astrocytes and Pericytes. PLoS ONE 2015, 10, e0134765. [Google Scholar] [CrossRef] [PubMed]
- Freese, C.; Hanada, S.; Fallier-Becker, P.; Kirkpatrick, C.J.; Unger, R.E. Identification of Neuronal and Angiogenic Growth Factors in an in Vitro Blood-Brain Barrier Model System: Relevance in Barrier Integrity and Tight Junction Formation and Complexity. Microvasc. Res. 2017, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Paradis, A.; Leblanc, D.; Dumais, N. MethodsX Optimization of an in Vitro Human Blood-Brain Barrier Model: Application to Blood Monocyte Transmigration Assays. MethodsX 2016, 3, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Megard, I.; Garrigues, A.; Orlowski, S.; Jorajuria, S.; Clayette, P.; Ezan, E.; Mabondzo, A. A Co-Culture-Based Model of Human Blood-Brain Barrier: Application to Active Transport of Indinavir and in Vivo-in Vitro Correlation. Brain Res. 2002, 927, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.; Lee, C.; Rajesh, R. Regulation of Human Brain Vascular Pericytes and Human Astrocytes in a Blood-Brain Barrier Model Using Human Brain Microvascular. J. Taiwan Inst. Chem. Eng. 2018, 86, 9–17. [Google Scholar] [CrossRef]
- Strazza, M.; Maubert, M.E.; Pirrone, V.; Wigdahl, B.; Nonnemacher, M.R. Co-Culture Model Consisting of Human Brain Microvascular Endothelial and Peripheral Blood Mononuclear Cells. J. Neurosci. Methods 2016, 269, 39–45. [Google Scholar] [CrossRef]
- Mendes, B.; Marques, C.; Carvalho, I.; Costa, P.; Martins, S.; Ferreira, D.; Sarmento, B. In Fl Uence of Glioma Cells on a New Co-Culture in Vitro Blood-Brain Barrier Model for Characterization and Validation of Permeability. Int. J. Pharm. 2015, 490, 94–101. [Google Scholar] [CrossRef]
- Gericke, B.; Römermann, K.; Noack, A.; Noack, S.; Kronenberg, J.; Blasig, I.E.; Löscher, W. A Face-to-Face Comparison of Claudin-5 Transduced Human Brain Endothelial (HCMEC/D3) Cells with Porcine Brain Endothelial Cells as Blood-Brain Barrier Models for Drug Transport Studies. Fluids Barriers CNS 2020, 17, 53. [Google Scholar] [CrossRef]
- Al-shehri, A.; Favretto, M.E.; Ioannou, P.V.; Romero, I.A.; Couraud, P.; Weksler, B.B.; Parker, T.L.; Kallinteri, P. Permeability of PEGylated Immunoarsonoliposomes Through In Vitro Blood Brain Barrier-Medulloblastoma Co-Culture Models for Brain Tumor Therapy. Pharm. Res. 2015, 32, 1072–1083. [Google Scholar] [CrossRef]
- Adhwa, N.; Nur, A.; Rasil, M.; Meyding-lamade, U.; Maria, E.; Diah, S.; Ani, A.; Hanna, S. Immortalized Endothelial Cell Lines for in Vitro Blood-Brain Barrier Models: A Systematic Review. Brain Res. 2016, 1642, 532–545. [Google Scholar] [CrossRef]
- Yang, S.; Mei, S.; Jin, H.; Zhu, B.; Tian, Y.; Huo, J.; Cui, X.; Guo, A.; Zhao, Z. Identification of Two Immortalized Cell Lines, ECV304 and BEnd3, for in Vitro Permeability Studies of Blood-Brain Barrier. PLoS ONE 2017, 12, e0187017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic Blood–Brain Barrier Model Provides in Vivo-like Barrier Properties for Drug Permeability Screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Delsing, L.; Dönnes, P.; Sánchez, J.; Clausen, M.; Voulgaris, D.; Falk, A.; Herland, A.; Brolén, G.; Zetterberg, H.; Hicks, R.; et al. Barrier Properties and Transcriptome Expression in Human IPSC-Derived Models of the Blood–Brain Barrier. Stem Cells 2018, 36, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, X.; Liu, H.; Huang, L.; Meng, G.; Ding, Y.; Su, W.; Lu, J.; Gong, S.; Terstappen, G.C.; et al. Development of Human in Vitro Brain-Blood Barrier Model from Induced Pluripotent Stem Cell-Derived Endothelial Cells to Predict the in Vivo Permeability of Drugs. Neurosci. Bull. 2019, 35, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Canfield, S.G.; Stebbins, M.J.; Morales, B.S.; Asai, S.W.; Vatine, G.D.; Svendsen, C.N.; Palecek, S.P.; Shusta, E.V. An Isogenic Blood–Brain Barrier Model Comprising Brain Endothelial Cells, Astrocytes, and Neurons Derived from Human Induced Pluripotent Stem Cells. J. Neurochem. 2017, 140, 874–888. [Google Scholar] [CrossRef] [PubMed]
- Canfield, S.G.; Stebbins, M.J.; Faubion, M.G.; Gastfriend, B.D.; Palecek, S.P.; Shusta, E.V. An Isogenic Neurovascular Unit Model Comprised of Human Induced Pluripotent Stem Cell-Derived Brain Microvascular Endothelial Cells, Pericytes, Astrocytes, and Neurons. Fluids Barriers CNS 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Noorani, B.; Bhalerao, A.; Raut, S.; Nozohouri, E.; Bickel, U.; Cucullo, L. A Quasi-Physiological Microfluidic Blood-Brain Barrier Model for Brain Permeability Studies. Pharmaceutics 2021, 13, 1474. [Google Scholar] [CrossRef]
- Di Marco, A.; Vignone, D.; Gonzalez Paz, O.; Fini, I.; Battista, M.R.; Cellucci, A.; Bracacel, E.; Auciello, G.; Veneziano, M.; Khetarpal, V.; et al. Establishment of an in Vitro Human Blood-Brain Barrier Model Derived from Induced Pluripotent Stem Cells and Comparison to a Porcine. Cells 2020, 9, 994. [Google Scholar] [CrossRef]
- Jamieson, J.J.; Linville, R.M.; Ding, Y.Y.; Gerecht, S.; Searson, P.C. Role of IPSC-Derived Pericytes on Barrier Function of IPSC-Derived Brain Microvascular Endothelial Cells in 2D and 3D. Fluids Barriers CNS 2019, 16, 15. [Google Scholar] [CrossRef]
- Neal, E.H.; Marinelli, N.A.; Shi, Y.; Mcclatchey, P.M.; Balotin, K.M.; Gullett, D.R.; Hagerla, K.A.; Bowman, A.B.; Ess, K.C.; Wikswo, J.P.; et al. Stem Cell Reports. Stem Cell Rep. 2019, 12, 1380–1388. [Google Scholar] [CrossRef]
- Xie, J.; Wettschurack, K.; Yuan, C. Review: In Vitro Cell Platform for Understanding Developmental Toxicity. Front. Genet. 2020, 11, 623117. [Google Scholar] [CrossRef]
- Deepika, D.; Sharma, R.P.; Schuhmacher, M.; Kumar, V. An Integrative Translational Framework for Chemical Induced Neurotoxicity–a Systematic Review. Crit. Rev. Toxicol. 2020, 50, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.Z.; Lehmann, M.; Gutbier, S.; Nembo, E.; Noel, S.; Smirnova, L.; Forsby, A.; Hescheler, J.; Avci, H.X.; Hartung, T.; et al. In Vitro Acute and Developmental Neurotoxicity Screening: An Overview of Cellular Platforms and High-Throughput Technical Possibilities. Arch. Toxicol. 2017, 91, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Lacher, S.E.; Skagen, K.; Veit, J.; Dalton, R.; Woodahl, E.L. P-Glycoprotein Transport of Neurotoxic Pesticides. J. Pharmacol. Exp. Ther. 2015, 355, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bal-Price, A.K.; Hogberg, H.T.; Buzanska, L.; Coecke, S. Relevance of in Vitro Neurotoxicity Testing for Regulatory Requirements: Challenges to Be Considered. Neurotoxicol Teratol. 2010, 32, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.S.; Cen, J.; Liu, L.; He, L. In Vitro and in Vivo Study of Dolichyl Phosphate on the Efflux Activity of P-Glycoprotein at the Blood-Brain Barrier. Int. J. Dev. Neurosci. 2013, 31, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Brás, J.P.; Bravo, J.; Freitas, J.; Barbosa, M.A.; Santos, S.G.; Summavielle, T.; Almeida, M.I. TNF-Alpha-Induced Microglia Activation Requires MiR-342: Impact on NF-KB Signaling and Neurotoxicity. Cell Death Dis. 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.L.; Kittel, Á.; Veszelka, S.; Palmela, I.; Tóth, A.; Brites, D.; Deli, M.A.; Brito, M.A. Exposure to Lipopolysaccharide and/or Unconjugated Bilirubin Impair the Integrity and Function of Brain Microvascular Endothelial Cells. PLoS ONE 2012, 7, e35919. [Google Scholar] [CrossRef]
- Chemmarappally, J.M.; Pegram, H.C.; Abeywickrama, N.; Fornari, E.; Hargreaves, A.J.; De Girolamo, L.A.; Stevens, B. OPEN A Co-Culture Nanofibre Scaffold Model of Neural Cell Degeneration in Relevance to Parkinson’ s Disease. Sci. Rep. 2020, 10, 2767. [Google Scholar] [CrossRef]
- De Simone, U.; Caloni, F.; Gribaldo, L.; Coccini, T. Human Co-Culture Model of Neurons and Astrocytes to Test Acute Cytotoxicity of Neurotoxic Compounds. Int. J. Toxicol. 2017, 36, 463–477. [Google Scholar] [CrossRef]
- Ariel, S.; Gisele, M.; Etcheverrito, A.; Gustavo, D.; Ruth, D. Journal of Trace Elements in Medicine and Biology Neurotoxicity Mediated by Oxidative Stress Caused by Titanium Dioxide Nanoparticles in Human Neuroblastoma (SH-SY5Y) Cells. J. Trace Elem. Med. Biol. 2020, 57, 126413. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, P.; Fan, T.; Yang, X.; Zheng, T.; Sun, C. The Effect of Glutamate-Induced Excitotoxicity on DNA Methylation in Astrocytes in a New in Vitro Neuron-Astrocyte-Endothelium Co-Culture System. Biochem. Biophys. Res. Commun. 2019, 508, 1209–1214. [Google Scholar] [CrossRef]
- Terrasso, A.P.; Silva, A.C.; Filipe, A.; Pedroso, P.; Ferreira, A.L.; Alves, P.M.; Brito, C. Human Neuron-Astrocyte 3D Co-Culture-Based Assay for Evaluation of Neuroprotective Compounds. J. Pharmacol. Toxicol. Methods 2017, 83, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chu, S.; Yang, Y.; Zhang, Z.; Pang, Z.; Chen, N. Neuroinflammatory In Vitro Cell Culture Models and the Potential Applications for Neurological Disorders. Front. Pharmacol. 2021, 12, 671734. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.P.; Fenart, L. Modelling of the Blood-Brain Barrier in Drug Discovery and Development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef]
- Gorshkov, K.; Aguisanda, F.; Thorne, N.; Zheng, W. Astrocytes as Targets for Drug Discovery. Drug Discov. Today 2018, 23, 673–680. [Google Scholar] [CrossRef]
- Juźwik, C.A.; Drake, S.S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. MicroRNA Dysregulation in Neurodegenerative Diseases: A Systematic Review. Prog. Neurobiol. 2019, 182, 101664. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of Neuroinflammation in Neurodegenerative Diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Enright, H.A.; Lam, D.; Sebastian, A.; Sales, A.P.; Cadena, J.; Hum, N.R.; Osburn, J.J.; Peters, S.K.G.; Petkus, B.; Soscia, D.A.; et al. Functional and Transcriptional Characterization of Complex Neuronal Co-Cultures. Sci. Rep. 2020, 10, 11007. [Google Scholar] [CrossRef]
- Mursaleen, L.; Noble, B.; Somavarapu, S.; Zariwala, M.G. Micellar Nanocarriers of Hydroxytyrosol Are Protective against Parkinson’s Related Oxidative Stress in an in Vitro Hcmec/D3-sh-sy5y Co-culture System. Antioxidants 2021, 10, 887. [Google Scholar] [CrossRef]
- Mayeux, R. Epidemiology of Neurodegeneration. Annu. Rev. Neurosci. 2003, 26, 81–104. [Google Scholar] [CrossRef]
- Gunnarsson, L.G.; Bodin, L. Occupational Exposures and Neurodegenerative Diseases—A Systematic Literature Review and Meta-Analyses. Int. J. Environ. Res. Public Health 2019, 16, 337. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Zhang, X.; Gao, S.; Wang, P. Role of Monocarboxylate Transporter 4 in Alzheimer Disease. Neurotoxicology 2020, 76, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Penney, J.; Ralvenius, W.T.; Tsai, L.H. Modeling Alzheimer’s Disease with IPSC-Derived Brain Cells. Mol. Psychiatry 2020, 25, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s Disease: Insights and New Prospects in Disease Pathophysiology, Biomarkers and Disease-Modifying Drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, A.R.; Korolainen, M.A.; Odero, G.; Miller, D.W.; Kauppinen, T.M. Poly(ADP-Ribose) Polymerase-1 Regulates Microglia Mediated Decrease of Endothelial Tight Junction Integrity. Neurochem. Int. 2017, 108, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Yadav, K.; Mantri, S.S.; Singhal, N.K.; Ganesh, S.; Sandhir, R. Evidence for Compromised Insulin Signaling and Neuronal Vulnerability in Experimental Model of Sporadic Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 8916–8935. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Merlo, S.; Fagone, E.; Fruciano, M.; Barbagallo, C.; Kanda, T.; Sano, Y.; Purrello, M.; Vancheri, C.; Ragusa, M.; et al. Astrocytes Modify Migration of Pbmcs Induced by β-Amyloid in a Blood-Brain Barrier in Vitro Model. Front. Cell Neurosci. 2019, 13, 337. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, B.; Kanekiyo, T.; Singh, J. ApoE-2 Brain-Targeted Gene Therapy Through Transferrin and Penetratin Tagged Liposomal Nanoparticles. Pharm. Res. 2019, 36, 161. [Google Scholar] [CrossRef]
- Topal, G.R.; Mészáros, M.; Porkoláb, G.; Szecskó, A.; Polgár, T.F.; Siklós, L.; Deli, M.A.; Veszelka, S.; Bozkir, A. ApoE-Targeting Increases the Transfer of Solid Lipid Nanoparticles with Donepezil Cargo across a Culture Model of the Blood–Brain Barrier. Pharmaceutics 2021, 13, 38. [Google Scholar] [CrossRef]
- Alvariño, R.; Alonso, E.; Lacret, R.; Oves-Costales, D.; Genilloud, O.; Reyes, F.; Alfonso, A.; Botana, L.M. Streptocyclinones A and B Ameliorate Alzheimer’s Disease Pathological Processes in Vitro. Neuropharmacology 2018, 141, 283–295. [Google Scholar] [CrossRef]
- Wasielewska, J.M.; Chaves, J.C.S.; Johnston, R.L.; Milton, L.A.; Hernández, D.; Chen, L.; Song, J.; Lee, W.; Leinenga, G.; Nisbet, R.M.; et al. A Sporadic Alzheimer’s Blood-Brain Barrier Model for Developing Ultrasound-Mediated Delivery of Aducanumab and Anti-Tau Antibodies. Theranostics 2022, 12, 6826–6847. [Google Scholar] [CrossRef] [PubMed]
- Mantle, J.L.; Lee, K.H. A Differentiating Neural Stem Cell-Derived Astrocytic Population Mitigates the Inflammatory Effects of TNF-α and IL-6 in an IPSC-Based Blood-Brain Barrier Model. Neurobiol. Dis. 2018, 119, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Haenseler, W.; Sansom, S.N.; Buchrieser, J.; Newey, S.E.; Moore, C.S.; Nicholls, F.J.; Chintawar, S.; Schnell, C.; Antel, J.P.; Allen, N.D.; et al. A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-Culture-Specific Expression Profile and Inflammatory Response. Stem Cell Rep. 2017, 8, 1727–1742. [Google Scholar] [CrossRef]
- Morrice, J.R.; Gregory-Evans, C.Y.; Shaw, C.A. Necroptosis in Amyotrophic Lateral Sclerosis and Other Neurological Disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Smethurst, P.; Risse, E.; Tyzack, G.E.; Mitchell, J.S.; Taha, D.M.; Chen, Y.R.; Newcombe, J.; Collinge, J.; Sidle, K.; Patani, R. Distinct Responses of Neurons and Astrocytes to TDP-43 Proteinopathy in Amyotrophic Lateral Sclerosis. Brain 2020, 143, 430–440. [Google Scholar] [CrossRef]
- Paul, P.; De Belleroche, J. The Role of D-Serine and Glycine as Co-Agonists of NMDA Receptors in Motor Neuron Degeneration and Amyotrophic Lateral Sclerosis (ALS). Front. Synaptic Neurosci. 2014, 6, 10. [Google Scholar] [CrossRef]
- Veyrat-Durebex, C.; Corcia, P.; Piver, E.; Devos, D.; Dangoumau, A.; Gouel, F.; Vourc’h, P.; Emond, P.; Laumonnier, F.; Nadal-Desbarats, L.; et al. Disruption of TCA Cycle and Glutamate Metabolism Identified by Metabolomics in an In Vitro Model of Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2016, 53, 6910–6924. [Google Scholar] [CrossRef]
- Lee, M.L.; Martinez-Lozada, Z.; Krizman, E.N.; Robinson, M.B. Brain Endothelial Cells Induce Astrocytic Expression of the Glutamate Transporter GLT-1 by a Notch-Dependent Mechanism. J. Neurochem. 2017, 143, 489–506. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Markandaiah, S.S.; Bonanno, S.; Pasinelli, P.; Trotti, D. Excess Glutamate Secreted from Astrocytes Drives Upregulation of P-Glycoprotein in Endothelial Cells in Amyotrophic Lateral Sclerosis. Exp. Neurol. 2019, 316, 27–38. [Google Scholar] [CrossRef]
- Efremova, L.; Schildknecht, S.; Adam, M.; Pape, R.; Gutbier, S.; Hanf, B.; Bürkle, A.; Leist, M. Prevention of the Degeneration of Human Dopaminergic Neurons in an Astrocyte Co-Culture System Allowing Endogenous Drug Metabolism. Br. J. Pharmacol. 2015, 172, 4119–4132. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Asanuma, M.; Kikkawa, Y.; Takeshima, M.; Murakami, S.; Miyoshi, K.; Sogawa, N.; Kita, T. Astrocyte-Derived Metallothionein Protects Dopaminergic Neurons from Dopamine Quinone Toxicity. Glia 2011, 59, 435–451. [Google Scholar] [CrossRef]
- Sergi, D.; Alex, G.; Beaulieu, J.; Renaud, J.; Tardif-pellerin, E.; Martinoli, M. ε-Viniferin in a Neuron-Glia Co-Culture Cellular Model of Parkinson’ s Disease. Foods 2021, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Park, H.Y.; Dasilva, N.A.; Ma, H.; Seeram, N.P. Levodopa-Reduced Mucuna Pruriens Seed Extract Shows Neuroprotective Effects against Parkinson’ s Disease in Murine Microglia and Human. Nutrients 2018, 10, 1139. [Google Scholar] [CrossRef]
- Kuan, W.L.; Bennett, N.; He, X.; Skepper, J.N.; Martynyuk, N.; Wijeyekoon, R.; Moghe, P.V.; Williams-Gray, C.H.; Barker, R.A. α-Synuclein Pre-Formed Fibrils Impair Tight Junction Protein Expression without Affecting Cerebral Endothelial Cell Function. Exp. Neurol. 2016, 285, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Dohgu, S.; Takata, F.; Matsumoto, J.; Kimura, I.; Yamauchi, A.; Kataoka, Y. Monomeric α-Synuclein Induces Blood–Brain Barrier Dysfunction through Activated Brain Pericytes Releasing Inflammatory Mediators in Vitro. Microvasc. Res. 2019, 124, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Dohgu, S.; Takata, F.; Nishioku, T.; Sumi, N.; Machida, T.; Takahashi, H.; Yamauchi, A.; Kataoka, Y. Lipopolysaccharide-Activated Microglia Lower P-Glycoprotein Function in Brain Microvascular Endothelial Cells. Neurosci. Lett. 2012, 524, 45–48. [Google Scholar] [CrossRef]
- Liu, Q.; Hou, J.; Chen, X.; Liu, G.; Zhang, D.; Sun, H.; Zhang, J. P-Glycoprotein Mediated Efflux Limits the Transport of the Novel Anti-Parkinson’s Disease Candidate Drug FLZ across the Physiological and PD Pathological in Vitro BBB Models. PLoS ONE 2014, 9, e102442. [Google Scholar] [CrossRef]
- Krach, F.; Bogiongko, M.-E.; Winner, B. Decoding Parkinson’s Disease—IPSC-Derived Models in the OMICs Era. Mol. Cell. Neurosci. 2020, 106, 103501. [Google Scholar] [CrossRef]
- Simmnacher, K.; Lanfer, J.; Rizo, T.; Kaindl, J.; Winner, B. Modeling Cell-Cell Interactions in Parkinson’s Disease Using Human Stem Cell-Based Models. Front. Cell Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef]
- Rostami, J.; Mothes, T.; Kolahdouzan, M.; Eriksson, O.; Moslem, M.; Bergström, J.; Ingelsson, M.; O’Callaghan, P.; Healy, L.M.; Falk, A.; et al. Crosstalk between Astrocytes and Microglia Results in Increased Degradation of α-Synuclein and Amyloid-β Aggregates. J. Neuroinflamm. 2021, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Katt, M.E.; Mayo, L.N.; Ellis, S.E.; Mahairaki, V.; Rothstein, J.D.; Cheng, L.; Searson, P.C. The Role of Mutations Associated with Familial Neurodegenerative Disorders on Blood–Brain Barrier Function in an IPSC Model. Fluids Barriers CNS 2019, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Leng, K.; Park, J.; Sorets, A.G.; Kim, S.; Shostak, A.; Embalabala, R.J.; Mlouk, K.; Katdare, K.A.; Rose, I.V.L.; et al. Reactive Astrocytes Transduce Inflammation in a Blood-Brain Barrier Model through a TNF-STAT3 Signaling Axis and Secretion of Alpha 1-Antichymotrypsin. Nat. Commun. 2022, 13, 6581. [Google Scholar] [CrossRef] [PubMed]
- Guttenplan, K.A.; Weigel, M.K.; Adler, D.I.; Couthouis, J.; Liddelow, S.A.; Gitler, A.D.; Barres, B.A. Knockout of Reactive Astrocyte Activating Factors Slows Disease Progression in an ALS Mouse Model. Nat. Commun. 2020, 11, 3753. [Google Scholar] [CrossRef]
- Yun, S.P.; Kam, T.-I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.-S.; Kwon, S.-H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 Astrocyte Conversion by Microglia Is Neuroprotective in Models of Parkinson’s Disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef]
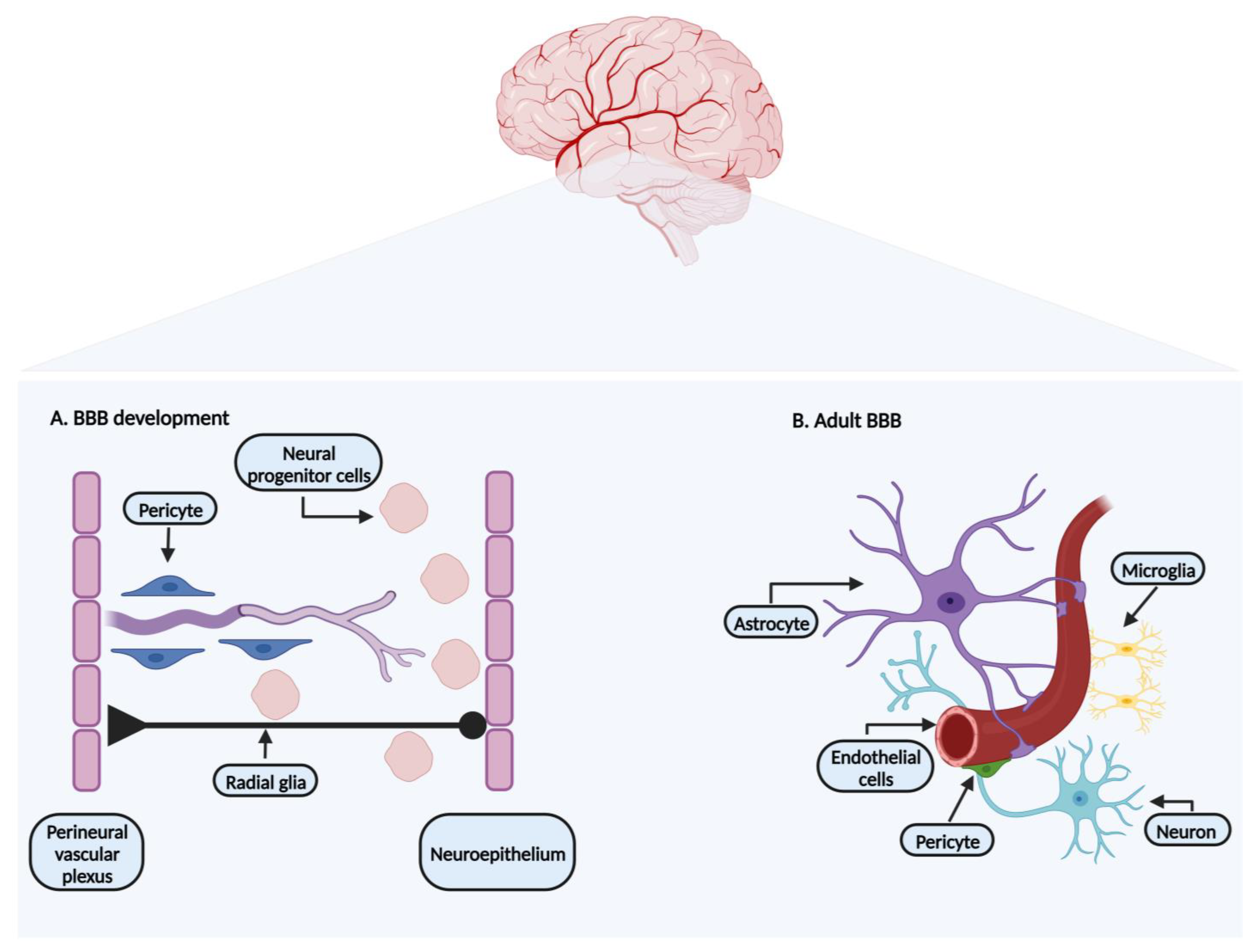

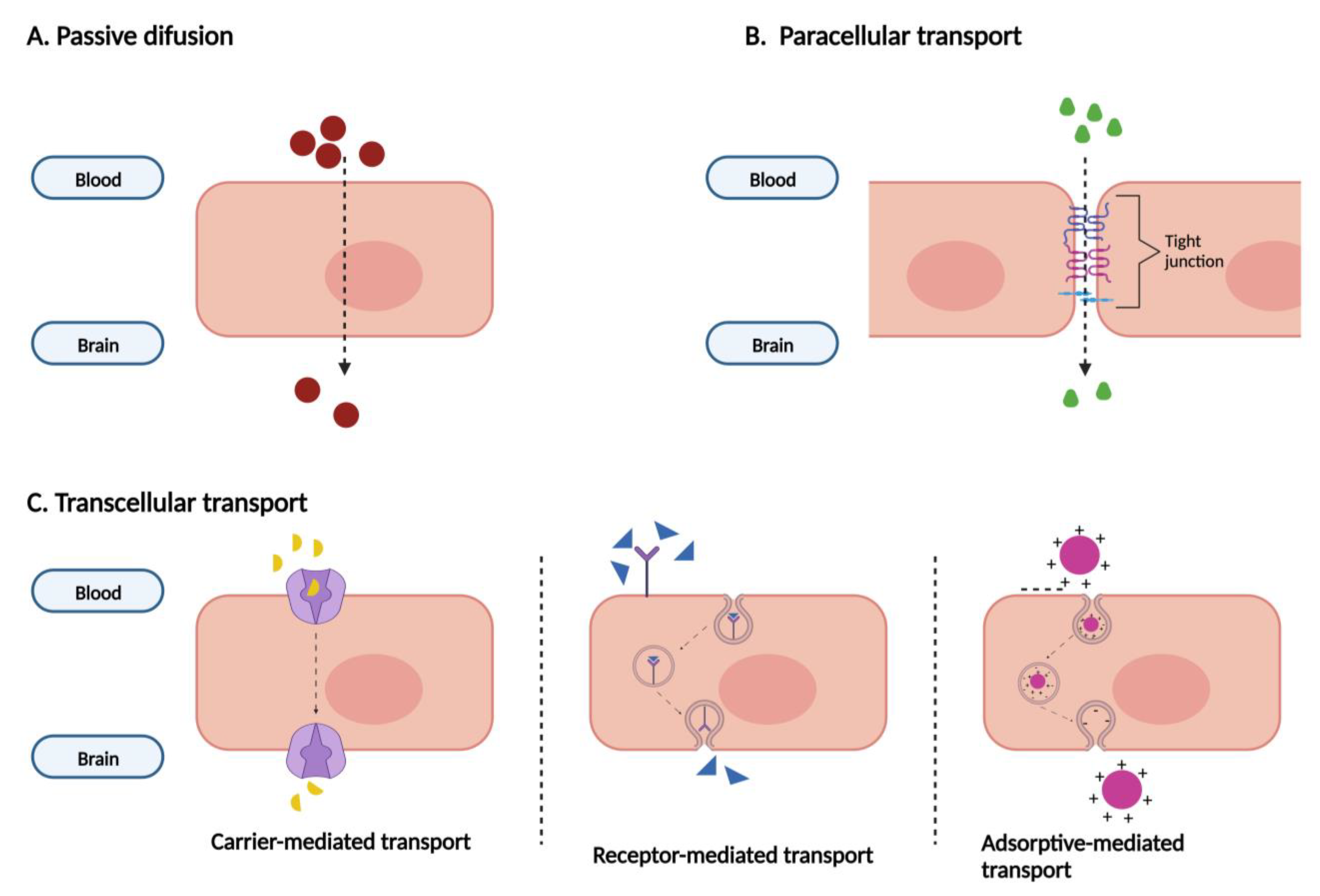
| Inter-Endothelial Junctions | Principal Proteins | Characteristics/Main Functions | References |
|---|---|---|---|
| Tight junctions | Claudins |
| [3,9,11,15] |
| Occludin |
| [15,16] | |
| JAM |
| [3,9,15] | |
| Adherens junctions | VE-cadherin |
| [3,9,15] |
| Catenins |
| [9,15] | |
| PECAMs |
| [3,15] |
| ABC Transporter | Main Functions/Characteristics | References |
|---|---|---|
| P-gp (ABCB1) |
| [21,22,26] |
| BCRP (ABCG2) |
| [12,21,22,26] |
| MRPs (ABCC) |
| [12,21,22,26] |
| ABC Transporter | Main Functions/Characteristics | References |
|---|---|---|
| GLUT-1 (SLC2A1) |
| [3,27] |
| SGLT1 (SLC5A1) |
| [27] |
| MCT1 (SLC16A1) |
| [3,27] |
| CRT (SLC6A8) |
| [27,28] |
| LAT-1 (SLC7A5) |
| [3,27] |
| ATB 0,+ (SLC6A14) |
| [27] |
| CAT-1 (SLC7A1) |
| [27] |
| EAAT (SLC1) |
| [3,27] |
| TAUT (SLC6A6) |
| [27] |
| CTL1 (SLC44A1) |
| [3,27] |
| OAT-3 (SLC22A8) |
| [27] |
| OATP (SLC21/SLCO) |
| [3,27] |
| OCT1 (SLC22A1) OCT2 (SLC22A2) |
| [27] |
| Markers | Essential Expression in a BBB Model | Validation | Observations/Limitations | Relevance | References |
|---|---|---|---|---|---|
| Tight junctions | Occludin Claudin-5 ZO-1 | Trans-endothelial electrical resistance (TEER) |
|
| [29,30] |
| Permeability |
| ||||
| ABC transporters | P-gp BCRP MRPs |
|
|
| [29,30] |
| SLC transporters | GLUT-1 LAT-1 MCT-1 |
|
|
| [29,30] |
| Receptor systems | Transferrin receptor |
|
|
| [29,30] |
| Model Type | Origin | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Primary cells | Animal |
|
| [13,31,32,33,34] |
| Human |
|
| ||
| Immortalized cell lines | Animal or Human |
|
| [13,31,32,33,34] |
| Category | Markers | References |
|---|---|---|
| Enzymes |
| [36,37] |
| Tight junctional proteins |
| [37,40,41] |
| ABC transporters |
| [37,40,41] |
| SLC transporters |
| [37,40,41] |
| Category | Markers | References |
|---|---|---|
| Enzymes |
| [43,44,45,46] |
| Tight junctional proteins |
| [43,44,45,46] |
| ABC transporters |
| [43,44,45,46] |
| SLC transporters |
| [43,44,45,46] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [47,48,49,50] |
| ABC transporters |
| [47,48,49,50] |
| SLC transporters |
| [47,48,49,50] |
| Category | Markers | References |
|---|---|---|
| Enzymes |
| [51,52,53] |
| Tight junctional proteins |
| [51,52,53] |
| ABC transporters |
| [51,52,53] |
| SLC transporters |
| [51,52,53] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [35,54] |
| ABC transporters |
| [35,54] |
| SLC transporters |
| [35,54] |
| Category | Markers | References |
|---|---|---|
| Enzymes |
| [34,54,55,56] |
| Tight junctional proteins |
| [34,54,55,56] |
| ABC transporters |
| [34,54,55,56] |
| SLC transporters |
| [34,54,55,56] |
| Receptor-mediated transcytosis |
| [56,57] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [57,58] |
| ABC transporters |
| [57,58] |
| SLC transporters |
| [57,58] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [37,60] |
| ABC transporters |
| [37,60] |
| SLC transporters |
| [37,60] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [61,62,63,64] |
| SLC transporters |
| [61,62,63,64] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [62,65] |
| ABC transporters |
| [29] |
| SLC transporters |
| [62,65] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [66,67,69] |
| ABC transporters |
| [66,67,69] |
| SLC transporters |
| [66,67,69] |
| Receptor-mediated transcytosis |
| [66,67,69] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [72,73] |
| ABC transporters |
| [72,73] |
| SLC transporters |
| [72,73] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [73] |
| ABC transporters |
| [73] |
| SLC transporters |
| [73] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [35,77,78] |
| ABC transporters |
| [35,77,78] |
| SLC transporters |
| [35,77,78] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [80,81,82] |
| ABC transporters |
| [80,81,82] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [66,83,84] |
| ABC transporters |
| [66,83,84] |
| SLC transporters |
| [66,83,84] |
| Receptor-mediated transcytosis |
| [66,83,84] |
| Category | Markers | References |
|---|---|---|
| Tight junctional proteins |
| [85,86] |
| ABC transporters |
| [85,86] |
| SLC transporters |
| [85,86] |
| Receptor-mediated transcytosis |
| [85,86] |
| Phenotype | Markers | References |
|---|---|---|
| Dopaminergic |
| [88,89,90] |
| Cholinergic |
| [88,89] |
| Adrenergic |
| [88,89] |
| Category | Markers | References |
|---|---|---|
| Enzymes |
| [55] |
| Tight junctional proteins |
| [55] |
| ABC transporters |
| [55] |
| SLC transporters |
| [55] |
| Category | Markers | References |
|---|---|---|
| Enzymes |
| [55,91] |
| Tight junctional proteins |
| [55,91] |
| ABC transporters |
| [55,91] |
| SLC transporters |
| [55,91] |
| Type of the Neurovascular Unit Cells Used in Co-Culture | Advantage(s) | References |
|---|---|---|
| Astrocytes |
| [29,32,88,100,101,102] |
| Pericytes |
| [29,32,100] |
| Neurons |
| [32,51,100] |
| Microglia |
| [51,103,104] |
| Types of Dynamic Models | Advantages | Disadvantages | References |
|---|---|---|---|
| Dynamic in vitro (DIV) system |
|
| [94,104] |
| Hanging-drop method |
|
| [106] |
| Forced-floating method |
|
| [106] |
| Matrices and scaffolds |
|
| [106] |
| Agitation-based approaches |
|
| [106] |
| Microfluidic technology “organ-on-a-chip” |
|
| [106,107,108,109] |
| Source of Endothelial Cells | Co-Cultivated with | Advantages/Observations | References |
|---|---|---|---|
| Bovine |
|
| [32,39,41,110,111] |
| Rat |
|
| [32,55] |
| Porcine |
|
| [32,42,43,44,111,112,113,114] |
|
| ||
|
| ||
| Human |
|
| [31,51,115,116,117] |
| Cell Line | Co-Cultivated with | Advantages/Observations | References |
|---|---|---|---|
| RBE4 |
|
| [32,56] |
|
| ||
| HCMEC/D3 |
|
| [35,42,46,68,70,118,119,120,121] |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
| TY10 |
|
| [35,122] |
|
| ||
| BB19 |
|
| [35,122] |
|
| ||
| b.END3 |
|
| [32,93,123] |
| CerebEND |
|
| [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Co-Culture Models: Key Players in In Vitro Neurotoxicity, Neurodegeneration and BBB Modeling Studies. Biomedicines 2024, 12, 626. https://doi.org/10.3390/biomedicines12030626
Monteiro AR, Barbosa DJ, Remião F, Silva R. Co-Culture Models: Key Players in In Vitro Neurotoxicity, Neurodegeneration and BBB Modeling Studies. Biomedicines. 2024; 12(3):626. https://doi.org/10.3390/biomedicines12030626
Chicago/Turabian StyleMonteiro, Ana Rita, Daniel José Barbosa, Fernando Remião, and Renata Silva. 2024. "Co-Culture Models: Key Players in In Vitro Neurotoxicity, Neurodegeneration and BBB Modeling Studies" Biomedicines 12, no. 3: 626. https://doi.org/10.3390/biomedicines12030626
APA StyleMonteiro, A. R., Barbosa, D. J., Remião, F., & Silva, R. (2024). Co-Culture Models: Key Players in In Vitro Neurotoxicity, Neurodegeneration and BBB Modeling Studies. Biomedicines, 12(3), 626. https://doi.org/10.3390/biomedicines12030626








