Higher Synovial Immunohistochemistry Reactivity of IL-17A, Dkk1, and TGF-β1 in Patients with Early Psoriatic Arthritis and Rheumatoid Arthritis Could Predict the Use of Biologics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Arthroscopies
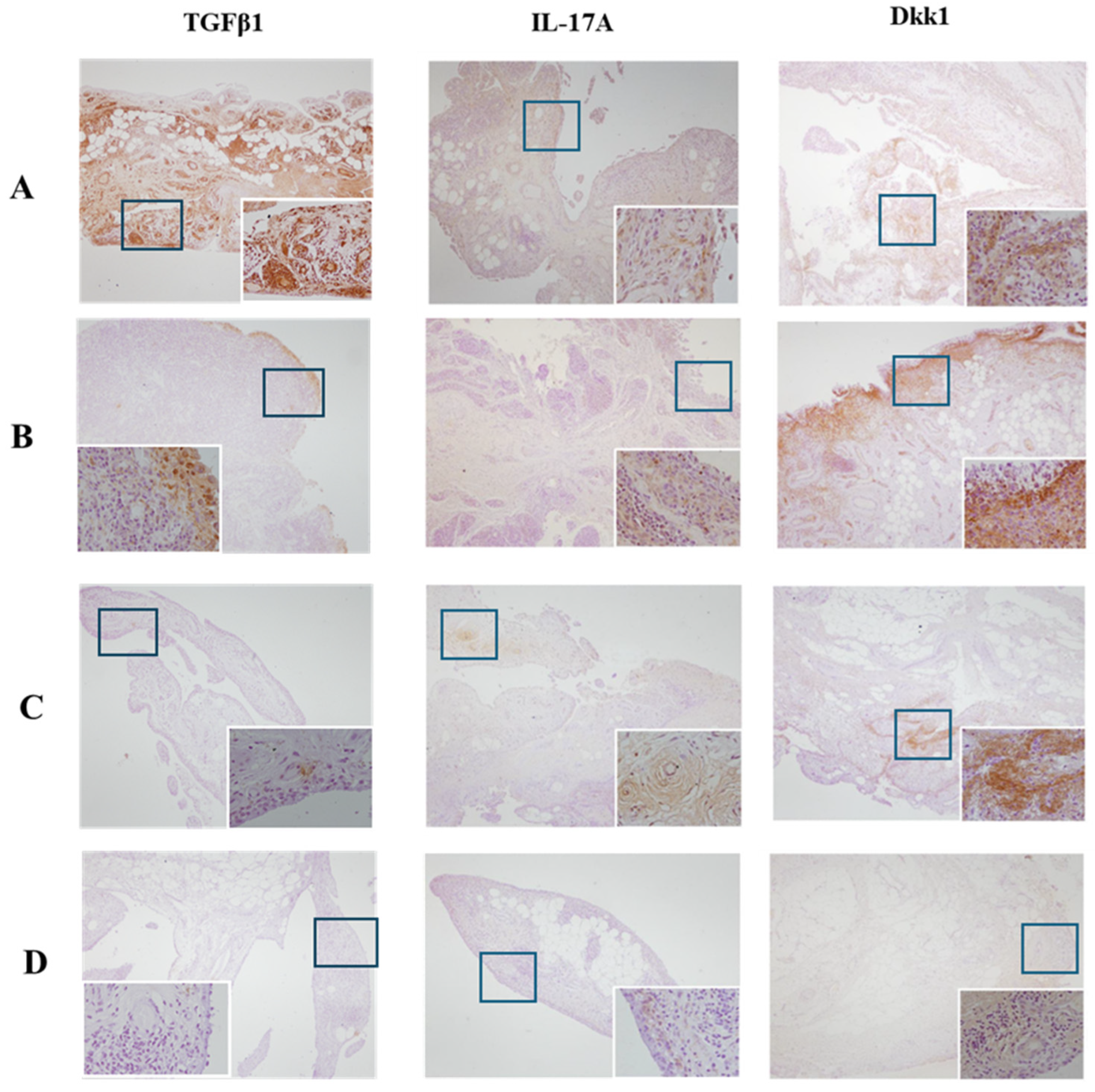
2.2. Histopathological Analysis and Immunohistochemistry: Quantification of Protein Expression in IHC Staining
2.3. Statistical Analysis
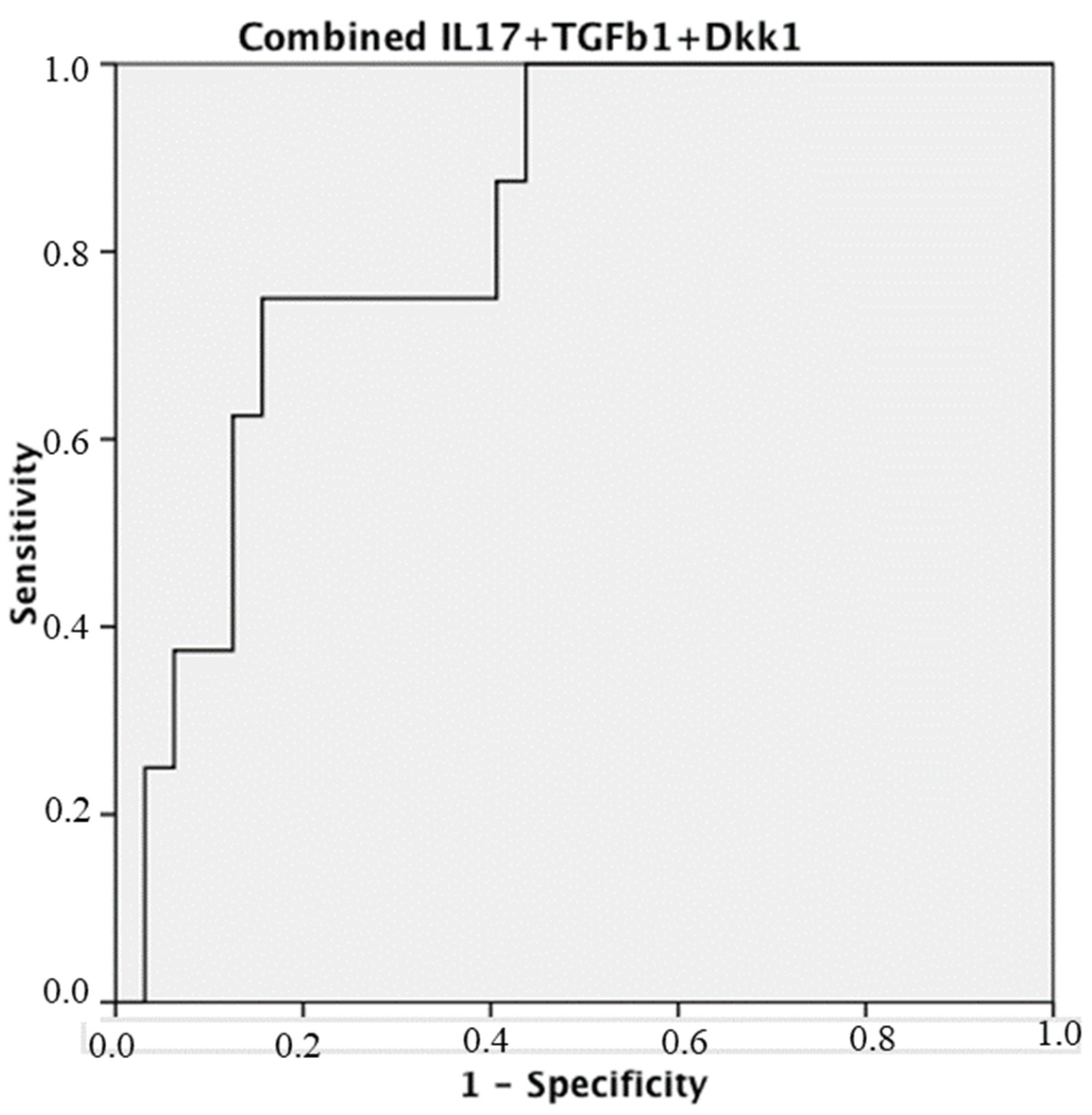
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giles, J.T. Extra-articular manifestations and comorbidity in rheumatoid arthritis: Potential impact of pre-rheumatoid arthritis prevention. Clin. Ther. 2019, 41, 1246–1255. [Google Scholar] [CrossRef]
- Campanati, A.; Marani, A.; Martina, E.; Diotallevi, F.; Radi, G.; Offidani, A. Psoriasis as an Immune-Mediated and Inflammatory Systemic Disease: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 1511. [Google Scholar] [CrossRef]
- Kane, D.; Stafford, L.; Bresnihan, B.; FitzGerald, O. A prospective, clinical and radiological study of early psoriatic arthritis: An early synovitis clinic experience. Rheumatology 2003, 42, 1460–1468. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Hioki, T.; Komine, M.; Ohtsuki, M. Diagnosis and Intervention in Early Psoriatic Arthritis. J. Clin. Med. 2022, 11, 2051. [Google Scholar] [CrossRef]
- Merola, J.F.; Espinoza, L.R.; Fleischmann, R. Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open 2018, 4, e000656. [Google Scholar] [CrossRef]
- Mc Ardle, A.; Flatley, B.; Pennington, S.R.; FitzGerald, O. Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res. Ther. 2015, 17, 141. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. Overseas Ed. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Yap, H.Y.; Tee, S.Z.; Wong, M.M.; Chow, S.K.; Peh, S.C.; Teow, S.Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018, 7, 161. [Google Scholar] [CrossRef]
- Vandooren, B.; Noordenbos, T.; Ambarus, C.; Krausz, S.; Cantaert, T.; Yeremenko, N.; Boumans, M.; Lutter, R.; Tak, P.P.; Baeten, D. Absence of a classically activated macrophage cytokine signature in peripheral spondylarthritis, including psoriatic arthritis. Arthritis Rheum. 2009, 60, 966–975. [Google Scholar] [CrossRef]
- Veale, D.J.; Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 2018, 391, 2273–2284. [Google Scholar] [CrossRef]
- Azuaga, A.B.; Ramírez, J.; Cañete, J.D. Psoriatic Arthritis: Pathogenesis and Targeted Therapies. Int. J. Mol. Sci. 2023, 24, 4901. [Google Scholar] [CrossRef]
- Navarro-Compán, V.; Puig, L.; Vidal, S.; Ramírez, J.; Llamas-Velasco, M.; Fernández-Carballido, C.; Almodóvar, R.; Pinto, J.S.; Galíndez-Aguirregoikoa, E.; Zarco, P.; et al. The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases. Front. Immunol. 2023, 14, 1332177. [Google Scholar] [CrossRef]
- Sharma, M.; Kaveri, S.V.; Bayry, J. Th17 cells, pathogenic or not? TGF-β3 imposes the embargo. Cell Mol. Immunol. 2013, 10, 101–102. [Google Scholar] [CrossRef]
- Esplugues, E.; Huber, S.; Gagliani, N.; Hauser, A.E.; Town, T.; Wan, Y.Y.; O’connor, W.; Rongvaux, A.; Van Rooijen, N.; Haberman, A.M.; et al. Control of TH17 cells occurs in the small intestine. Nature 2011, 475, 514–518. [Google Scholar] [CrossRef]
- Celis, R.; Planell, N.; Fernández-Sueiro, J.L.; Sanmartí, R.; Ramírez, J.; González-Álvaro, I.; Pablos, J.L.; Cañete, J.D. Synovial cytokine expression in psoriatic arthritis and associations with lymphoid neogenesis and clinical features. Arthritis Res. Ther. 2012, 14, R93. [Google Scholar] [CrossRef]
- Kraan, M.C.; Reece, R.J.; Smeets, T.J.; Veale, D.J.; Emery, P.; Tak, P.P. Comparison of synovial tissues from the knee joints and the small joints of rheumatoid arthritis patients: Implication for pathogenesis and evaluation of treatment. Arthritis Rheum. 2002, 46, 2034–2038. [Google Scholar] [CrossRef]
- Tasende, J.A.P.; Fernandez-Moreno, M.; Vazquez-Mosquera, M.E.; Fernandez-Lopez, J.C.; Oreiro-Villar, N.; Santos, F.J.D.T.; Blanco-García, F.J. Increased synovial immunohistochemistry reactivity of TGF-β1 in erosive peripheral psoriatic arthritis. BMC Musculoskelet. Disord. 2023, 24, 246. [Google Scholar]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef]
- Coates, L.C.; Kavanaugh, A.; Mease, P.J.; Soriano, E.R.; Acosta-Felquer, M.L.; Armstrong, A.W.; Bautista-Molano, W.; Boehncke, W.; Campbell, W.; Cauli, A.; et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016, 68, 1060–1071. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis: A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Morel, J.; Combe, B. How to predict prognosis in early rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 2005, 19, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Visser, H.; le Cessie, S.; Vos, K.; Breedveld, F.C.; Hazes, J.M.W. How to diagnose rheumatoid arthritis early: A prediction model for persistent (erosive) arthritis. Arthritis Rheum. 2002, 46, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Balandraud, N.; Boyer, L.; Lafforgue, P.; Pham, T. Biomarkers in psoriatic arthritis: A meta-analysis and systematic review. Front. Immunol. 2022, 13, 1054539. [Google Scholar] [CrossRef] [PubMed]
- Kimak, A.; Robak, E.; Makowska, J.; Woźniacka, A. Psoriatic Arthritis: Development, Detection and Prevention: A Scoping Review. J. Clin. Med. 2023, 12, 3850. [Google Scholar] [CrossRef] [PubMed]
- Burska, A.; Boissinot, M.; Ponchel, F. Cytokines as biomarkers in rheumatoid arthritis. Mediators Inflamm. 2014, 2014, 545493. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.H.; Yoon, S.J.; Kim, M.; Cho, S.; Lim, J.; Park, Y.; Kim, H.S.; Kwon, S.W.; Kim, W.U. Lipidome profile predictive of disease evolution and activity in rheumatoid arthritis. Exp. Mol. Med. 2022, 54, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Jadon, D.R.; Stober, C.; Pennington, S.R.; FitzGerald, O. Applying precision medicine to unmet clinical needs in psoriatic disease. Nat. Rev. Rheumatol. 2020, 16, 609–627. [Google Scholar] [CrossRef]
- Ritchlin, C.T.; Pennington, S.R.; Reynolds, N.J.; FitzGerald, O. Moving Toward Precision Medicine in Psoriasis and Psoriatic Arthritis. J. Rheumatol. Suppl. 2020, 96, 19–24. [Google Scholar] [CrossRef]
- FitzGerald, O.; Behrens, F.; Barton, A.; Bertheussen, H.; Boutouyrie-Dumont, B.; Coates, L.; Davies, O.; de Wit, M.; Fagni, F.; Goodyear, C.S.; et al. Application of clinical and molecular profiling data to improve patient outcomes in psoriatic arthritis. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231192315. [Google Scholar] [CrossRef]
- González-Álvaro, I.; Ortiz, A.M.; Seoane, I.V.; García-Vicuña, R.; Martínez, C.; Gomariz, R.P. Biomarkers predicting a need for intensive treatment in patients with early arthritis. Curr. Pharm. Des. 2015, 21, 170–181. [Google Scholar] [CrossRef]
- Verstappen, S.M.; Lunt, M.; Bunn, D.K.; Scott, D.G.; Symmons, D.P. In patients with early inflammatory polyarthritis, ACPA positivity, younger age and inefficacy of the first non-biological DMARD are predictors for receiving biological therapy: Results from the Norfolk Arthritis Register. Ann. Rheum. Dis. 2011, 70, 1428–1432. [Google Scholar] [CrossRef]
- Teitsma, X.M.; Jacobs, J.W.G.; de Jong, P.H.P.; Hazes, J.M.W.; Weel, A.E.A.M.; Welsing, P.M.J.; Pethö-Schramm, A.; Borm, M.E.A.; van Laar, J.M.; Bijlsma, J.W.J.; et al. Adding baseline protein biomarkers to clinical predictors does not enhance prediction of treatment response to a methotrexate strategy in early rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 142–144. [Google Scholar] [CrossRef]
- Da Mota, L.M.; dos Santos Neto, L.L.; Pereira, I.A.; Burlingame, R.; Ménard, H.A.; Laurindo, I.M. Autoanticorpos como preditores da necessidade de terapia biológica na artrite reumatóide inicial [Autoantibodies as predictors of biological therapy for early rheumatoid arthritis]. Acta Reumatol. Port. 2010, 35, 156–166. [Google Scholar]
- Nam, J.; Villeneuve, E.; Emery, P. The role of biomarkers in the management of patients with rheumatoid arthritis. Curr. Rheumatol. Rep. 2009, 11, 371–377. [Google Scholar] [CrossRef]
- Rosengren, S.; Firestein, G.S.; Boyle, D.L. Measurement of inflammatory biomarkers in synovial tissue extracts by enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 2003, 10, 1002–1010. [Google Scholar] [CrossRef]
- MBromley, D.E. Woolley. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 1984, 27, 857–863. [Google Scholar]
- Pohlers, D.; Beyer, A.; Koczan, D.; Wilhelm, T.; Thiesen, H.J.; Kinne, R.W. Constitutive upregulation of the transforming growth factor-beta pathway in rheumatoid arthritis synovial fibroblasts. Arthritis Res. Ther. 2007, 9, R59. [Google Scholar] [CrossRef]
- Moustakas, A.; Pardali, K.; Gaal, A.; Heldin, C.H. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol. Lett. 2002, 82, 85–91. [Google Scholar] [CrossRef]
- Kehrl, J.H.; Wakefield, L.M.; Roberts, A.B.; Jakowlew, S.; Alvarez-Mon, M.; Derynck, R.; Sporn, M.B.; Fauci, A.S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 1986, 163, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Gil, E.; Criado, G.; Santiago, B.; Dotor, J.; Pablos, J.L.; Galindo, M. Transforming growth factor (TGF)-β signalling is increased in rheumatoid synovium but TGF-β blockade does not modify experimental arthritis. Clin. Exp. Immunol. 2013, 174, 245–255. [Google Scholar] [CrossRef]
- Ruiz de Morales, J.M.G.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Baruuel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef] [PubMed]
- Akitsu, A.; Iwakura, Y. Interleukin-17-producing γδ T (γδ17) cells in inflammatory diseases. Immunology 2018, 155, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.H.; Srenathan, U.; Durham, L.E.; Lalnunhlimi, S.; Steel, K.J.A.; Catrina, A.; Kirkham, B.W.; Taams, L.S. Human in vitro-induced IL-17A+ CD8+ T-cells exert pro-inflammatory effects on synovial fibroblasts. Clin. Exp. Immunol. 2023, 214, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Zhang, Y.; Wang, S.; Kang, S.; Mi, N.; Li, R.; Zou, Y. Identification immune response genes in psoriasis after treatment with secukinumab. BMC Med. Genom. 2023, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Blijdorp, I.C.; van Mens, L.J.J.; Bowcutt, R.; Latuhihin, T.E.; van de Sande, M.G.H.; Shaw, S.; Yeremenko, N.G.; Baeten, D.L.P. Interleukin 17A and IL-17F Expression and Functional Responses in Rheumatoid Arthritis and Peripheral Spondyloarthritis. J. Rheumatol. 2020, 47, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Crotti, C.; Cincinelli, G.; Di Taranto, R.; Amati, A.; Ferrito, M.; Varenna, M.; Caporali, R. Bone Involvement in Rheumatoid Arthritis and Spondyloarthritis: An Updated Review. Biology 2023, 12, 1320. [Google Scholar] [CrossRef] [PubMed]
- Yeremenko, N.; Polzer, K.; Noordenhos, T.; Zwerina, J.; Tak, P.P.; Schett, G.; Baeten, D. DKK-1, a master regulator of bone remodeling, is abundantly expressed in peripheral inflammatory arthritis. Arthritis Rheum. 2008, 58 (Suppl. S9), S161–S950. [Google Scholar]
- Biedroń, G.; Czepiel, M.; Siedlar, M.; Korkosz, M. Serum concentration of Dickkopf-related protein 1 (DKK1) in psoriatic arthritis in the context of bone remodelling. Rheumatol. Int. 2023, 43, 2175–2183. [Google Scholar] [CrossRef]
- Dalbeth, N.; Pool, B.; Smith, T.; Callon, K.E.; Lobo, M.; Taylor, W.J.; Jones, P.B.; Cornish, J.; McQueen, F.M. Circulating mediators of bone remodelling in psoriatic arthritis: Implications for disordered osteoclastogenesis and bone erosion. Arthritis Res. Ther. 2010, 12, R164. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Wang, M.; Xia, Q.; Yang, J.; Wu, M.; Han, R.; Chen, M.; Hu, X.; Yuan, Y.; et al. The serum level of Dickkopf-1 in patients with rheumatoid arthritis: A systematic review and meta-analysis. Int. Immunopharmacol. 2018, 59, 227–232. [Google Scholar] [CrossRef]
- Klavdianou, K.; Liossis, S.N.; Daoussis, D. Dkk1: A key molecule in joint remodelling and fibrosis. Mediterr. J. Rheumatol. 2017, 28, 174–182. [Google Scholar] [CrossRef]
- Klavdianou, K.; Kanellou, A.; Daoussis, D. Molecular Mechanisms of New Bone Formation in Axial Spondyloarthritis. Mediterr. J. Rheumatol. 2022, 33 (Suppl. S1), 115–125. [Google Scholar] [CrossRef]
- Chung, Y.; Li, Z.C.; Sun, X.L.; Liu, Y.Y.; Shao, M.; Gan, Y.Z.; Li, Y.M.; Li, Y.H.; Zhang, X.W. Elevated serum Dickkopf-1 is a biomarker for bone erosion in patients with psoriatic arthritis. Chin. Med. J. 2021, 134, 2583–2588. [Google Scholar] [CrossRef]
- Karmakar, S.; Kay, J.; Gravallese, E.M. Bone damage in rheumatoid arthritis: Mechanistic insights and approaches to prevention. Rheum. Dis. Clin. N. Am. 2010, 36, 385–404. [Google Scholar] [CrossRef]
- Yuliasih, Y.; Permatasari, A.; Rahmawati, L.D.; Wahyudi, M.I.; Nisa’, N. The Increasing Level of DKK-1 as a New Bone Formation Factor in Patients with Early Spondyloarthritis. Autoimmune Dis. 2023, 2023, 5543234. [Google Scholar] [CrossRef]
- Silvagni, E.; Bortoluzzi, A.; Ciancio, G.; Govoni, M. Biological and synthetic target DMARDs in psoriatic arthritis. Pharmacol. Res. 2019, 149, 104473. [Google Scholar] [CrossRef]
- Hutton, J.; Mease, P.; Jadon, D. Horizon scan: State-of-the-art therapeutics for psoriatic arthritis. Best. Pract. Res. Clin. Rheumatol. 2022, 36, 101809. [Google Scholar] [CrossRef]
- Magee, C.; Jethwa, H.; FitzGerald, O.M.; Jadon, D.R. Biomarkers predictive of treatment response in psoriasis and psoriatic arthritis: A systematic review. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X2110140. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; Mclnnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Garaffoni, C.; Adinolfi, A.; Bortoluzzi, A.; Filippou, G.; Giollo, A.; Sakellariou, G.; Sirotti, S.; Ughi, N.; Scirè, C.A.; Silvagni, E. Novel insights into the management of rheumatoid arthritis: One year in review 2022. Ann. Rheum. Dis. 2022, 40, 1247–1257. [Google Scholar] [CrossRef]

| Total n = 44 * | PsA n = 13 | RA n = 9 | OA n = 18 | r-axSpA n = 4 | p ** |
|---|---|---|---|---|---|
| Male, n (%) | 10 (66.7) | 4 (50.0) | 7 (30.9) | 4 (100) | 0.036 |
| Age, years | 48.0 (40.5–55.5) | 42.5 (32.2–58.7) | 69.0 (64.5–74.7) | 57.5 (49.0–65.2) | <0.0001 |
| Time course of the disease, years | 3.0 (1.0–9.0) | 2.0 (1.0–5.7) | 12.5 (6.0–18.5) | 19.5 (8.0–31.7) | 0.002 |
| Tender joint count | 2 (1–3) | 1 (1–2) | 1 (1–1) | 1 (1–1.7) | 0.033 |
| Swollen joint count | 1 (1–2) | 1 (1–1.7) | 1 (1–1) | 1 (1–1) | 0.236 |
| ESR, mm/first hour | 20 (8–36) | 15 (7–29) | 22 (14–33) | 45 (23–69) | 0.181 |
| C-reactive protein, mg/dL | 0.70 (0.29–1.74) | 0.36 (0.21–0.61) | 0.44 (0.25–0.53) | 2.20 (0.52–3.80) | 0.05 |
| Uricaemia, mg/dL | 4.8 (4.1–6.3) | 5.7 (4.2–7.7) | 5.5 (4.5–6.0) | 5.2 (4.2–6.9) | 0.715 |
| RF and/or ACPA+, n (%) | 0 | 4 (50) | 0 | 0 | 0.001 |
| Erosions on X-rays, n (%) | 4 (26.7) | 0 | 0 | 0 | 0.032 |
| Sacroiliitis on X-rays, n (%) | 0 | 0 | 0 | 4 (100%) | 0.001 |
| Metotrexate, n (%) | 8 (53.3) | 0 | 0 | 0 | 0.004 |
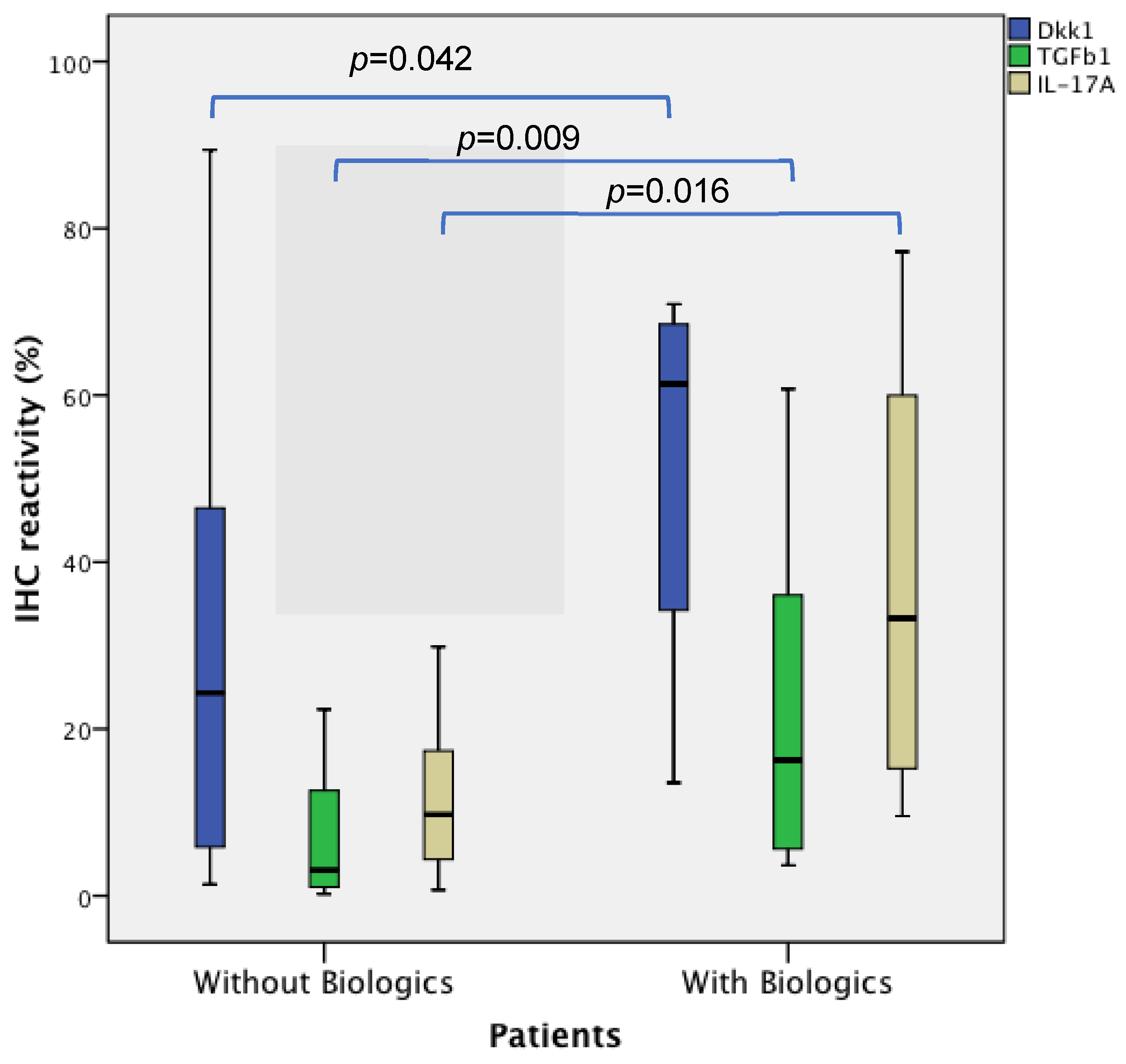
| IHC * | Controls n = 20 | PsA/RA with Biologic n = 6 | PsA/RA without Biologics n = 14 | p ** |
|---|---|---|---|---|
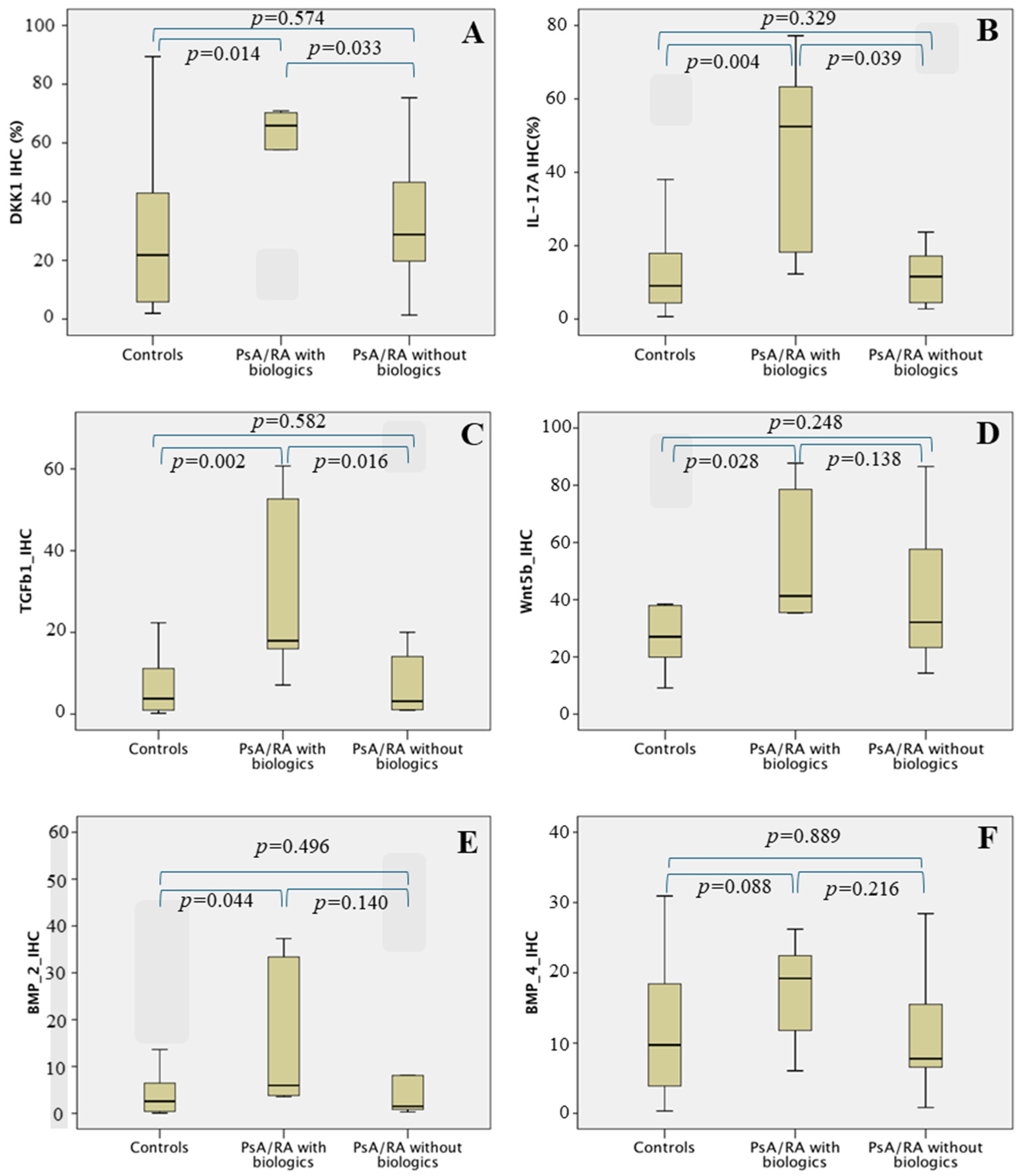
| DKK1 | 21.82 (5.36–43.37) | 61.37 (30.51–69.40) | 24.31 (5.36–46.54) | 0.042 |
| BMP2 | 2.57 (0.38–7.29) | 4.18 (3.62–26.98) | 1.82 (0.59–8.13) | 0.127 |
| BMP4 | 9.72 (3.44–18.91) | 16.54 (10.32–21.71) | 8.00 (4.81–18.91) | 0.260 |
| Wnt5b | 27.06 (18.80–38.16) | 36.32 (26.07–70.28) | 30.81 (22.53–50.58) | 0.067 |
| TGFβ1 | 3.82 (0.94–11.55) | 16.24 (4.88–44.33) | 3.06 (1.06–12.94) | 0.009 |
| IL17A | 9.07 (4.30–18.09) | 33.26 (13.78–61.63) | 9.75 (4.31–17.47) | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto-Tasende, J.A.; Fernandez-Moreno, M.; Rego Perez, I.; Fernandez-Lopez, J.C.; Oreiro-Villar, N.; De Toro Santos, F.J.; Blanco-García, F.J. Higher Synovial Immunohistochemistry Reactivity of IL-17A, Dkk1, and TGF-β1 in Patients with Early Psoriatic Arthritis and Rheumatoid Arthritis Could Predict the Use of Biologics. Biomedicines 2024, 12, 815. https://doi.org/10.3390/biomedicines12040815
Pinto-Tasende JA, Fernandez-Moreno M, Rego Perez I, Fernandez-Lopez JC, Oreiro-Villar N, De Toro Santos FJ, Blanco-García FJ. Higher Synovial Immunohistochemistry Reactivity of IL-17A, Dkk1, and TGF-β1 in Patients with Early Psoriatic Arthritis and Rheumatoid Arthritis Could Predict the Use of Biologics. Biomedicines. 2024; 12(4):815. https://doi.org/10.3390/biomedicines12040815
Chicago/Turabian StylePinto-Tasende, Jose A., Mercedes Fernandez-Moreno, Ignacio Rego Perez, J. Carlos Fernandez-Lopez, Natividad Oreiro-Villar, F. Javier De Toro Santos, and Francisco J. Blanco-García. 2024. "Higher Synovial Immunohistochemistry Reactivity of IL-17A, Dkk1, and TGF-β1 in Patients with Early Psoriatic Arthritis and Rheumatoid Arthritis Could Predict the Use of Biologics" Biomedicines 12, no. 4: 815. https://doi.org/10.3390/biomedicines12040815
APA StylePinto-Tasende, J. A., Fernandez-Moreno, M., Rego Perez, I., Fernandez-Lopez, J. C., Oreiro-Villar, N., De Toro Santos, F. J., & Blanco-García, F. J. (2024). Higher Synovial Immunohistochemistry Reactivity of IL-17A, Dkk1, and TGF-β1 in Patients with Early Psoriatic Arthritis and Rheumatoid Arthritis Could Predict the Use of Biologics. Biomedicines, 12(4), 815. https://doi.org/10.3390/biomedicines12040815








