Gender-Specific Effects on the Cardiorespiratory System and Neurotoxicity of Intermittent and Permanent Low-Level Lead Exposures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model of Long-Term Lead Exposure
2.2. Behavioral Evaluation
2.2.1. Elevated plus Maze Test
2.2.2. Open-Field Exploration Test
2.2.3. Novel Object Recognition Test
2.3. Metabolic Evaluation
2.4. Functional Evaluation
2.4.1. Physiological and Autonomic Evaluation
2.4.2. Data Acquisition and Analysis
2.4.3. Baro- and Chemoreceptor Reflex Analysis
2.5. Immunohistochemistry (IHC)
2.6. Statistical Analysis
3. Results
3.1. Male Lead Exposure Groups Have an Increase in Their Weight
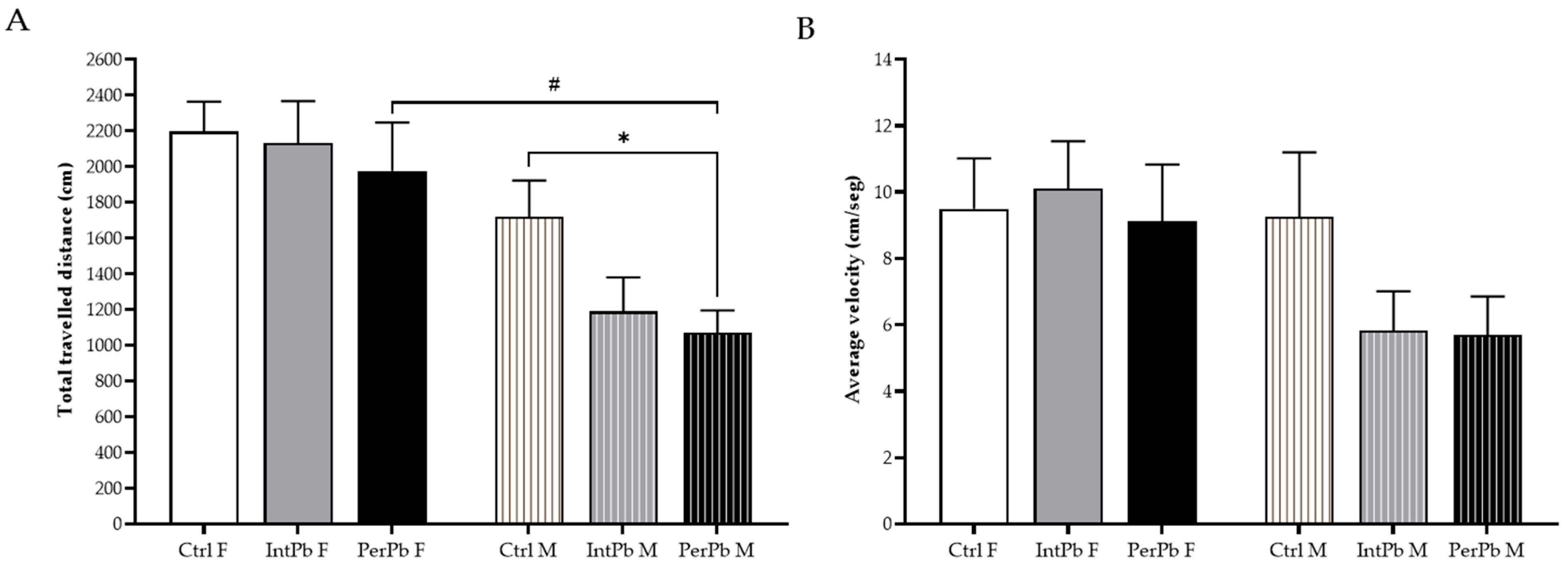
3.2. Male Permanent Exposure Group Shows a Significant Decrease in the Travelled Distance
3.3. Permanent Lead Exposure, Independent of Gender Causes Anxiety-like Behavior
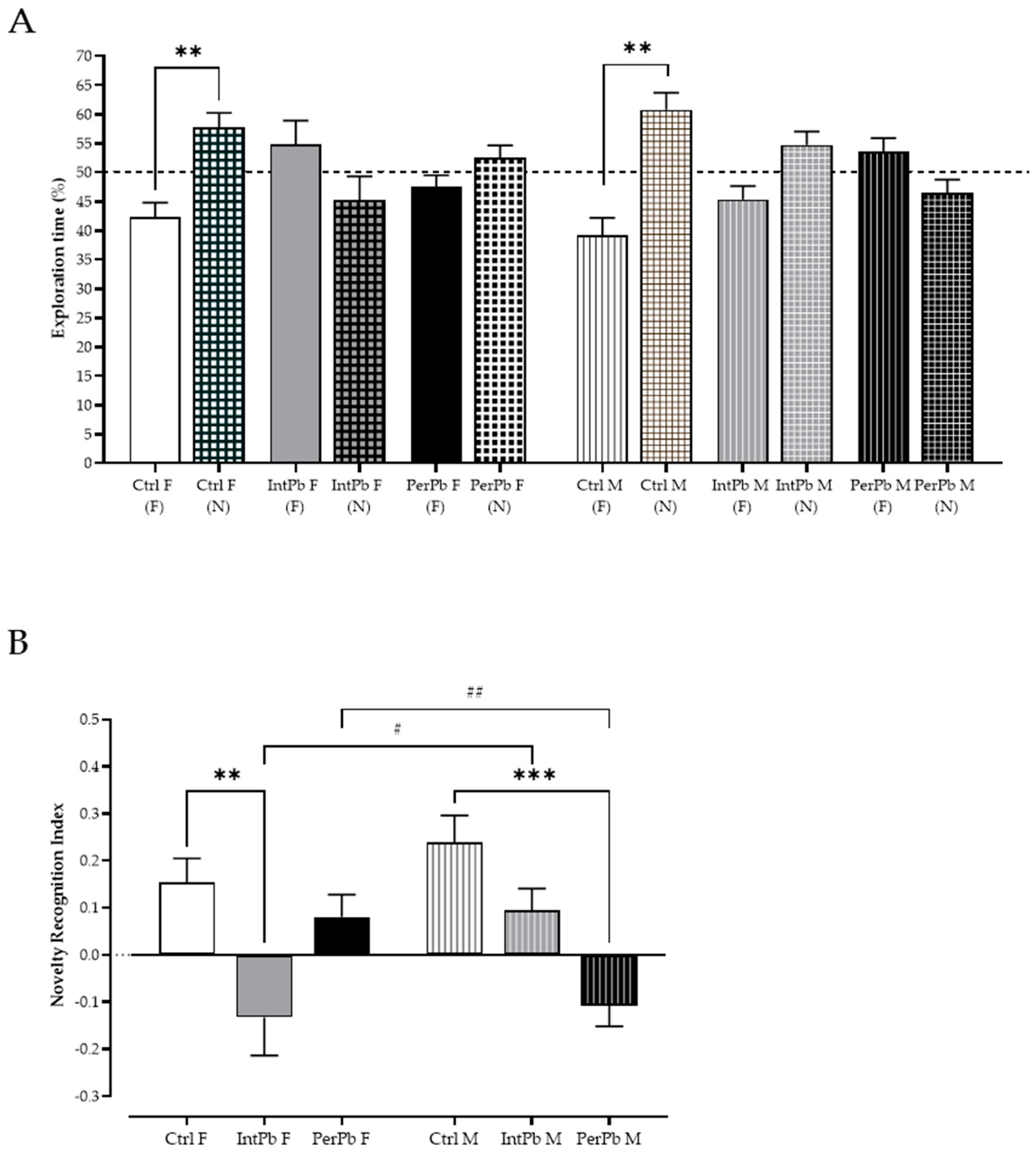
3.4. Female Intermittent Lead Exposure and Male Permanent Lead Exposure Groups Show a Strong Decline in Episodic Long-Term Memory
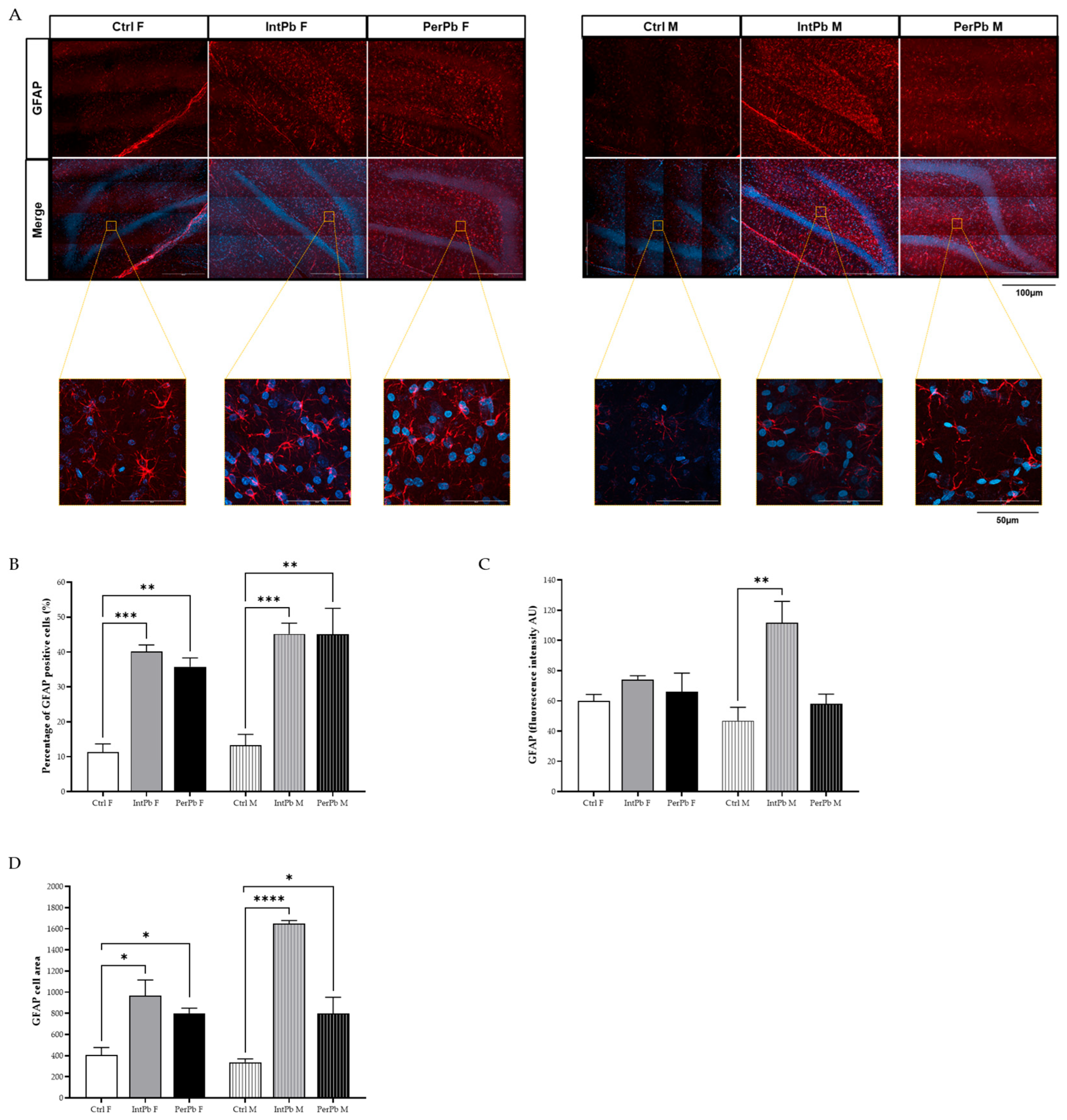
3.5. Lead Exposure, Independent of Gender, Causes Astrocytic Activation with Stronger Effects in the Male Groups
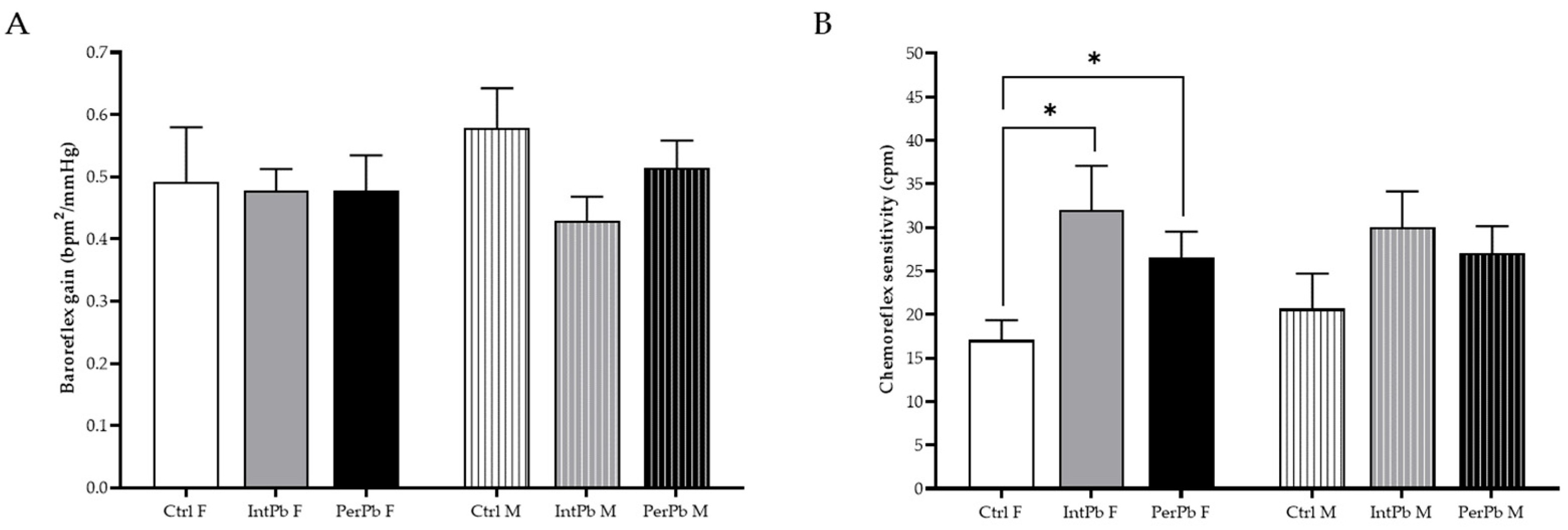
3.6. Permanent Lead Exposure, Regardless of the Gender Shows a Stronger Effect on Blood Pressure and Respiratory Frequency
3.7. Lead Exposure in Female Animals, Regardless of the Type, Increases the Chemoreflex Sensitivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Exposure to Lead: A Major Public Health Concern. 2010, Volume 6. Available online: https://iris.who.int/bitstream/handle/10665/346775/9789240037656-eng.pdf?sequence=1 (accessed on 15 August 2023).
- World Health Organization. Lead Poisoning and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 7 August 2023).
- World Health Organization. International Lead Poisoning Prevention Week of Action. Available online: https://www.who.int/ipcs/lead_campaign/en/ (accessed on 15 August 2023).
- Tempowski, J.; World Health Organization. WHO Guideline for Clinical Management of Exposure to Lead. 2021. Available online: https://www.who.int/publications/i/item/9789240037045 (accessed on 15 August 2023).
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Sani, A.H.; Amanabo, M. Lead: A Concise Review of Its Toxicity, Mechanism and Health Effect. GSC Biol. Pharm. Sci. 2021, 15, 55–062. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of Lead: A Review with Recent Updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead Toxicity: A Review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Todd, A.C.; Wetmur, J.G.; Moline, J.M.; Godbold, J.H.; Levin, S.M.; Landrigan, P.J. Unraveling the Chronic Toxicity of Lead: An Essential Priority for Environmental Health. Environ. Health Perspect. 1996, 104, 141–146. [Google Scholar] [CrossRef]
- Gunnarsson, L.G.; Bodin, L. Occupational Exposures and Neurodegenerative Diseases—A Systematic Literature Review and Meta-Analyses. Int. J. Environ. Res. Public Health 2019, 16, 337. [Google Scholar] [CrossRef]
- Gidlow, D.A. Lead Toxicity. Occup. Med. 2015, 65, 348–356. [Google Scholar] [CrossRef]
- Needleman, H. Lead Poisoning. Annu. Rev. Med. 2004, 55, 209–222. [Google Scholar] [CrossRef]
- Mayans, L. Lead Poisoning in Children. Am. Fam. Physician 2019, 100, 24–30. [Google Scholar]
- World Health Organization. Childhood Lead Poisoning Prevention. JAMA J. Am. Med. 2010, 89, 1129–1130. [Google Scholar]
- Rastogi, S. Renal Effects of Environmental and Occupational Lead Exposure. Indian J. Occup. Environ. Med. 2008, 12, 103. [Google Scholar] [CrossRef]
- Loghman-Adham, M. Renal Effects of Environmental and Occupational Lead Exposure. Environ. Health Perspect. 2008, 105, 103–106. [Google Scholar] [CrossRef]
- Roncal, C.; Mu, W.; Reungjui, S.; Kim, K.M.; Henderson, G.N.; Ouyang, X.; Nakagawa, T.; Johnson, R.J. Lead, at Low Levels, Accelerates Arteriolopathy and Tubulointerstitial Injury in Chronic Kidney Disease. Am. J. Physiol.-Ren. Physiol. 2007, 293, F1391–F1396. [Google Scholar] [CrossRef]
- Cullen, M.R.; Kayne, R.D.; Robins, J.M. Endocrine and Reproductive Dysfunction in Men Associated with Occupational Inorganic Lead Intoxication. Arch. Environ. Health Int. J. 1984, 39, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Sharma, S.; Purohit, P.; Sharma, P. Clinical and Molecular Aspects of Lead Toxicity: An Update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 506–528. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.R.; Preti, S.C.; Azevedo, B.F.; Fiorim, J.; Freire, D.D.; Covre, E.P.; Vassallo, D.V.; dos Santos, L. Low-Level Chronic Lead Exposure Impairs Neural Control of Blood Pressure and Heart Rate in Rats. Cardiovasc. Toxicol. 2017, 17, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Shvachiy, L.; Amaro-Leal, Â.; Outeiro, T.F.; Rocha, I.; Geraldes, V. Intermittent Lead Exposure Induces Behavioral and Cardiovascular Alterations Associated with Neuroinflammation. Cells 2023, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Lamas, G.A.; Thurston, G.; Solenkova, N.V.; Newman, J.D.; Berger, J.S.; Hochman, J.S. Metal Pollutants and Cardiovascular Disease: Mechanisms and Consequences of Exposure. Am. Heart J. 2014, 168, 812–822. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead Exposure and Cardiovascular Disease—A Systematic Review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. Mechanisms of Lead-Induced Hypertension and Cardiovascular Disease. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H454–H465. [Google Scholar] [CrossRef]
- Mason, L.H.; Harp, J.P.; Han, D.Y.; Mason, L.H.; Harp, J.P.; Han, D.Y. Pb Neurotoxicity: Neuropsychological Effects of Lead Toxicity. BioMed Res. Int. 2014, 2014, 840547. [Google Scholar] [CrossRef] [PubMed]
- Toscano, C.D.; Guilarte, T.R. Lead Neurotoxicity: From Exposure to Molecular Effects. Brain Res. Rev. 2005, 49, 529–554. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, Y.; Markowitz, M.E.; Rosen, J.F. Low-Level Lead-Induced Neurotoxicity in Children: An Update on Central Nervous System Effects. Brain Res. Rev. 1998, 27, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, M.B.; Aschner, M. Molecular Mechanisms of Lead Neurotoxicity. In Advances in Neurotoxicology; Elsevier Inc.: Amsterdam, The Netherlands, 2021; Volume 5, pp. 159–213. ISBN 9780128237755. [Google Scholar]
- Liu, K.S.; Hao, J.H.; Zeng, Y.; Dai, F.C.; Gu, P.Q. Neurotoxicity and Biomarkers of Lead Exposure: A Review. Chin. Med. Sci. J. 2013, 28, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.R.; Esquivel, D.F.G.; Ayala, T.B.; Pineda, B.; Manzo, S.G.; Quino, J.M.; Mora, P.C.; de la Cruz, V.P. Cognitive Impairment Induced by Lead Exposure during Lifespan: Mechanisms of Lead Neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef]
- Rocha, A.; Trujillo, K.A. Neurotoxicity of Low-Level Lead Exposure: History, Mechanisms of Action, and Behavioral Effects in Humans and Preclinical Models. Neurotoxicology 2019, 73, 58–80. [Google Scholar] [CrossRef]
- Shvachiy, L.; Geraldes, V.; Amaro-Leal, Â.; Rocha, I. Persistent Effects on Cardiorespiratory and Nervous Systems Induced by Long-Term Lead Exposure: Results from a Longitudinal Study. Neurotox. Res. 2020, 37, 857–870. [Google Scholar] [CrossRef]
- Du, Y.; Ge, M.-M.; Xue, W.; Yang, Q.-Q.; Wang, S.; Xu, Y. Chronic Lead Exposure and Mixed Factors of Gender×Age×Brain Regions Interactions on Dendrite Growth, Spine Maturity and NDR Kinase. PLoS ONE 2015, 10, 138112. [Google Scholar] [CrossRef]
- Vahter, M.; Åkesson, A.; Lidén, C.; Ceccatelli, S.; Berglund, M. Gender Differences in the Disposition and Toxicity of Metals. Environ. Res. 2007, 104, 85–95. [Google Scholar] [CrossRef]
- Dart, A.M.; Du, X.-J.; Kingwell, B.A. Gender, Sex Hormones and Autonomic Nervous Control of the Cardiovascular System. Cardiovasc. Res. 2002, 53, 678–687. [Google Scholar] [CrossRef]
- Stone, S.V.; Dembroski, T.M.; Costa, P.T.; Macdougalp, J.M. Gender Differences in Cardiovascular Reactivity. J. Behav. Med. 1990, 13, 137–156. [Google Scholar] [CrossRef]
- Gaignard, P.; Savouroux, S.; Liere, P.; Pianos, A.; Thérond, P.; Schumacher, M.; Slama, A.; Guennoun, R. Effect of Sex Differences on Brain Mitochondrial Function and Its Suppression by Ovariectomy and in Aged Mice. Endocrinology 2015, 156, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, V.; Sobolewski, M.; Cory-Slechta, D.A.; Schneider, J.S. Sex-Dependent Effects of Developmental Lead Exposure on the Brain. Front. Genet. 2018, 9, 351268. [Google Scholar] [CrossRef] [PubMed]
- Kasten-Jolly, J.; Lawrence, D.A. Sex-Specific Effects of Developmental Lead Exposure on the Immune-Neuroendocrine Network. Toxicol. Appl. Pharmacol. 2017, 334, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Polanska, K.; Hanke, W.; Pawlas, N.; Wesolowska, E.; Jankowska, A.; Jagodic, M.; Mazej, D.; Dominowska, J.; Grzesiak, M.; Mirabella, F.; et al. Sex-Dependent Impact of Low-Level Lead Exposure during Prenatal Period on Child Psychomotor Functions. Int. J. Environ. Res. Public Health 2018, 15, 2263. [Google Scholar] [CrossRef] [PubMed]
- Faulk, C.; Barks, A.; Sánchez, B.N.; Zhang, Z.; Anderson, O.S.; Peterson, K.E.; Dolinoy, D.C. Perinatal Lead (Pb) Exposure Results in Sex-Specific Effects on Food Intake, Fat, Weight, and Insulin Response across the Murine Life-Course. PLoS ONE 2014, 9, e104273. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rojas, C.; Jones, B.C. Sex Differences in Neurotoxicogenetics. Front. Genet. 2018, 9, 351720. [Google Scholar] [CrossRef] [PubMed]
- Llop, S.; Lopez-Espinosa, M.J.; Rebagliato, M.; Ballester, F. Gender Differences in the Neurotoxicity of Metals in Children. Toxicology 2013, 311, 3–12. [Google Scholar] [CrossRef]
- Schnaas, L.; Rothenberg, S.J.; Flores, M.-F.; Martinez, S.; Hernandez, C.; Osorio, E.; Velasco, S.R.; Perroni, E. Reduced Intellectual Development in Children with Prenatal Lead Exposure. Environ. Health Perspect. 2006, 114, 791. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Z.-Y.; Yan, J.; Ying, X.-L.; Tong, S.-L.; Yan, C.-H. Sex Differences in the Effects of Prenatal Lead Exposure on Birth Outcomes. Environ. Pollut. 2017, 225, 193–200. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Perera, F.P.; Jankowski, J.; Mrozek-Budzyn, D.; Mroz, E.; Flak, E.; Edwards, S.; Skarupa, A.; Lisowska-Miszczyk, I. Very Low Prenatal Exposure to Lead and Mental Development of Children in Infancy and Early Childhood. Neuroepidemiology 2009, 32, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Koss, W.A.; Frick, K.M. Activation of Androgen Receptors Protects Intact Male Mice from Memory Impairments Caused by Aromatase Inhibition. Horm. Behav. 2019, 111, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Shvachiy, L.; Geraldes, V.; Amaro-Leal, Â.; Rocha, I. Intermittent Low-Level Lead Exposure Provokes Anxiety, Hypertension, Autonomic Dysfunction and Neuroinflammation. Neurotoxicology 2018, 69, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C. Very Low Lead Exposures and Children’s Neurodevelopment. Curr. Opin. Pediatr. 2008, 20, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Njati, S.Y.; Maguta, M.M. Lead-Based Paints and Children’s PVC Toys Are Potential Sources of Domestic Lead Poisoning—A Review. Environ. Pollut. 2019, 249, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, V.; Carvalho, M.; Goncalves-Rosa, N.; Tavares, C.; Laranjo, S.; Rocha, I. Lead Toxicity Promotes Autonomic Dysfunction with Increased Chemoreceptor Sensitivity. Neurotoxicology 2016, 54, 170–177. [Google Scholar] [CrossRef]
- Guimarães, D.; Carvalho, M.L.; Geraldes, V.; Rocha, I.; Alves, L.C.; Santos, J.P. Lead in Liver and Kidney of Exposed Rats: Aging Accumulation Study. J. Trace Elem. Med. Biol. 2012, 26, 285–290. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice; Neuromethods; Gould, T., Ed.; Humana Press: Totowa, NJ, USA, 2009; Volume 42. [Google Scholar]
- Naolapo, O.J.O.; Naolapo, A.Y.O.; Josiah, T.; Osaku, M.; Akanji, O.O.; Abiodun, O.R. Elevated Plus Maze and Y-Maze Behavioral Effects of Subchronic, Oral Low Dose Monosodium Glutamate in Swiss Albino Mice. J. Pharm. Biol. Sci. 2012, 3, 21–27. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The Novel Object Recognition Memory: Neurobiology, Test Procedure, and Its Modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Costa, R.; Tamascia, M.L.; Nogueira, M.D.; Casarini, D.E.; Marcondes, F.K. Handling of Adolescent Rats Improves Learning and Memory and Decreases Anxiety. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 548–553. [Google Scholar]
- Schmitt, U.; Hiemke, C. Strain Differences in Open-Field and Elevated plus-Maze Behavior of Rats without and with Pretest Handling. Pharmacol. Biochem. Behav. 1998, 59, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Ho, Y.-J.; Spanagel, R.; Pawlak, C.R. A Novel Elevated Plus-Maze Procedure to Avoid the One-Trial Tolerance Problem. Front. Behav. Neurosci. 2011, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Buccafusco, J.J. Methods of Behavior Analysis in Neurosciences; CRC Tress LLC: Boca Raton, FL, USA, 2001; Volume 3, ISBN 084930704X. [Google Scholar]
- Ramos, A. Animal Models of Anxiety: Do I Need Multiple Tests? Trends Pharmacol. Sci. 2008, 29, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.G.; Vassilieff, I.; Vassilieff, V.S. Developmental Lead Exposure: Behavioral Alterations in the Short and Long Term. Neurotoxicol. Teratol. 2001, 23, 489–495. [Google Scholar] [CrossRef]
- Shvachiy, L.; Amaro-Leal, Â.; Outeiro, T.F.; Rocha, I.; Geraldes, V. From Molecular to Functional Effects of Different Environmental Lead Exposure Paradigms. Biology 2022, 11, 1164. [Google Scholar] [CrossRef]
- Mouro, F.M.; Batalha, V.L.; Ferreira, D.G.; Coelho, J.E.; Baqi, Y.; Müller, C.E.; Lopes, L.V.; Ribeiro, J.A.; Sebastião, A.M. Chronic and Acute Adenosine A2A Receptor Blockade Prevents Long-Term Episodic Memory Disruption Caused by Acute Cannabinoid CB1 Receptor Activation. Neuropharmacology 2017, 117, 316–327. [Google Scholar] [CrossRef]
- Chegão, A.; Guarda, M.; Alexandre, B.M.; Shvachiy, L.; Temido-Ferreira, M.; Marques-Morgado, I.; Fernandes Gomes, B.; Matthiesen, R.; Lopes, L.V.; Florindo, P.R.; et al. Glycation Modulates Glutamatergic Signalling and Exacerbates Parkinson’s Disease-like Phenotypes. Npj Park. Dis. 2022, 8, 51. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 479091. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Hol, E.M.; Pekny, M. Glial Fibrillary Acidic Protein (GFAP) and the Astrocyte Intermediate Filament System in Diseases of the Central Nervous System. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tu, R.; Wang, Y.; Hu, Y.; Li, X.; Cheng, X.; Yin, Y.; Li, W.; Huang, H. Early-Life Exposure to Lead Induces Cognitive Impairment in Elder Mice Targeting SIRT1 Phosphorylation and Oxidative Alterations. Front. Physiol. 2017, 8, 264546. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Amateau, S.K.; Mong, J.A. Steroid Modulation of Astrocytes in the Neonatal Brain: Implications for Adult Reproductive Function. Biol. Reprod. 2002, 67, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.A.; Grant, L.D.; Sloan, C.S.; Gladen, B.C. Chronic Low-Level Lead Toxicity in the Rat. Toxicol. Appl. Pharmacol. 1980, 56, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.W.; Stegner, S.E. Lead-Produced Changes in the Relative Rate of Open Field Activity of Laboratory Rats. Pharmacol. Biochem. Behav. 1978, 8, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Repko, J. Behavioral Toxicology of Inorganic Lead Exposure. In Health Effects of Occupational Lead and Arsenic Exposure, a Symposium, Division of Surveillance, Hazard Evaluation and Field Studies; Contract No. 210-75-0026, HEW Publication No. (NIOSH) 76–134; NIOSH: Cincinnati, OH, USA, 1976; pp. 59–72. [Google Scholar]
- Driscoll, J.W.; Stegner, S.E. Behavioral Effects of Chronic Lead Ingestion on Laboratory Rats. Pharmacol. Biochem. Behav. 1976, 4, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, J. Rats and Mice. In Ferrets, Rabbits and Rodents; Elsevier: St. Louis, MO, EUA, 2020; pp. 234–367. [Google Scholar]
- Hidayat, R. Patricia Wulandari Anatomy and Physiology of Animal Model Rats in Biomedical Research. Biomed. J. Indones. 2021, 7, 265–269. [Google Scholar] [CrossRef]
- Nistiar, F.; Racz, O.; Lukacinova, A.; Hubkova, B.; Novakova, J.; Lovasova, E.; Sedlakova, E. Age Dependency on Some Physiological and Biochemical Parameters of Male Wistar Rats in Controlled Environment. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2012, 47, 1224–1233. [Google Scholar] [CrossRef]
- Khalil-Manesh, F.; Gonick, H.C.; Weiler, E.W.J.; Prins, B.; Weber, M.A.; Purdy, R.E. Lead-Induced Hypertension: Possible Role of Endothelial Factors. Am. J. Hypertens. 1993, 6, 723–729. [Google Scholar] [CrossRef]
- Gonick, H.C.; Ding, Y.; Bondy, S.C.; Ni, Z.; Vaziri, N.D. Lead-Induced Hypertension. Hypertension 1997, 30, 1487–1492. [Google Scholar] [CrossRef]
- Wildemann, T.M.; Siciliano, S.D.; Weber, L.P. The Mechanisms Associated with the Development of Hypertension after Exposure to Lead, Mercury Species or Their Mixtures Differs with the Metal and the Mixture Ratio. Toxicology 2016, 339, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G. Sympathetic and Baroreflex Function in Hypertension: Implications for Current and New Drugs. Curr. Pharm. Des. 2004, 10, 3579–3589. [Google Scholar] [CrossRef] [PubMed]
- Albaghdadi, M. Baroreflex Control of Long-Term Arterial Pressure. Rev. Bras. Hipertens. 2007, 14, 212–225. [Google Scholar]
- Swenne, C.A. Baroreflex Sensitivity: Mechanisms and Measurement. Neth. Heart J. 2013, 21, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Diz, D.I.; Garcia-Espinosa, M.A.; Gallagher, P.E.; Ganten, D.; Ferrario, C.M.; Averill, D.B. Angiotensin-(1-7) and Baroreflex Function in Nucleus Tractus Solitarii of (MRen2)27 Transgenic Rats. J. Cardiovasc. Pharmacol. 2008, 51, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.F.R.; Wang, S.; Polson, J.W.; Kasparov, S. Signalling across the Blood Brain Barrier by Angiotensin II: Novel Implications for Neurogenic Hypertension. J. Mol. Med. 2008, 86, 705. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Rosário, L.B.; De Oliveira, E.I.; Barros, M.A.; Silva-Carvallho, L. Enhancement of Carotid Chemoreceptor Reflex and Cardiac Chemosensitive Reflex in the Acute Phase of Myocardial Infarction of the Anesthetized Rabbit. Basic Res. Cardiol. 2003, 98, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, V.; Goncalves-Rosa, N.; Liu, B.; Paton, J.F.R.; Rocha, I. Essential Role of RVL Medullary Neuronal Activity in the Long Term Maintenance of Hypertension in Conscious SHR. Auton. Neurosci. 2014, 186, 22–31. [Google Scholar] [CrossRef]
- Geraldes, V.; Gonçalves-Rosa, N.; Liu, B.; Paton, J.F.R.; Rocha, I. Chronic Depression of Hypothalamic Paraventricular Neuronal Activity Produces Sustained Hypotension in Hypertensive Rats. Exp. Physiol. 2014, 99, 89–100. [Google Scholar] [CrossRef]
- Kara, T.; Narkiewicz, K.; Somers, V.K. Chemoreflexes—Physiology and Clinical Implications. Acta Physiol. Scand. 2003, 177, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.-J. Peripheral Chemoreceptors in Health and Disease. J. Appl. Physiol. 2004, 96, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Trzebski, A.; Tafil, M.; Zoltowski, M.; Przybylski, J. Central and Peripheral Chemosensitivity in Early Essential Hypertension in Man. In Central Neurone Environment and the Control Systems of Breathing and Circulation; Springer: Berlin/Heidelberg, Germany, 1983; pp. 204–213. [Google Scholar]
- Chen, X.; Zhang, X.; Zhang, X. Smog in our Brains: Gender Differences in the Impact of Exposure to Air Pollution on Cognitive Performance in China; IFPRI Discussion Paper 1619; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2017. [Google Scholar]
- Obisesan, T.O. Hypertension and Cognitive Function. Clin. Geriatr. Med. 2009, 25, 259. [Google Scholar] [CrossRef] [PubMed]


| Blood Lead Levels (μg/dL) | Weight (g) | Food Intake (g) | Water Intake (mL) | Produced Faeces (g) | Produced Urine (mL) | |
|---|---|---|---|---|---|---|
| Ctrl F | 0.425 ± 0.085 | 329 ± 8 | 24.9 ± 1.3 | 40.0 ± 2.4 | 28.30 ± 3.07 | 16.31 ± 1.77 |
| IntPb F | 17.85 ± 5.350 * | 302 ± 6 | 23.6 ± 1.7 | 45.9 ± 6.1 | 21.00 ± 4.25 | 16.36 ± 2.23 |
| PerPb F | 26.43 ± 3.798 *** | 324 ± 7 | 23.2 ± 1.9 | 37.3 ± 4.6 | 21.31 ± 3.62 | 16.00 ± 1.99 |
| Ctrl M | 0.48 ± 0.086 | 620 ± 15 | 36.3 ± 3.4 | 47.1 ± 2.6 | 29.14 ± 4.96 | 21.00 ± 2.38 |
| IntPb M | 18.77 ± 0.612 ** | 501 ± 7 **** | 31.5 ± 4.6 | 37.5 ± 5.2 | 24.33 ± 3.44 | 12.83 ± 0.58 ** |
| PerPb M | 21.77 ± 5.871 *** | 555 ± 17 ** | 29.7 ± 1.3 | 34.3 ± 3.6 | 21.43 ± 3.96 | 17.00 ± 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shvachiy, L.; Amaro-Leal, Â.; Machado, F.; Rocha, I.; Outeiro, T.F.; Geraldes, V. Gender-Specific Effects on the Cardiorespiratory System and Neurotoxicity of Intermittent and Permanent Low-Level Lead Exposures. Biomedicines 2024, 12, 711. https://doi.org/10.3390/biomedicines12040711
Shvachiy L, Amaro-Leal Â, Machado F, Rocha I, Outeiro TF, Geraldes V. Gender-Specific Effects on the Cardiorespiratory System and Neurotoxicity of Intermittent and Permanent Low-Level Lead Exposures. Biomedicines. 2024; 12(4):711. https://doi.org/10.3390/biomedicines12040711
Chicago/Turabian StyleShvachiy, Liana, Ângela Amaro-Leal, Filipa Machado, Isabel Rocha, Tiago F. Outeiro, and Vera Geraldes. 2024. "Gender-Specific Effects on the Cardiorespiratory System and Neurotoxicity of Intermittent and Permanent Low-Level Lead Exposures" Biomedicines 12, no. 4: 711. https://doi.org/10.3390/biomedicines12040711






