Pharmacogenetic Approaches in Personalized Medicine for Postoperative Pain Management
Abstract
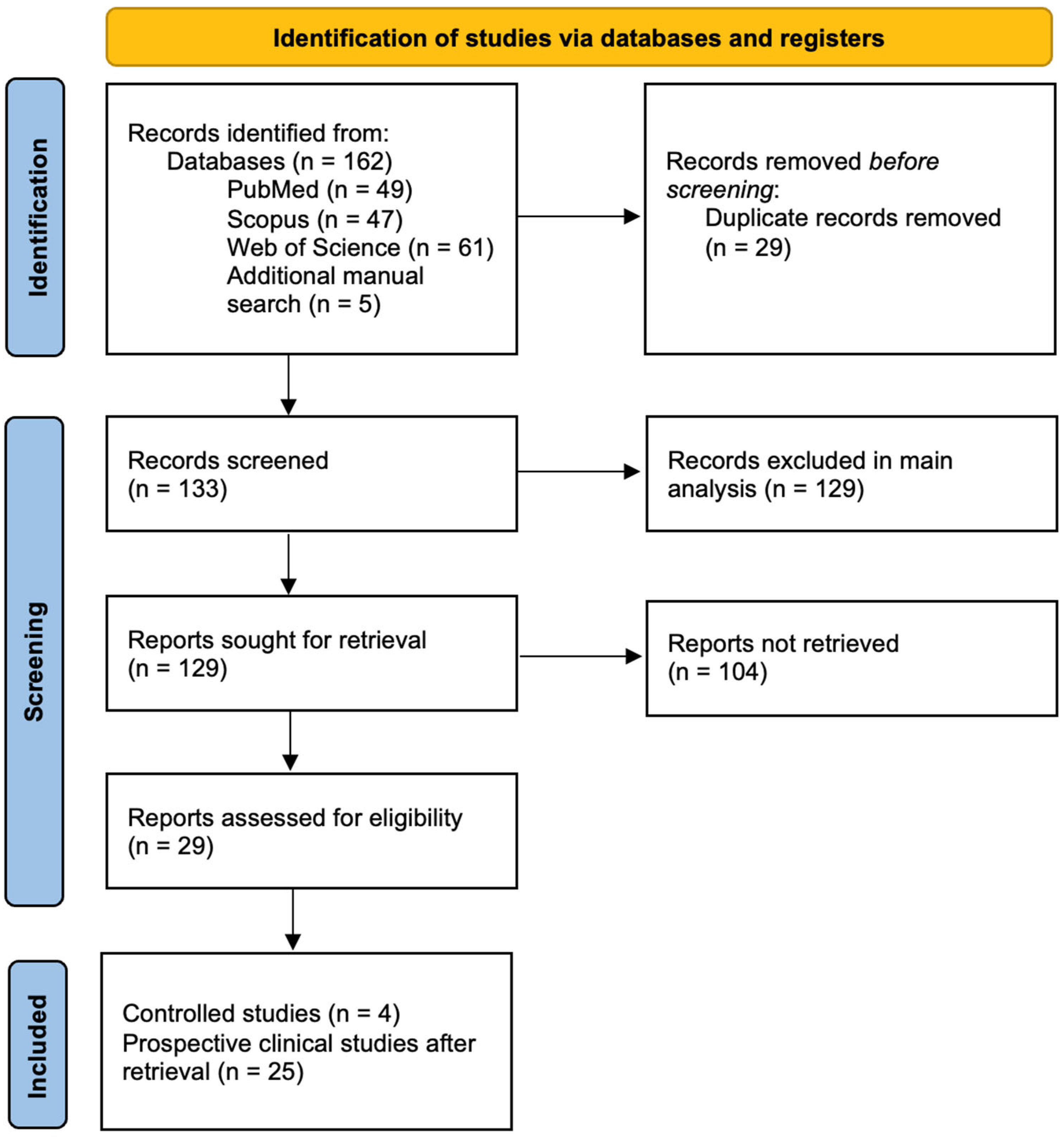
:1. Introduction
2. Postoperative Pain Management and Pharmacogenetics
3. Comprehensive Analysis of Clinical Studies
4. Main Genes Related to Postoperative Pain
4.1. OPRM1
4.2. CYP2D6
4.3. CYP2C, CYP2C19, CYP2C9 and CYP2D6
4.4. ABCB1
4.5. CYP3
4.6. COMT
5. Precision Personalized Medicine
6. Postoperative Pain and Pharmacogenetics Cost-Effectiveness
7. Platforms and Evidence-Based Protocols
8. Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goldstein, D.H.; Ellis, J.; Brown, R.; Wilson, R.; Penning, J.; Chisom, K.; VanDenKerkhof, E. Recommendations for improved acute pain services: Canadian collaborative acute pain initiative. Pain Res. Manag. 2004, 9, 123–130. [Google Scholar] [CrossRef]
- Hinrichs-Rocker, A.; Schulz, K.; Jarvinen, I.; Lefering, R.; Simanski, C.; Neugebauer, E.A. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP)—A systematic review. Eur. J. Pain 2009, 13, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Viterbori, P.; Flego, G. Prevalence of pain in Italian hospitals: Results of a regional cross-sectional survey. J. Pain Symptom Manag. 2002, 23, 221–230. [Google Scholar] [CrossRef]
- Lin, C.C. Applying the American Pain Society’s QA standards to evaluate the quality of pain management among surgical, oncology, and hospice inpatients in Taiwan. Pain 2000, 87, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.; Tcherny-Lessenot, S.; Collin, E.; Coutaux, A.; Levy-Soussan, M.; Legeron, M.C.; Bourgeois, P.; Cesselin, F.; Desfosses, G.; Rosenheim, M. Pain prevalence in a French teaching hospital. J. Pain Symptom Manag. 2002, 24, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Strohbuecker, B.; Mayer, H.; Evers, G.C.; Sabatowski, R. Pain prevalence in hospitalized patients in a German university teaching hospital. J. Pain Symptom Manag. 2005, 29, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.; de Rijke, J.M.; van Kleef, M.; Kessels, A.G.; Peters, M.L.; Geurts, J.W.; Gramke, H.F.; Marcus, M.A. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. Eur. J. Anaesthesiol. 2008, 25, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Lui, J.C. Postoperative pain management: Study of patients’ level of pain and satisfaction with health care providers’ responsiveness to their reports of pain. Nurs. Health Sci. 2003, 5, 13–21. [Google Scholar] [CrossRef]
- Strassels, S.A.; Chen, C.; Carr, D.B. Postoperative analgesia: Economics, resource use, and patient satisfaction in an urban teaching hospital. Anesth. Analg. 2002, 94, 130–137. [Google Scholar] [CrossRef]
- Apfelbaum, J.L.; Chen, C.; Mehta, S.S.; Gan, T.J. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth. Analg. 2003, 97, 534–540. [Google Scholar] [CrossRef]
- Svensson, I.; Sjostrom, B.; Haljamae, H. Influence of expectations and actual pain experiences on satisfaction with postoperative pain management. Eur. J. Pain 2001, 5, 125–133. [Google Scholar] [CrossRef]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Macrae, W.A. Chronic pain after surgery. Br. J. Anaesth. 2001, 87, 88–98. [Google Scholar] [CrossRef]
- Wilder-Smith, O.H.; Tassonyi, E.; Arendt-Nielsen, L. Preoperative back pain is associated with diverse manifestations of central neuroplasticity. Pain 2002, 97, 189–194. [Google Scholar] [CrossRef]
- Gordon, D.B.; Pellino, T.A.; Miaskowski, C.; McNeill, J.A.; Paice, J.A.; Laferriere, D.; Bookbinder, M. A 10-year review of quality improvement monitoring in pain management: Recommendations for standardized outcome measures. Pain Manag. Nurs. 2002, 3, 116–130. [Google Scholar] [CrossRef]
- Awad, M.E.; Padela, M.T.; Sayeed, Z.; Abaab, L.; El-Othmani, M.M.; Saleh, K.J. Pharmacogenomics Testing for Postoperative Pain Optimization Before Total Knee and Total Hip Arthroplasty. JBJS Rev. 2018, 6, e3. [Google Scholar] [CrossRef]
- Schug, S.; Ting, S. The pharmacogenomics of pain management: Prospects for personalized medicine. J. Pain Res. 2016, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Garcia, A.J.; Hall, O.M.; Jeha, G.M.; Cramer, K.D.; Granier, A.L.; Kallurkar, A.; Cornett, E.M.; Urman, R.D. Update on the pharmacogenomics of pain management. Pharmgenom. Pers. Med. 2019, 12, 125–143. [Google Scholar] [CrossRef]
- Webster, L.R.; Belfer, I. Pharmacogenetics and Personalized Medicine in Pain Management. Clin. Lab. Med. 2016, 36, 493–506. [Google Scholar] [CrossRef]
- Meredith, P.; Ownsworth, T.; Strong, J. A review of the evidence linking adult attachment theory and chronic pain: Presenting a conceptual model. Clin. Psychol. Rev. 2008, 28, 407–429. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Schwarz, J. Pain management. A moral imperative. Am. J. Nurs. 2000, 100, 49–50. [Google Scholar] [PubMed]
- Lome, B. Acute pain and the critically ill trauma patient. Crit. Care Nurs. Q 2005, 28, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Harmer, M. Consent and ethics in postoperative pain management. Anaesthesia 2002, 57, 1153–1154. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.M. Association Between Human Pain-Related Genotypes and Variability in Opioid Analgesia: An Updated Review. Pain Pract. 2015, 15, 580–594. [Google Scholar] [CrossRef]
- Yoshida, K.; Nishizawa, D.; Ide, S.; Ichinohe, T.; Fukuda, K.I.; Ikeda, K. A pharmacogenetics approach to pain management. Neuropsychopharmacol. Rep. 2018, 38, 2–8. [Google Scholar] [CrossRef]
- Hamilton, W.G. Prospective Randomized Study Using Pharmacogenetics to Customize Postoperative Pain Medication Following Hip and Knee Arthroplasty. J. Arthroplast. 2022, 37, S76–S81. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Parvataneni, H.K.; Gray, C.F.; Deen, J.T.; Prieto, H.A.; Pulido, L.F.; Elsey, A.R.; Elwood, E.N.; Starostik, P.; Gong, Y.; et al. A hybrid implementation-effectiveness randomized trial of CYP2D6-guided postoperative pain management. Genet. Med. 2021, 23, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.G. Using pharmacogenetics to structure individual pain management protocols in total knee arthroplasty a randomized pilot study. Bone Jt. J. 2020, 102, 73–78. [Google Scholar] [CrossRef]
- Senagore, A.J.; Champagne, B.J.; Dosokey, E.; Brady, J.; Steele, S.R.; Reynolds, H.L.; Stein, S.L.; Delaney, C.P. Pharmacogenetics-guided analgesics in major abdominal surgery: Further benefits within an enhanced recovery protocol. Am. J. Surg. 2017, 213, 467–472. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, L.; Yang, Y.; Gao, Y.; Li, Y.; Wang, B.; Pan, B.; Huang, J.; Guo, W. Is OPRM1 genotype a valuable predictor of VAS in patients undergoing laparoscopic radical resection of colorectal cancer with fentanyl? BMC Anesth. 2023, 23, 173. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Rodríguez, M.; Valdez-Acosta, S.; Borobia, A.M.; Burgueño, M.; Gálvez-Múgica, M.; Acero, J.; Cabaleiro, T.; Muñoz-Guerra, M.F.; Puerro, M.; Llanos, L.; et al. Influence of Genetic Polymorphisms on the Response to Tramadol, Ibuprofen, and the Combination in Patients With Moderate to Severe Pain After Dental Surgery. Clin. Ther. 2021, 43, e86–e102. [Google Scholar] [CrossRef] [PubMed]
- Matic, M.; de Hoogd, S.; de Wildt, S.N.; Tibboel, D.; Knibbe, C.A.; van Schaik, R.H. OPRM1 and COMT polymorphisms: Implications on postoperative acute, chronic and experimental pain after cardiac surgery. Pharmacogenomics 2020, 21, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Stamer, U.M.; Musshoff, F.; Stüber, F.; Brockmöller, J.; Steffens, M.; Tzvetkov, M.V. Loss-of-function polymorphisms in the organic cation transporter OCT1 are associated with reduced postoperative tramadol consumption. Pain 2016, 157, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.C.; Lim, E.C.; Ocampo, C.E.; Allen, J.C.; Sng, B.L.; Sia, A.T. Common variants of catechol-O-methyltransferase influence patient-controlled analgesia usage and postoperative pain in patients undergoing total hysterectomy. Pharmacogenomics J. 2016, 16, 186–192. [Google Scholar] [CrossRef]
- Dong, H.; Lu, S.-J.; Zhang, R.; Liu, D.-D.; Zhang, Y.-Z.; Song, C.-Y. Effect of the CYP2D6 gene polymorphism on postoperative analgesia of tramadol in Han nationality nephrectomy patients. Eur. J. Clin. Pharmacol. 2015, 71, 681–686. [Google Scholar] [CrossRef]
- Seripa, D.; Latina, P.; Fontana, A.; Gravina, C.; Lattanzi, M.; Savino, M.; Gallo, A.P.; Melchionda, G.; Santini, S.A.; Margaglione, M.; et al. Role of CYP2D6 Polymorphisms in the Outcome of Postoperative Pain Treatment. Pain Med. 2015, 16, 2012–2023. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.B.; Cai, L.N.; Yang, X.H.; Fu, H.G.; Sun, K.; Yuan, F.; Dong, T.L. Impact of CYP2D6 Polymorphisms on Postoperative Fentanyl Analgesia in Gastric Cancer Patients. Genet. Test. Mol. Biomark. 2015, 19, 248–252. [Google Scholar] [CrossRef]
- Zhang, F.; Tong, J.; Hu, J.; Zhang, H.; Ouyang, W.; Huang, D.; Tang, Q.; Liao, Q. COMT gene haplotypes are closely associated with postoperative fentanyl dose in patients. Anesth. Analg. 2015, 120, 933–940. [Google Scholar] [CrossRef]
- Boswell, M.V.; Stauble, M.E.; Loyd, G.E.; Langman, L.; Ramey-Hartung, B.; Baumgartner, R.N.; Tucker, W.W.; Jortani, S.A. The role of hydromorphone and OPRM1 in postoperative pain relief with hydrocodone. Pain Physician 2013, 16, E227–E235. [Google Scholar]
- Candiotti, K.; Yang, Z.; Xue, L.; Zhang, Y.; Rodriguez, Y.; Wang, L.; Hao, S.; Gitlin, M. Single-nucleotide polymorphism C3435T in the ABCB1 gene is associated with opioid consumption in postoperative pain. Pain Med. 2013, 14, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Henker, R.A.; Lewis, A.; Dai, F.; Lariviere, W.R.; Meng, L.; Gruen, G.S.; Sereika, S.M.; Pape, H.; Tarkin, I.S.; Gowda, I.; et al. The Associations between OPRM1 and COMT Genotypes and Postoperative Pain, Opioid Use, and Opioid-Induced Sedation. Biol. Res. Nurs. 2013, 15, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Slanar, O.; Dupal, P.; Matouskova, O.; Vondrackova, H.; Pafko, P.; Perlik, F. Tramadol efficacy in patients with postoperative pain in relation to CYP2D6 and MDR1 polymorphisms. Bratisl. Med. J. 2012, 113, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.C.; Hassan, S.K.; Mohamad, N.A.; Gan, S.H. Cytochrome P450 3A4 genetic polymorphisms and post-operative fentanyl requirements. J. Clin. Pharm. Ther. 2012, 37, 100–104. [Google Scholar] [CrossRef] [PubMed]
- De Gregori, M.; Garbin, G.; De Gregori, S.; Minella, C.E.; Bugada, D.; Lisa, A.; Govoni, S.; Regazzi, M.; Allegri, M.; Ranzani, G.N. Genetic variability at COMT but not at OPRM1 and UGT2B7 loci modulates morphine analgesic response in acute postoperative pain. Eur. J. Clin. Pharmacol. 2013, 69, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yuan, J.J.; Kan, Q.C.; Zhang, L.R.; Chang, Y.Z.; Wang, Z.Y.; Li, Z.S. Influence of CYP3A5*3 polymorphism and interaction between CYP3A5*3 and CYP3A4*1G polymorphisms on post-operative fentanyl analgesia in Chinese patients undergoing gynaecological surgery. Eur. J. Anaesthesiol. 2011, 28, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Zhang, X.; Deng, Q.; Wu, Y.; Xiang, G. Impact of CYP3A4*1G polymorphism on metabolism of fentanyl in Chinese patients undergoing lower abdominal surgery. Clin. Chim. Acta 2011, 412, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Zwisler, S.T.; Enggaard, T.P.; Mikkelsen, S.; Brosen, K.; Sindrup, S.H. Impact of the CYP2D6 genotype on post-operative intravenous oxycodone analgesia. Acta Anaesthesiol. Scand. 2010, 54, 232–240. [Google Scholar] [CrossRef]
- Fukuda, K.; Hayashida, M.; Ide, S.; Saita, N.; Kokita, Y.; Kasai, S.; Nishizawa, D.; Ogai, Y.; Hasegawa, J.; Nagashima, M.; et al. Association between OPRM1 gene polymorphisms and fentanyl sensitivity in patients undergoing painful cosmetic surgery. Pain 2009, 147, 194–201. [Google Scholar] [CrossRef]
- Tan, E.C.; Lim, E.C.; Teo, Y.Y.; Lim, Y.; Law, H.Y.; Sia, A.T. Ethnicity and OPRM variant independently predict pain perception and patient-controlled analgesia usage for post-operative pain. Mol. Pain 2009, 5, 32. [Google Scholar] [CrossRef]
- Sia, A.T.; Lim, Y.; Lim, E.C.; Goh, R.W.; Law, H.Y.; Landau, R.; Teo, Y.Y.; Tan, E.C. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology 2008, 109, 520–526. [Google Scholar] [CrossRef]
- Chou, W.Y.; Yang, L.C.; Lu, H.F.; Ko, J.Y.; Wang, C.H.; Lin, S.H.; Lee, T.H.; Concejero, A.; Hsu, C.J. Association of μ-opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol. Scand. 2006, 50, 787–792. [Google Scholar] [CrossRef]
- Chou, W.Y.; Wang, C.H.; Liu, P.H.; Liu, C.C.; Tseng, C.C.; Jawan, B. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology 2006, 105, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, H.; He, F.; Fang, X. Effect of the CYP2D6*10 C188T polymorphism on postoperative tramadol analgesia in a Chinese population. Eur. J. Clin. Pharmacol. 2006, 62, 927–931. [Google Scholar] [CrossRef]
- Stamer, U.M.; Lehnen, K.; Höthker, F.; Bayerer, B.; Wolf, S.; Hoeft, A.; Stuber, F. Impact of CYP2D6 genotype on postoperative tramadol analgesia. Pain 2003, 105, 231–238. [Google Scholar] [CrossRef]
- Koren, G.; Cairns, J.; Chitayat, D.; Gaedigk, A.; Leeder, S.J. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 2006, 368, 704. [Google Scholar] [CrossRef]
- Willmann, S.; Edginton, A.N.; Coboeken, K.; Ahr, G.; Lippert, J. Risk to the breast-fed neonate from codeine treatment to the mother: A quantitative mechanistic modeling study. Clin. Pharmacol. Ther. 2009, 86, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Bagher, A.M. Association of CYP2C9 *3 and CYP2C8 *3 Non-Functional Alleles with Ibuprofen-Induced Upper Gastrointestinal Toxicity in a Saudi Patient. Case Rep. Med. 2023, 2023, 6623269. [Google Scholar] [CrossRef] [PubMed]
- Zobdeh, F.; Eremenko, I.I.; Akan, M.A.; Tarasov, V.V.; Chubarev, V.N.; Schioth, H.B.; Mwinyi, J. Pharmacogenetics and Pain Treatment with a Focus on Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Antidepressants: A Systematic Review. Pharmaceutics 2022, 14, 1190. [Google Scholar] [CrossRef]
- Macias, Y.; Garcia-Menaya, J.M.; Marti, M.; Cordobes, C.; Jurado-Escobar, R.; Cornejo-Garcia, J.A.; Torres, M.J.; Blanca-Lopez, N.; Canto, G.; Blanca, M.; et al. Lack of Major Involvement of Common CYP2C Gene Polymorphisms in the Risk of Developing Cross-Hypersensitivity to NSAIDs. Front. Pharmacol. 2021, 12, 648262. [Google Scholar] [CrossRef]
- Frangakis, S.G.; MacEachern, M.; Akbar, T.A.; Bolton, C.; Lin, V.; Smith, A.V.; Brummett, C.M.; Bicket, M.C. Association of Genetic Variants with Postsurgical Pain: A Systematic Review and Meta-analyses. Anesthesiology 2023, 139, 827–839. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, L.; Carr, D.F.; Pirmohamed, M. Pharmacogenomics of NSAID-Induced Upper Gastrointestinal Toxicity. Front. Pharmacol. 2021, 12, 684162. [Google Scholar] [CrossRef] [PubMed]
- Musumba, C.O.; Jorgensen, A.; Sutton, L.; Van Eker, D.; Zhang, E.; O’Hara, N.; Carr, D.F.; Pritchard, D.M.; Pirmohamed, M. CYP2C19*17 gain-of-function polymorphism is associated with peptic ulcer disease. Clin. Pharmacol. Ther. 2013, 93, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Dean, L. Amitriptyline Therapy and CYP2D6 and CYP2C19 Genotype. In Medical Genetics Summarie; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kattman, B.L., Malheiro, A.J., Eds.; Medical Genetics Summaries: Bethesda, MD, USA, 2012. [Google Scholar]
- Sadhasivam, S.; Chidambaran, V.; Zhang, X.; Meller, J.; Esslinger, H.; Zhang, K.; Martin, L.J.; McAuliffe, J. Opioid-induced respiratory depression: ABCB1 transporter pharmacogenetics. Pharmacogenomics J. 2015, 15, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Branford, R.; Droney, J.; Ross, J.R. Opioid genetics: The key to personalized pain control? Clin. Genet. 2012, 82, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Korczeniewska, O.A.; Kuo, F.; Huang, C.Y.; Nasri-Heir, C.; Khan, J.; Benoliel, R.; Hirschberg, C.; Eliav, E.; Diehl, S.R. Genetic variation in catechol-O-methyltransferase is associated with individual differences in conditioned pain modulation in healthy subjects. J. Gene Med. 2021, 23, e3374. [Google Scholar] [CrossRef] [PubMed]
- Vetterlein, A.; Monzel, M.; Reuter, M. Are catechol-O-methyltransferase gene polymorphisms genetic markers for pain sensitivity after all? A review and meta-analysis. Neurosci. Biobehav. Rev. 2023, 148, 105112. [Google Scholar] [CrossRef] [PubMed]
- Diatchenko, L.; Slade, G.D.; Nackley, A.G.; Bhalang, K.; Sigurdsson, A.; Belfer, I.; Goldman, D.; Xu, K.; Shabalina, S.A.; Shagin, D.; et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum. Mol. Genet. 2005, 14, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Meloto, C.B.; Bortsov, A.V.; Bair, E.; Helgeson, E.; Ostrom, C.; Smith, S.B.; Dubner, R.; Slade, G.D.; Fillingim, R.B.; Greenspan, J.D.; et al. Modification of COMT-dependent pain sensitivity by psychological stress and sex. Pain 2016, 157, 858–867. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, X.; Xu, G.; Zhang, Q.; Qian, P.; Liu, S.; Zhu, J.; Shen, R. Association between COMT Polymorphism Val158Met and Opioid Consumption in Patients with Postoperative Pain: A Meta-Analysis. Neurosignals 2018, 26, 11–21. [Google Scholar] [CrossRef]
- Smietanska, I.; Adrian, E.; Smietanski, M.; Kitowski, J. Does the Pain-free hospital certification improve the management of pain following hernioplasty? Anestezjol. Intens. Ter. 2010, 42, 190–193. [Google Scholar] [PubMed]
- Skinner, H.B.; Shintani, E.Y. Results of a multimodal analgesic trial involving patients with total hip or total knee arthroplasty. Am. J. Orthop. 2004, 33, 85–92; discussion 92. [Google Scholar]
- Wells, N.; Pasero, C.; McCaffery, M. Improving the Quality of Care through Pain Assessment and Management; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008.
- American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2004, 100, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, B.; Fonseca, S.; Pozza, D.H.; Xara, D.; Sa Rodrigues, A. Relationship between Postoperative Pain and Sociocultural Level in Major Orthopedic Surgery. Adv. Orthop. 2022, 2022, 7867719. [Google Scholar] [CrossRef] [PubMed]
- Bardiau, F.M.; Taviaux, N.F.; Albert, A.; Boogaerts, J.G.; Stadler, M. An intervention study to enhance postoperative pain management. Anesth. Analg. 2003, 96, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Ernst, F.R.; Grizzle, A.J. Drug-related morbidity and mortality: Updating the cost-of-illness model. J. Am. Pharm. Assoc. 2001, 41, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, C.J.; Visser, K.; Moen, J.; Bonsel, G.J.; Grobbee, D.E.; Moons, K.G. Preoperative prediction of severe postoperative pain. Pain 2003, 105, 415–423. [Google Scholar] [CrossRef]
- Severino, G.; Del Zompo, M. Adverse drug reactions: Role of pharmacogenomics. Pharmacol. Res. 2004, 49, 363–373. [Google Scholar] [CrossRef]
- Stephanie, N.; Schatz, R.J.W. PSAP: CNS/Pharmacy Practice; American College of Clinical Pharmacy: Lenexa, KS, USA, 2015. [Google Scholar]
- Pozza, D.H.; Azevedo, L.F.; Castro Lopes, J.M. Pain as the fifth vital sign-A comparison between public and private healthcare systems. PLoS ONE 2021, 16, e0259535. [Google Scholar] [CrossRef]
- McNeill, J.A.; Sherwood, G.D.; Starck, P.L.; Thompson, C.J. Assessing clinical outcomes: Patient satisfaction with pain management. J. Pain Symptom Manag. 1998, 16, 29–40. [Google Scholar] [CrossRef]
- Bright, D.R.; Petry, N.; Roath, E.; Gibb, T. Engaging pharmacogenomics in pain management and opioid selection. Pharmacogenomics 2021, 22, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.A.; Alsaidi, A.T.; Verbyla, A.; Cruz, A.; Macfarlane, C.; Bauer, J.; Patel, J.N. Cost Effectiveness of Pharmacogenetic Testing for Drugs with Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines: A Systematic Review. Clin. Pharmacol. Ther. 2022, 112, 1318–1328. [Google Scholar] [CrossRef]
- Lazarou, J.; Pomeranz, B.H.; Corey, P.N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998, 279, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Ionova, Y.; Ashenhurst, J.; Zhan, J.; Nhan, H.; Kosinski, C.; Tamraz, B.; Chubb, A. CYP2C19 Allele Frequencies in Over 2.2 Million Direct-to-Consumer Genetics Research Participants and the Potential Implication for Prescriptions in a Large Health System. Clin. Transl. Sci. 2020, 13, 1298–1306. [Google Scholar] [CrossRef]
- Alrajeh, K.Y.; Roman, Y.M. The frequency of major CYP2C19 genetic polymorphisms in women of Asian, Native Hawaiian and Pacific Islander subgroups. Pers. Med. 2022, 19, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Anderson, K.O.; Baker, T.A.; Campbell, L.C.; Decker, S.; Fillingim, R.B.; Kalauokalani, D.A.; Lasch, K.E.; Myers, C.; Tait, R.C.; et al. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Med. 2003, 4, 277–294. [Google Scholar] [CrossRef]
- Salari, P.; Larijani, B. Ethical Issues Surrounding Personalized Medicine: A Literature Review. Acta Med. Iran. 2017, 55, 209–217. [Google Scholar]
- Relling, M.V.; Klein, T.E. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin. Pharmacol. Ther. 2011, 89, 464–467. [Google Scholar] [CrossRef]
- Crews, K.R.; Monte, A.A.; Huddart, R.; Caudle, K.E.; Kharasch, E.D.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Callaghan, J.T.; Samer, C.F.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin. Pharmacol. Ther. 2021, 110, 888–896. [Google Scholar] [CrossRef]
- Truong, T.M.; Apfelbaum, J.; Shahul, S.; Anitescu, M.; Danahey, K.; Knoebel, R.W.; Liebovitz, D.; Karrison, T.; Van Wijk, X.M.R.; Yeo, K.T.J.; et al. The ImPreSS Trial: Implementation of Point-of-Care Pharmacogenomic Decision Support in Perioperative Care. Clin. Pharmacol. Ther. 2019, 106, 1179–1183. [Google Scholar] [CrossRef]
- Coulbault, L.; Beaussier, M.; Verstuyft, C.; Weickmans, H.; Dubert, L.; Tregouet, D.; Descot, C.; Parc, Y.; Lienhart, A.; Jaillon, P.; et al. Environmental and genetic factors associated with morphine response in the postoperative period. Clin. Pharmacol. Ther. 2006, 79, 316–324. [Google Scholar] [CrossRef] [PubMed]

| Ref. | Participants/Country/Tools | Intervention/Treatment | Pharmacogenetic | Main Results |
|---|---|---|---|---|
| Hamilton 2022 [27] |
|
| 16 genes, including CYP2D6, CYP2C9, OPRM1, CYP3A4 and CYP1A2 |
|
| Thomas 2021 [28] |
|
| CYP2D6 |
|
| Hamilton 2020 [29] |
|
| 7 genes, including CYP2D6, CYP2C9, OPRM1, CYP3A4 and CYP1A2 |
|
| Senagore 2017 [30] |
|
| 9 genes, including CYP2D6, CYP2C9, OPRM1, CYP3A4 and CYP1A2 |
|
| Ref. | Participants/Country/Pain Assessm. | Intervention/Treatment | Pharmacogenetic | Main Results |
|---|---|---|---|---|
| Zhou 2023 [31] |
| 2 Groups based on OPRM1 A118G polymorphisms: wild-type (A/A) and heterozygous (G/A), and mutant homozygous (G/G) | OPRM1 A118G genotypes |
|
| Saiz-Rodríguez 2021 [32] |
| 3 Groups:
| 12 genes, including CYP2C9, CYP2C19, CYP3A4, CYP2D6, ABCB1 and OPRM1 |
|
| Matic 2020 [33] |
| 2 Groups based on intraoperative medication:
| COMT and OPRM1 |
|
| Stamer 2016 [34] |
| 3 genotype groups: 0 active OCT1 allele (OCT1 poor transporter), 1 active OCT1 allele (heterozygous for 1 inactive allele), and 2 active OCT1 alleles (OCT1 extensive transporter). 4 CYP2D6 activity groups: CYP2D6 PM, CYP2D6 IM, CYP2D6 EM and CYP2D6 UM | SLC22A1 (OCT1) and CYP2D6 |
|
| Tan 2016 [35] |
| Groups were created based on COMT rs4633, rs4818, rs4680 polymorphisms and COMT haplotype | COMT |
|
| Dong 2015 [36] |
| 3 Groups according to CYP2D6*10 allele: wild-type (CYP2D6*1/*1), heterozygous (CYP2D6*1/*10) and mutant homozygous (CYP2D6*10/*10) | CYP2D6 |
|
| Seripa 2015 [37] |
| 4 Groups according to CYP2D6 activity: UM, EM, IM and PM POP treatment was multimodal, including opioids (tramadol, morphine), NSAIDs (ketoprofen) plus metoclopramide, and ranitidine | CYP2D6 |
|
| Wu 2015 [38] |
| 3 Groups according to CYP2D6 polymorphisms: wild-type (W/W), heterozygous (M/W) and mutant homozygous (M/M) Cumulative Analgesic Consumption of Fentanyl during Postoperative Period | CYP2D6 |
|
| Zhang 2015 [39] |
| Groups were created based on polymorphisms in COMT SNPs (rs6269, rs4633, rs4818, rs4680) and their haplotypes | COMT |
|
| Boswell 2013 [40] |
| 2 Groups according to OPRM1 polymorphisms: wild-type (AA): both heterozygous and mutant homozygous (AG/GG) | OPRM1 |
|
| Candiotti 2013 [41] |
| 3 Groups according to ABCB1 C3435T genotype: wild-type (CC), heterozygous (CT) and mutant homozygous (TT) | ABCB1 |
|
| Henker 2013 [42] |
| Groups were created based on polymorphisms in OPRM1 A118G rs1799971, rs1799972, COMT SNPs (rs6269, rs4633, rs4818, rs4680) and their haplotypes | COMT and OPRM1 |
|
| Slanar 2012 [43] |
| 3 groups according to ABCB1 genotype: wild-type (3435CC), heterozygous (3435CT) and mutant homozygous (3435TT) 4 CYP2D6 phenotype groups: CYP2D6 PM, homozygous CYP2D6 EM, heterozygous CYP2D6 EM and CYP2D6 UM | CYP2D6 and ABCB1 (MDR1) |
|
| Tan 2012 [44] |
| 2 Groups according to CYP3A4 polymorphisms: wild-type (CYP3A4*1/*1) and mutant heterozygous (CYP3A4*1/*18) | CYP3A4 |
|
| De Gregori 2012 [45] |
| Groups were created based on polymorphisms in OPRM1 A118G rs1799971, COMT SNPs (rs6269, rs4633, rs4818, rs4680) and their haplotypes, and 7 other UGT2B7 SNPs | COMT, OPRM1 and UGT2B7 |
|
| Zhang 2011 [46] |
| 3 groups according to CYP3A5 genotype: wild-type (CYP3A5*1/*1), heterozygous (CYP3A5*1/*3) and mutant homozygous (CYP3A5*3/*3) | CYP3A4 and CYP3A5 |
|
| Yuan 2011 [47] |
| 3 Groups according to CYP3A4 polymorphisms: wild-type (*1/*1), heterozygous (*1/*1G) and mutant homozygous (*1G/*1G) | CYP3A4 |
|
| Zwisler 2010 [48] |
| 2 Groups according to CYP2D6 activity: 8.9% Poor Metabolizers (PM), 91.1% Extensive Metabolizers (EM). | CYP2D6 genotypes |
|
| Fukuda 2009 [49] |
| SNP genotypes: A118G and IVS3 + A8449G grouped into major allele homozygote (AA) and combined heterozygote/minor allele homozygote (AG + GG), resulting in 4 genotype groups. | OPRM1 |
|
| Tan 2009 [50] |
| 3 Groups according to OPRM1 polymorphisms: wild-type (AA) heterozygous (AG) and mutant homozygous (GG) | OPRM1 |
|
| Sia 2008 [51] |
| 3 Groups according to OPRM1 polymorphisms: wild-type A118 homozygous (AA), heterozygous (AG) and mutant G118 homozygous (GG) | OPRM1 |
|
| Chou 2006 [52] |
| 3 Groups according to OPRM1 polymorphisms: wild-type A118 homozygous (AA), heterozygous (AG) and mutant G118 homozygous (GG) | OPRM1 |
|
| Chou 2006 [53] |
| 3 Groups according to OPRM1 polymorphisms: wild-type A118 homozygous (AA), heterozygous (AG) and mutant G118 homozygous (GG) | OPRM1 |
|
| Wang 2006 [54] |
| 3 Groups were created according to CYP2D6*10 polymorphism: wild-type (Group I), heterozygous (Group II) and mutant homozygous (Group III) | CYP2D6 |
|
| Stamer 2003 [55] |
| 2 Groups according to CYP2D6 activity: CYP2D6 EM (mutant heterozygous) and CYP2D6 PM (mutant homozygous) | CYP2D6 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira do Couto, M.L.; Fonseca, S.; Pozza, D.H. Pharmacogenetic Approaches in Personalized Medicine for Postoperative Pain Management. Biomedicines 2024, 12, 729. https://doi.org/10.3390/biomedicines12040729
Ferreira do Couto ML, Fonseca S, Pozza DH. Pharmacogenetic Approaches in Personalized Medicine for Postoperative Pain Management. Biomedicines. 2024; 12(4):729. https://doi.org/10.3390/biomedicines12040729
Chicago/Turabian StyleFerreira do Couto, Maria Leonor, Sara Fonseca, and Daniel Humberto Pozza. 2024. "Pharmacogenetic Approaches in Personalized Medicine for Postoperative Pain Management" Biomedicines 12, no. 4: 729. https://doi.org/10.3390/biomedicines12040729
APA StyleFerreira do Couto, M. L., Fonseca, S., & Pozza, D. H. (2024). Pharmacogenetic Approaches in Personalized Medicine for Postoperative Pain Management. Biomedicines, 12(4), 729. https://doi.org/10.3390/biomedicines12040729





