Early Growth Response Protein 1 Exacerbates Murine Inflammatory Bowel Disease by Transcriptional Activation of Matrix Metalloproteinase 12
Abstract
1. Introduction
2. Materials and Methods
2.1. DSS-Induced IBD Model
2.2. Histological Observations and Scoring
2.3. Immunohistochemistry (IHC)
2.4. Enzyme-Linked Immunosorbent Assay (ELISA) for Cytokine Detection in the Colonic Tissue
2.5. Cell Lines
2.6. Production of Lentiviral Vectors
2.7. Lentiviral Infection and Stable Transfectant Selection
2.8. Cloning for Human MMP12 Promoter
2.9. Cell Transfection
2.10. Reporter Gene Assay
2.11. Chromatin Immunoprecipitation (ChIP) Assay
2.12. Statistics
3. Results
3.1. Egr1 Is Highly Expressed in Colon Epithelial Cells of IBD Mice
3.2. Clinical Signs of DSS-Induced Egr1 Gene KO and Wild-Type Mice
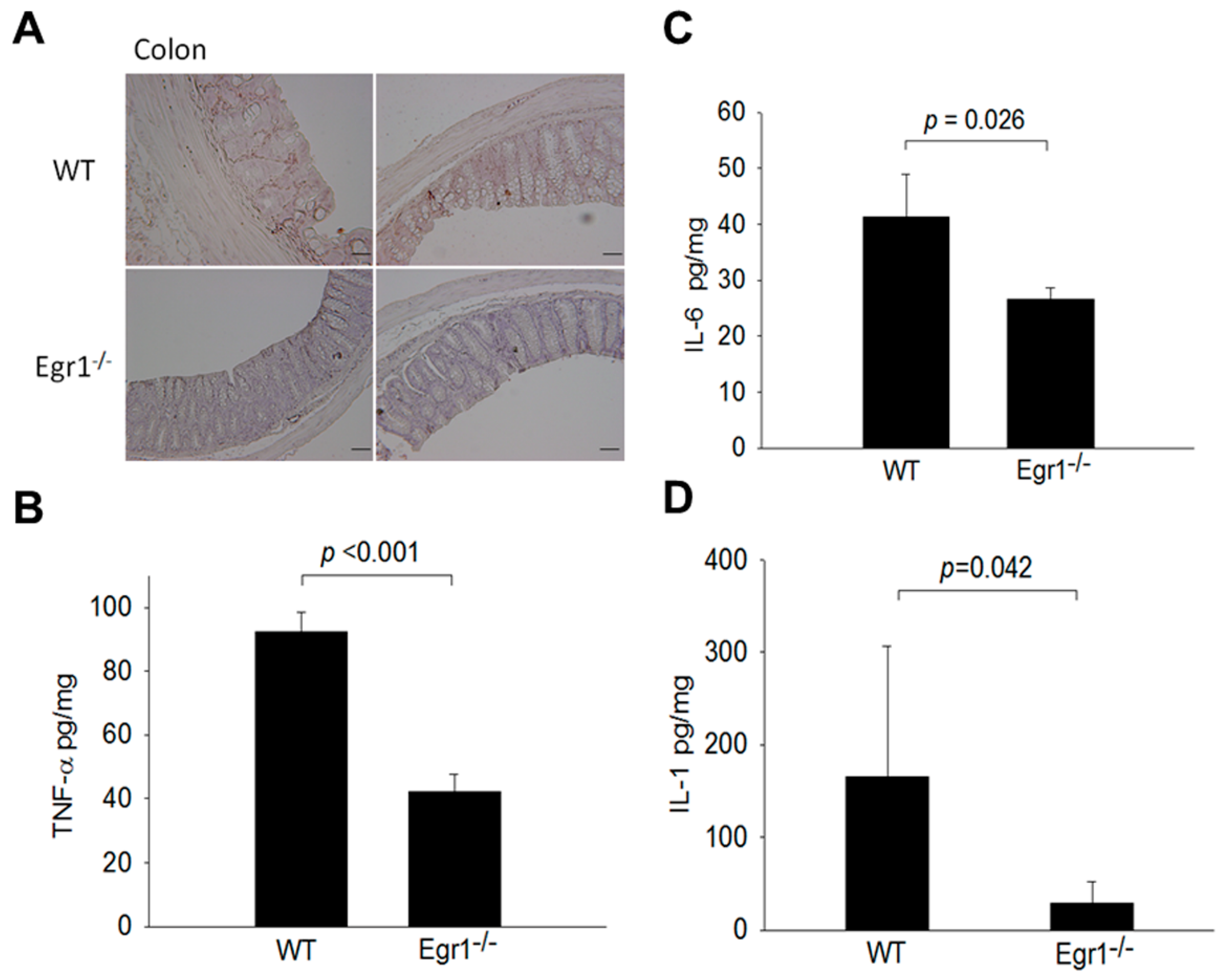
3.3. Reduced Expression Levels of MMPs and Proinflammatory Cytokines in the Intestines of Egr1 KO Colitis Mice
3.4. Egr1 Directly Binds to the MMP12 Promoter and Increases MMP12 Promoter Activity
3.5. Egr1 Induces MMP12 Expression at the Transcriptional Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lennard-Jones, J. Classification of inflammatory bowel disease. Scand. J. Gastroenterol. 1989, 24 (Suppl. S170), 2–6. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Svrcek, M.; Seksik, P.; Bouvier, A.M.; Simon, T.; Allez, M.; Brixi, H.; Gornet, J.M.; Altwegg, R.; Beau, P.; et al. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology 2013, 145, 166–175.e8. [Google Scholar] [CrossRef] [PubMed]
- Gashler, A.; Sukhatme, V.P. Early growth response protein 1 (Egr-1): Prototype of a zinc-finger family of transcription factors. Prog. Nucleic Acid Res. Mol. Biol. 1995, 50, 191–224. [Google Scholar] [PubMed]
- Xu, X.C.; Gao, H.; Zhang, W.B.; Abuduhadeer, X.; Wang, Y.H. Clinical significance of immunogenic cell death biomarker rage and early growth response 1 in human primary gastric adenocarcinoma. Int. J. Immunopathol. Pharmacol. 2013, 26, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Tureyen, K.; Brooks, N.; Bowen, K.; Svaren, J.; Vemuganti, R. Transcription factor early growth response-1 induction mediates inflammatory gene expression and brain damage following transient focal ischemia. J. Neurochem. 2008, 105, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Lin, Z.; Hegarty, J.P.; Chen, X.; Kelly, A.A.; Wang, Y.; Poritz, L.S.; Koltun, W.A. Genes differentially regulated by NKX2-3 in B cells between ulcerative colitis and Crohn’s disease patients and possible involvement of EGR1. Inflammation 2012, 35, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Howe, C.L.; Mayoral, S.; Rodriguez, M. Activated microglia stimulate transcriptional changes in primary oligodendrocytes via IL-1beta. Neurobiol. Dis. 2006, 23, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, Y.; Matsushima, M.; Minaguchi, T.; Kobayashi, K.; Sagae, S.; Kudo, R.; Terakawa, N.; Nakamura, Y. Correlation between expression of the matrix metalloproteinase-1 gene in ovarian cancers and an insertion/deletion polymorphism in its promoter region. Cancer Res. 1999, 59, 4225–4227. [Google Scholar]
- Macsharry, J.; O’Mahony, L.; Fanning, A.; Bairead, E.; Sherlock, G.; Tiesman, J.; Fulmer, A.; Kiely, B.; Dinan, T.G.; Shanahan, F.; et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand. J. Gastroenterol. 2008, 43, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Nagase, H.; Harris, E.D., Jr. Matrix metalloproteinases 1, 2, and 3 from rheumatoid synovial cells are sufficient to destroy joints. J. Rheumatol. 1987, 14, 41–42. [Google Scholar]
- Kirkegaard, T.; Hansen, A.; Bruun, E.; Brynskov, J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn’s disease. Gut 2004, 53, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Mäkitalo, L.; Kolho, K.L.; Karikoski, R.; Anthoni, H.; Saarialho-Kere, U. Expression profiles of matrix metalloproteinases and their inhibitors in colonic inflammation related to pediatric inflammatory bowel disease. Scand. J. Gastroenterol. 2010, 45, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Hayden, D.M.; Forsyth, C.; Keshavarzian, A. The role of matrix metalloproteinases in intestinal epithelial wound healing during normal and inflammatory states. J. Surg. Res. 2011, 168, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Neurath, M.F. Mouse models of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2007, 59, 1073–1083. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chu, C.-T.; Yang, M.-L.; Lin, J.-D.; Wang, C.-T.; Lee, C.-H.; Lin, I.-C.; Shiau, A.-L.; Ling, P.; Wu, C.-L. Amelioration of Murine Colitis by Attenuated Salmonella choleraesuis Encoding Interleukin-19. Microorganisms 2023, 11, 1530. [Google Scholar] [CrossRef]
- Melgar, S.; Karlsson, A.; Michaëlsson, E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: Correlation between symptoms and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1328–G1338. [Google Scholar] [CrossRef]
- Chen, S.Y.; Shiau, A.L.; Li, Y.T.; Lin, C.C.; Jou, I.M.; Liu, M.F.; Wu, C.L.; Wang, C.R. Transcription factor snail regulates tumor necrosis factor α-mediated synovial fibroblast activation in the rheumatoid joint. Arthritis Rheumatol. 2015, 67, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Jou, I.-M.; Ko, P.-Y.; Hsu, K.-L.; Su, W.-R.; Kuo, L.-C.; Lee, P.-Y.; Wu, C.-L.; Wu, P.-T. Amelioration of experimental tendinopathy by lentiviral CD44 gene therapy targeting senescence-associated secretory phenotypes. Mol. Ther. Methods Clin. Dev. 2022, 26, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Chen, S.-Y.; Ko, P.-Y.; Kwan, F.-C.; Su, W.-R.; Jou, I.-M.; Wu, P.-T. MicroRNA-146a gene transfer ameliorates senescence and senescence-associated secretory phenotypes in tendinopathic tenocytes. Aging 2024, 16, 2702. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-C.; Chen, Y.-C.; Tseng, Y.-L.; Shieh, G.-S.; Wu, P.; Shiau, A.-L.; Wu, C.-L. The pro-survival Oct4/Stat1/Mcl-1 axis is associated with poor prognosis in lung adenocarcinoma patients. Cells 2021, 10, 2642. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Yoshikawa, T. Role of matrix metalloproteinases in inflammatory bowel disease. Mol. Asp. Med. 2005, 26, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Christy, B.; Nathans, D. DNA binding site of the growth factor-inducible protein Zif268. Proc. Natl. Acad. Sci. USA 1989, 86, 8737–8741. [Google Scholar] [CrossRef] [PubMed]
- Sukhatme, V.P. Early transcriptional events in cell growth: The Egr family. J. Am. Soc. Nephrol. 1990, 1, 859–866. [Google Scholar] [CrossRef]
- Calogero, A.; Lombari, V.; De Gregorio, G.; Porcellini, A.; Ucci, S.; Arcella, A.; Caruso, R.; Gagliardi, F.M.; Gulino, A.; Lanzetta, G. Inhibition of cell growth by EGR-1 in human primary cultures from malignant glioma. Cancer Cell Int. 2004, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.-F.; Fujita, T.; Lu, J.; Okada, K.; Shan Zou, Y.; Mackman, N.; Pinsky, D.J.; Stern, D.M. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat. Med. 2000, 6, 1355–1361. [Google Scholar] [CrossRef]
- Reynolds, P.R.; Cosio, M.G.; Hoidal, J.R. Cigarette smoke–induced Egr-1 upregulates proinflammatory cytokines in pulmonary epithelial cells. Am. J. Respir. Cell Mol. Biol. 2006, 35, 314–319. [Google Scholar] [CrossRef]
- Dieckgraefe, B.K.; Weems, D.M. Epithelial injury induces egr-1 and fos expression by a pathway involving protein kinase C and ERK. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999, 276, G322–G330. [Google Scholar] [CrossRef] [PubMed]
- Nebbaki, S.-S.; El Mansouri, F.E.; Afif, H.; Kapoor, M.; Benderdour, M.; Duval, N.; Pelletier, J.-P.; Martel-Pelletier, J.; Fahmi, H. Egr-1 contributes to IL-1-mediated down-regulation of peroxisome proliferator-activated receptor γ expression in human osteoarthritic chondrocytes. Arthritis Res. Ther. 2012, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Ashouri, J.; Wolter, S.; Doerrie, A.; Dittrich-Breiholz, O.; Schneider, H.; Wagner, E.F.; Troppmair, J.; Mackman, N.; Kracht, M. Transcriptional regulation of EGR-1 by the interleukin-1-JNK-MKK7-c-Jun pathway. J. Biol. Chem. 2008, 283, 12120–12128. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, S.; Ma, W.; Teng, Y.; Tian, Y.; Huang, X.; Zhang, Y. A newly identified microRNA, mmu-miR-7578, functions as a negative regulator on inflammatory cytokines tumor necrosis factor-α and interleukin-6 via targeting Egr1 in vivo. J. Biol. Chem. 2013, 288, 4310–4320. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.; Garg, P.; Sitaraman, S.V. Matrix metalloproteinases in inflammatory bowel disease: Boon or a bane? Inflamm. Bowel Dis. 2007, 13, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, N.; MacDonald, T. The role of matrix metalloproteinases in stromal/epithelial interactions in the gut. Physiology 2007, 22, 401–409. [Google Scholar] [CrossRef]
- Chen, A.; Xu, J.; Johnson, A. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene 2006, 25, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Glasgow, W.C.; Eling, T.E. Curcumin suppresses interleukin 1β-mediated microsomal prostaglandin E synthase 1 by altering early growth response gene 1 and other signaling pathways. J. Pharmacol. Exp. Ther. 2005, 315, 788–795. [Google Scholar] [CrossRef]
- Meijer, M.J.; Mieremet-Ooms, M.A.; van Duijn, W.; van der Zon, A.M.; Hanemaaijer, R.; Verheijen, J.H.; van Hogezand, R.A.; Lamers, C.B.; Verspaget, H.W. Effect of the anti-tumor necrosis factor-α antibody infliximab on the ex vivo mucosal matrix metalloproteinase–proteolytic phenotype in inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 200–210. [Google Scholar] [CrossRef]
- Kostadinova, R.M.; Nawrocki, A.R.; Frey, F.J.; Frey, B.M. Tumor necrosis factor alpha and phorbol 12-myristate-13-acetate down-regulate human 11beta-hydroxysteroid dehydrogenase type 2 through p50/p50 NF-kappaB homodimers and Egr-1. FASEB J. 2005, 19, 650–652. [Google Scholar] [CrossRef]
- Grimbacher, B.; Aicher, W.K.; Peter, H.H.; Eibel, H. TNF-alpha induces the transcription factor Egr-1, pro-inflammatory cytokines and cell proliferation in human skin fibroblasts and synovial lining cells. Rheumatol. Int. 1998, 17, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Son, S.W.; Min, B.W.; Lim, Y.; Lee, Y.H.; Shin, S.Y. Regulatory mechanism of TNFalpha autoregulation in HaCaT cells: The role of the transcription factor EGR-1. Biochem. Biophys. Res. Commun. 2008, 374, 777–782. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-Y.; Fang, C.-Y.; Su, B.-H.; Chen, H.-M.; Huang, S.-C.; Wu, P.-T.; Shiau, A.-L.; Wu, C.-L. Early Growth Response Protein 1 Exacerbates Murine Inflammatory Bowel Disease by Transcriptional Activation of Matrix Metalloproteinase 12. Biomedicines 2024, 12, 780. https://doi.org/10.3390/biomedicines12040780
Chen S-Y, Fang C-Y, Su B-H, Chen H-M, Huang S-C, Wu P-T, Shiau A-L, Wu C-L. Early Growth Response Protein 1 Exacerbates Murine Inflammatory Bowel Disease by Transcriptional Activation of Matrix Metalloproteinase 12. Biomedicines. 2024; 12(4):780. https://doi.org/10.3390/biomedicines12040780
Chicago/Turabian StyleChen, Shih-Yao, Chuan-Yin Fang, Bing-Hwa Su, Hao-Ming Chen, Shih-Chi Huang, Po-Ting Wu, Ai-Li Shiau, and Chao-Liang Wu. 2024. "Early Growth Response Protein 1 Exacerbates Murine Inflammatory Bowel Disease by Transcriptional Activation of Matrix Metalloproteinase 12" Biomedicines 12, no. 4: 780. https://doi.org/10.3390/biomedicines12040780
APA StyleChen, S.-Y., Fang, C.-Y., Su, B.-H., Chen, H.-M., Huang, S.-C., Wu, P.-T., Shiau, A.-L., & Wu, C.-L. (2024). Early Growth Response Protein 1 Exacerbates Murine Inflammatory Bowel Disease by Transcriptional Activation of Matrix Metalloproteinase 12. Biomedicines, 12(4), 780. https://doi.org/10.3390/biomedicines12040780






