Atorvastatin Treatment Significantly Increased the Concentration of Bone Marrow-Derived Mononuclear Cells and Transcutaneous Oxygen Pressure and Lowered the Pain Scale after Bone Marrow Cells Treatment in Patients with “No-Option” Critical Limb Ischaemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Bone Marrow Cell Isolation and Administration
2.3. Pre-Procedure Assessment and Follow-Up
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Baseline Information and Endpoints
3.2. Characteristics of Responders and Non-Responders
3.3. Characteristics of Super-Responders and Super-Non-Responders
3.4. Characteristics of Transplanted Bone Marrow Cells
3.5. Prognostic Factors and Predictors of BMCs Treatment Outcomes
3.6. Effect of Atorvastatin Therapy before BMCs Treatment on the Outcomes of Stem Cell Treatment
3.7. Parameters of Limb Ischaemia after BMCs Delivery in Atorvastatin or Non-Atorvastatin Group
3.8. Characteristics of Transplanted Bone Marrow Cells in ATV and Non-ATV Group
3.9. Effect of RAS-Acting Agents Therapy Prior to BMCs Treatment on the Outcomes of Stem Cell Treatment
3.10. Parameters of Limb Ischaemia after BMCs Delivery in RAS or Non-RAS Group
3.11. Characteristics of Transplanted Bone Marrow Cells in RAS or Non-RAS Group
3.12. Effect of Atorvastatin and RAS-Acting Agents Therapy Prior to BMCs Treatment on the Outcomes of Stem Cell Treatment
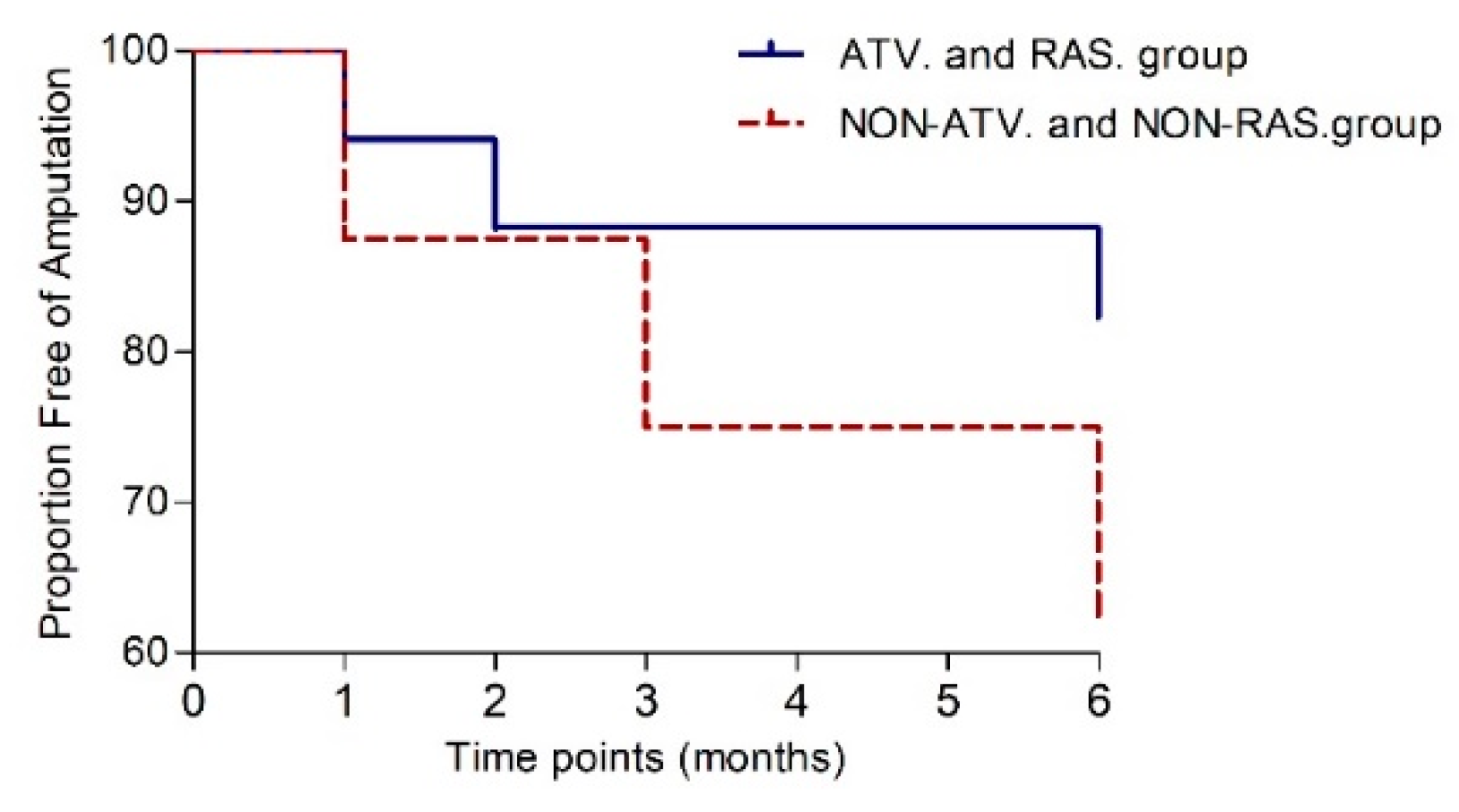
3.13. Parameters of Limb Ischaemia after BMCs Delivery in ATV and RAS Group or Non-ATV and Non-RAS Group
3.14. Characteristics of Transplanted Bone Marrow Cells in ATV and RAS Group or Non-ATV and Non-RAS Group
3.15. Results of Spearman Correlation Analysis for the Investigated Treatment Option
4. Discussion
4.1. Summary of the Results
4.2. Prognostic Factors of the Therapeutic Responses to Autologous BMCs Treatment
4.3. Subgroup Analysis of Treatment Approach before BMCs Transplant
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABI | ankle-brachial index |
| ACEIs | angiotensin-converting enzyme inhibitors |
| ARBs | angiotensin receptor blockers |
| ATV | atorvastatin |
| AUC | area under the ROC curve |
| BMCs | bone marrow cells |
| BM-MNCs | bone marrow-derived mononuclear cells |
| CI | confidence interval |
| CLI | critical limb ischaemia |
| CRP | C-reactive protein |
| EPC | endothelial progenitor cells |
| HR | hazard ratio |
| MI | myocardial infarct |
| MSCs | mesenchymal stem cells |
| NO-CLI | “no-option” critical limb ischaemia |
| non-RAS | non-renin-angiotensin system |
| OR | odds ratio |
| PAD | peripheral arterial disease |
| RAS | renin-angiotensin system |
| ROC | receiver operating characteristics |
| SD | standard deviation |
| SEM | standard error |
| TASC | Transatlantic Inter-Society Consensus |
| TcPO2 | transcutaneous oxygen pressure |
| VAS | Visual analogue scale |
References
- Paola, L.D.; Cimaglia, P.; Carone, A.; Scavone, G.; Boscarino, G.; Bernucci, D.; Sbarzaglia, P.; Censi, S.; Ferrari, R.; Campo, G. Limb salvage in diabetic patients with no-option critical limb ischemia: Outcomes of a specialized center experience. Diabet. Foot Ankle 2019, 10, 1696012. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef]
- Wahid, S.F.A.; Ismail, N.A.; Jamaludin, W.F.W.; Muhamad, N.A.; Hamid, M.K.A.A.; Harunarashid, H.; Lai, N.M. Autologous cells derived from different sources and administered using different regimens for “no-option” critical lower limb ischaemia patients. Cochrane Database Syst. Rev. 2018, 2018, CD010747. [Google Scholar] [CrossRef]
- Hassanshahi, M.; Khabbazi, S.; Peymanfar, Y.; Hassanshahi, A.; Hosseini-Khah, Z.; Su, Y.W.; Xian, C.J. Critical limb ischemia: Current and novel therapeutic strategies. J. Cell. Physiol. 2019, 234, 14445–14459. [Google Scholar] [CrossRef]
- Uccioli, L.; Meloni, M.; Izzo, V.; Giurato, L.; Merolla, S.; Gandini, R. Critical limb ischemia: Current challenges and future prospects. Vasc. Heal. Risk Manag. 2018, 14, 63–74. [Google Scholar] [CrossRef]
- Fang, G.; Jiang, X.; Fang, Y.; Pan, T.; Liu, H.; Ren, B.; Wei, Z.; Gu, S.; Chen, B.; Jiang, J.; et al. Autologous peripheral blood-derived stem cells transplantation for treatment of no-option angiitis-induced critical limb ischemia: 10-year management experience. Stem Cell Res. Ther. 2020, 11, 458. [Google Scholar] [CrossRef]
- Lu, D.; Jiang, Y.; Deng, W.; Zhang, Y.; Liang, Z.; Wu, Q.; Jiang, X.; Zhang, L.; Gao, F.; Cao, Y.; et al. Long-Term Outcomes of BMMSC Compared with BMMNC for Treatment of Critical Limb Ischemia and Foot Ulcer in Patients with Diabetes. Cell Transplant. 2019, 28, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Procházka, V.; Gumulec, J.; Jalůvka, F.; Šalounová, D.; Jonszta, T.; Czerný, D.; Krajča, J.; Urbanec, R.; Klement, P.; Martinek, J.; et al. Cell Therapy, a New Standard in Management of Chronic Critical Limb Ischemia and Foot Ulcer. Cell Transplant. 2010, 19, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.V.; Kövesd, Z.; Cserepes, J.; Daróczy, J.; Belkin, M.; Acsády, G. Peripheral blood-derived autologous stem cell therapy for the treatment of patients with late-stage peripheral artery disease—Results of the short- and long-term follow-up. Cytotherapy 2013, 15, 1245–1252. [Google Scholar] [CrossRef]
- Ozturk, A.; Kucukardali, Y.; Tangi, F.; Erikci, A.; Uzun, G.; Bashekim, C.; Sen, H.; Terekeci, H.; Narin, Y.; Ozyurt, M.; et al. Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J. Diabetes Complicat. 2012, 26, 29–33. [Google Scholar] [CrossRef]
- Teraa, M.; Sprengers, R.W.; Schutgens, R.E.; Slaper-Cortenbach, I.C.; Van Der Graaf, Y.; Algra, A.; Van Der Tweel, I.; Doevendans, P.A.; Mali, W.P.; Moll, F.L.; et al. Effect of Repetitive Intra-Arterial Infusion of Bone Marrow Mononuclear Cells in Patients with No-Option Limb Ischaemia: The Randomized, Double-Blind, Placebo-Controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) Trial. Circulation 2015, 131, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.H.; Krankenberg, H.; Balzer, J.O.; Kalka, C.; Baumgartner, I.; Schlüter, M.; Tonn, T.; Seeger, F.; Dimmeler, S.; Lindhoff-Last, E.; et al. Intraarterial Administration of Bone Marrow Mononuclear Cells in Patients with Critical Limb Ischemia. Circ. Cardiovasc. Interv. 2011, 4, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Xu, Q.; Luo, R.; Gao, J.; Chen, H.; Deng, Y.; Li, Y.; Wang, Y.; Yuan, W.; Wu, X. Atorvastatin treatment improves effects of implanted mesenchymal stem cells: Meta-analysis of animal models with acute myocardial infarction. BMC Cardiovasc. Disord. 2015, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Kokkinidis, D.G.; Arfaras-Melainis, A.; Giannopoulos, S.; Katsaros, I.; Jawaid, O.; Jonnalagadda, A.K.; Parikh, S.A.; Secemsky, E.A.; Giri, J.; Kumbhani, D.J.; et al. Statin therapy for reduction of cardiovascular and limb-related events in critical limb ischemia: A systematic review and meta-analysis. Vasc. Med. 2020, 25, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Samakova, A.; Gazova, A.; Sabova, N.; Valaskova, S.; Jurikova, M.; Kyselovic, J. The pi3k/Akt Pathway Is Associated With Angiogenesis, Oxidative Stress and Survival of Mesenchymal Stem Cells in Pathophysiologic Condition in Ischaemia 2019;68:8. Physiol. Res. 2019, 68, 8. [Google Scholar]
- Ridker, P.M.; Bhatt, D.L.; Pradhan, A.D.; Glynn, R.J.; MacFadyen, J.G.; Nissen, S.E. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: A collaborative analysis of three randomised trials. Lancet 2023, 401, 1293–1301. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Baker, J.; Ernst, C.; Johnston, K.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef] [PubMed]
- Shirbaghaee, Z.; Hassani, M.; Heidari Keshel, S.; Soleimani, M. Emerging roles of mesenchymal stem cell therapy in patients with critical limb ischemia. Stem Cell Res. Ther. 2022, 13, 462. [Google Scholar] [CrossRef]
- Weem, S.P.; Teraa, M.; de Borst, G.; Verhaar, M.; Moll, F. Bone Marrow derived Cell Therapy in Critical Limb Ischemia: A Meta-analysis of Randomized Placebo Controlled Trials. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 775–783. [Google Scholar] [CrossRef]
- Jaluvka, F.; Ihnat, P.; Madaric, J.; Vrtkova, A.; Janosek, J.; Prochazka, V. Current Status of Cell-Based Therapy in Patients with Critical Limb Ischemia. Int. J. Mol. Sci. 2020, 21, 8999. [Google Scholar] [CrossRef] [PubMed]
- Compagna, R.; Amato, B.; Massa, S.; Amato, M.; Grande, R.; Butrico, L.; de Franciscis, S.; Serra, R. Cell Therapy in Patients with Critical Limb Ischemia. Stem Cells Int. 2015, 2015, 931420. [Google Scholar] [CrossRef] [PubMed]
- Klepanec, A.; Mistrik, M.; Altaner, C.; Valachovicova, M.; Olejarova, I.; Slysko, R.; Balazs, T.; Urlandova, T.; Hladikova, D.; Liska, B.; et al. No Difference in Intra-Arterial and Intramuscular Delivery of Autologous Bone Marrow Cells in Patients with Advanced Critical Limb Ischemia. Cell Transplant. 2012, 21, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Tatsumi, T.; Murohara, T.; Imaizumi, T.; Katsuda, Y.; Ito, M.; Saito, Y.; Uemura, S.; Suzuki, H.; Fukumoto, S.; et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am. Hear. J. 2008, 156, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Benoit, E.; O’Donnell, T.F.; Patel, A.N.; O’Donnell, J.T.F. Safety and Efficacy of Autologous Cell Therapy in Critical Limb Ischemia: A Systematic Review. Cell Transplant. 2013, 22, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chullikana, A.; Parakh, R.; Desai, S.; Das, A.; Gottipamula, S.; Krishnamurthy, S.; Anthony, N.; Pherwani, A.; Majumdar, A.S. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow-derived mesenchymal stem cell in critical limb ischaemia. J. Transl. Med. 2013, 11, 143. [Google Scholar] [CrossRef]
- Zafarghandi, M.R.; Ravari, H.; Aghdami, N.; Namiri, M.; Moazzami, K.; Taghiabadi, E.; Fazel, A.; Pournasr, B.; Farrokhi, A.; Sharifian, R.A.; et al. Safety and efficacy of granulocyte–colony-stimulating factor administration following autologous intramuscular implantation of bone marrow mononuclear cells: A randomized controlled trial in patients with advanced lower limb ischemia. Cytotherapy 2010, 12, 783–791. [Google Scholar] [CrossRef]
- Leenstra, B.; Wijnand, J.; Verhoeven, B.; Koning, O.; Teraa, M.; Verhaar, M.C.; de Borst, G.J. Applicability of Transcutaneous Oxygen Tension Measurement in the Assessment of Chronic Limb-Threatening Ischemia. Angiology 2019, 71, 208–216. [Google Scholar] [CrossRef]
- Pan, T.; Liu, H.; Fang, Y.; Wei, Z.; Gu, S.; Fang, G.; Liu, Y.; Luo, Y.; Guo, D.; Xu, X.; et al. Predictors of responders to mononuclear stem cell-based therapeutic angiogenesis for no-option critical limb ischemia. Stem Cell Res. Ther. 2019, 10, 15. [Google Scholar] [CrossRef]
- Got, I. Transcutaneous oxygen pressure (TcPO2): Advantages and limitations. Diabetes Metab. 1998, 24, 379–384. [Google Scholar]
- Yang, C.; Weng, H.; Chen, L.; Yang, H.; Luo, G.; Mai, L.; Jin, G.; Yan, L. Transcutaneous oxygen pressure measurement in diabetic foot ulcers: Mean values and cut-point for wound healing. J. Wound Ostomy Cont. Nurs. Publ. Wound Ostomy Cont. Nurses Soc. 2013, 40, 585–589. [Google Scholar] [CrossRef]
- Madaric, J.; Klepanec, A.; Valachovicova, M.; Mistrik, M.; Bucova, M.; Olejarova, I.; Necpal, R.; Madaricova, T.; Paulis, L.; Vulev, I. Characteristics of responders to autologous bone marrow cell therapy for no-option critical limb ischemia. Stem Cell Res. Ther. 2016, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, N.; Nishinari, M.; Ohtani, S.; Kanai, A.; Noda, C.; Hirata, M.; Miyamoto, A.; Watanabe, M.; Minamino, T.; Izumi, T.; et al. Clinical features and predictors of patients with critical limb ischemia who responded to autologous mononuclear cell transplantation for therapeutic angiogenesis. Hear. Vessel. 2017, 32, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, S.; Annex, B.H. Biomarkers and Genetics in Peripheral Artery Disease. Clin. Chem. 2017, 63, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Ridker, P.M.; Belkin, M.; Hamdan, A.D.; Pomposelli, F.; Logerfo, F.; Creager, M.A.; Conte, M.S. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J. Vasc. Surg. 2007, 45, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kuliszewski, M.A.; Li, S.-H.; Szmitko, P.E.; Zucco, L.; Wang, C.-H.; Badiwala, M.V.; Mickle, D.A.; Weisel, R.D.; Fedak, P.W.; et al. C-Reactive Protein Attenuates Endothelial Progenitor Cell Survival, Differentiation, and Function: Further Evidence of a Mechanistic Link Between C-Reactive Protein and Cardiovascular Disease. Circulation 2004, 109, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, F.M.; Kajikawa, M.; Takaeko, Y.; Kishimoto, S.; Hashimoto, H.; Maruhashi, T.; Nakashima, A.; Wahid, S.F.S.A.; Higashi, Y. Relationship between cell number and clinical outcomes of autologous bone-marrow mononuclear cell implantation in critical limb ischemia. Sci. Rep. 2020, 10, 19891. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Barrera-Ramirez, J.; Ranasinghe, I.; Pilon, S.; Sy, R.; Fergusson, D.; Allan, D.S. Use of Statins to Augment Progenitor Cell Function in Preclinical and Clinical Studies of Regenerative Therapy: A Systematic Review. Stem Cell Rev. Rep. 2016, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhao, Y.; Yang, J.; Wang, W.; Wang, X.; Ling, L.; Ge, L. Simvastatin Promotes Dental Pulp Stem Cell–induced Coronal Pulp Regeneration in Pulpotomized Teeth. J. Endod. 2016, 42, 1049–1054. [Google Scholar] [CrossRef]
- Tahamtan, S.; Shirban, F.; Bagherniya, M.; Johnston, T.P.; Sahebkar, A. The effects of statins on dental and oral health: A review of preclinical and clinical studies. J. Transl. Med. 2020, 18, 155. [Google Scholar] [CrossRef]
- Bodewes, T.C.; Darling, J.D.; O’Donnell, T.F.; Deery, S.E.; Shean, K.E.; Mittleman, M.A.; Moll, F.L.; Schermerhorn, M.L. Long-term mortality benefit of renin-angiotensin system inhibitors in patients with chronic limb-threatening ischemia undergoing vascular intervention. J. Vasc. Surg. 2018, 67, 800–808. [Google Scholar] [CrossRef] [PubMed]

| All Patients (N = 33) | Responders (N = 22) | Non-Responders (N = 11) | p Value | |
|---|---|---|---|---|
| Age (years) | 64.9 ± 10 | 67.4 ± 9.3 | 59.8 ± 10.7 | 0.014 * |
| Sex (males) | 31 (94%) | 21 (95%) | 10 (91%) | 1.000 |
| Rutherford class (1–6) | 5.0 ± 0.30 | 5.0 | 4.9 ± 0.54 | 0.208 |
| Body mass index (kg/m2) | 27 ± 4.1 | 27.2 ± 3.8 | 27.6 ± 4.9 | 0.756 |
| Risk factors of limb ischaemia | ||||
| Diabetes mellitus | 11 (33%) | 7 (32%) | 4 (36%) | 1.000 |
| Arterial hypertension | 27 (82%) | 18 (82%) | 9 (82%) | 1.000 |
| Hyperlipidaemia | 23 (70%) | 15 (68%) | 8 (73%) | 1.000 |
| Smoker | 10 (30%) | 6 (27%) | 4 (36%) | 0.696 |
| Blood examination | ||||
| CRP (mg/L) | 13.3 ± 21.3 | 8.1 ± 12.3 | 23.3 ± 30.5 | 0.013 * |
| Creatinine (µmol/L) | 95.9 ± 34.6 | 95.4 ± 35.7 | 96.7 ± 34.2 | 0.946 |
| Treatment history | ||||
| Statins | 22 (67%) | 16 (73%) (NNT 5.5) | 6 (55%) | 0.255 |
| Antiplatelet drugs | 24 (73%) | 17 (77%) | 7 (64%) | 0.438 |
| Aspirin | 16 (48%) | 11 (50%) | 5 (45%) | 0.4074 |
| Clopidogrel | 17 (52%) | 10 (45%) | 7 (64%) | 0.1711 |
| RAS-acting agents | 20 (61%) | 16 (73%) (NNT 2.75) | 4 (36%) | 0.065 |
| Statins and RAS-acting agents | 17 (52%) | 13 (59%) (NNT4.4) | 4 (36%) | 0.282 |
| Naftidrofuryl | 28 (85%) | 18 (82%) | 10 (91%) | 0.643 |
| Post-MI | 31 (94%) | 22 (100%) | 9 (82%) | 0.104 |
| Parameters of limb ischaemia | ||||
| TcPO2 < 10 mmHg | 19 (58%) | 11 (50%) | 8 (73%) | 0.278 |
| ABI | 0.51 ± 0.38 | 0.57 ± 0.35 | 0.37 ± 0.43 | 0.183 |
| Pain scale (0–10) | 5.75 ± 1.6 | 5.62 ± 1.75 | 6.14 ± 1.07 | 0.366 |
| Parameters of Limb Ischaemia | Responders | Non-Responders | ||||
|---|---|---|---|---|---|---|
| Baseline (N = 22) | Six Months (N = 17) | p Value | Baseline (N = 11) | Six Months (N = 11) | p Value | |
| Rutherford class (1–6) | 5.0 ± 0.30 | 4.2 ± 1.0 | 0.008 * | 4.9 ± 0.54 | 5.55 ± 0.52 | 0.059 |
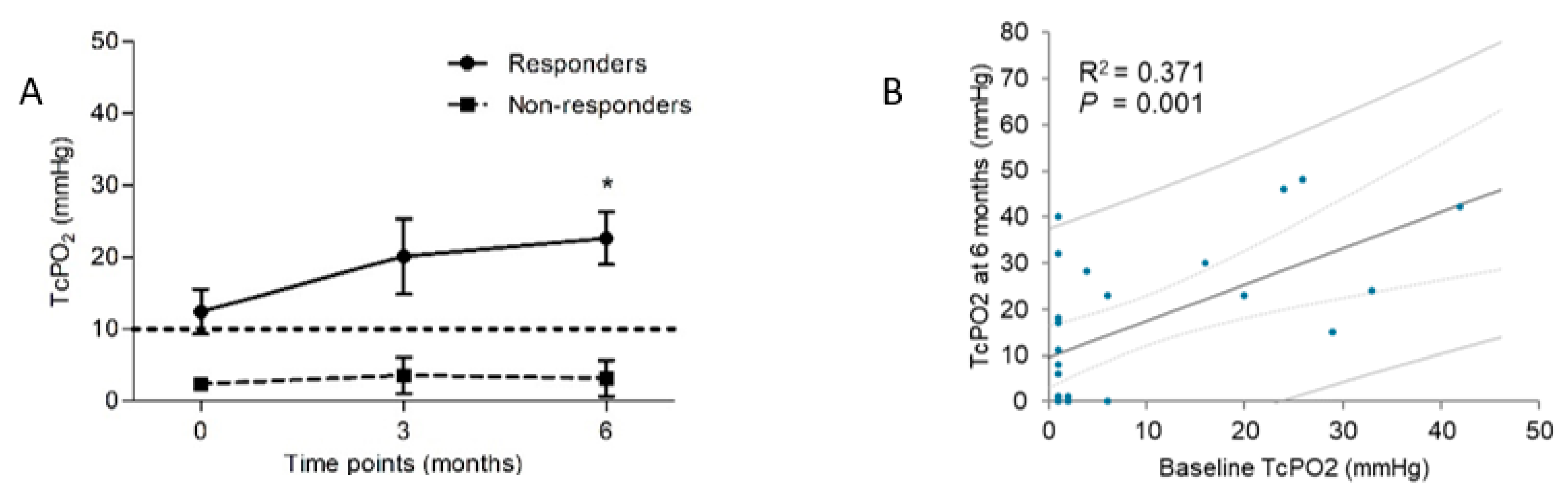
| TcPO2 (mmHg) | 12.5 ± 14.0 | 22.7 ± 15.2 | 0.008 * | 2.4 ± 1.77 | 3.18 ± 8.4 | 0.573 |
| ABI | 0.57 ± 0.35 | 0.60 ± 0.34 | 0.480 | 0.37 ± 0.43 | 0.029 ± 0.08 | 0.043 * |
| Pain scale (0–10) | 5.62 ± 1.75 | 1.78 ± 1.22 | <0.001 * | 6.14 ± 1.07 | 6.86 ± 3.76 | 0.445 |
| All Patients (N = 33) | Super-Responders (N = 13) | Super-Non-Responders (N = 6) | p Value | |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 64.9 ± 10 | 64.8 ± 10.4 | 66.8 ± 4.0 | 0.690 |
| Sex (males) | 31 (94%) | 12 (92%) | 6 (100%) | 1.000 |
| Rutherford class (1–6) | 5.0 ± 0.30 | 5.0 | 4.83 ± 0.75 | 0.175 |
| Body mass index (kg/m2) | 27 ± 4.1 | 27.1 ± 4.04 | 25.6 ± 3.76 | 0.450 |
| Risk factors of limb ischaemia | ||||
| Diabetes mellitus (N, %) | 11 (33%) | 5 (38%) | 3 (50%) | 1.000 |
| Arterial hypertension | 27 (82%) | 11 (85%) | 6 (100%) | 1.000 |
| Hyperlipidaemia | 23 (70%) | 10 (77%) | 5 (83%) | 1.000 |
| Smoker | 10 (30%) | 3 (23%) | 2 (33%) | 1.000 |
| Blood examination | ||||
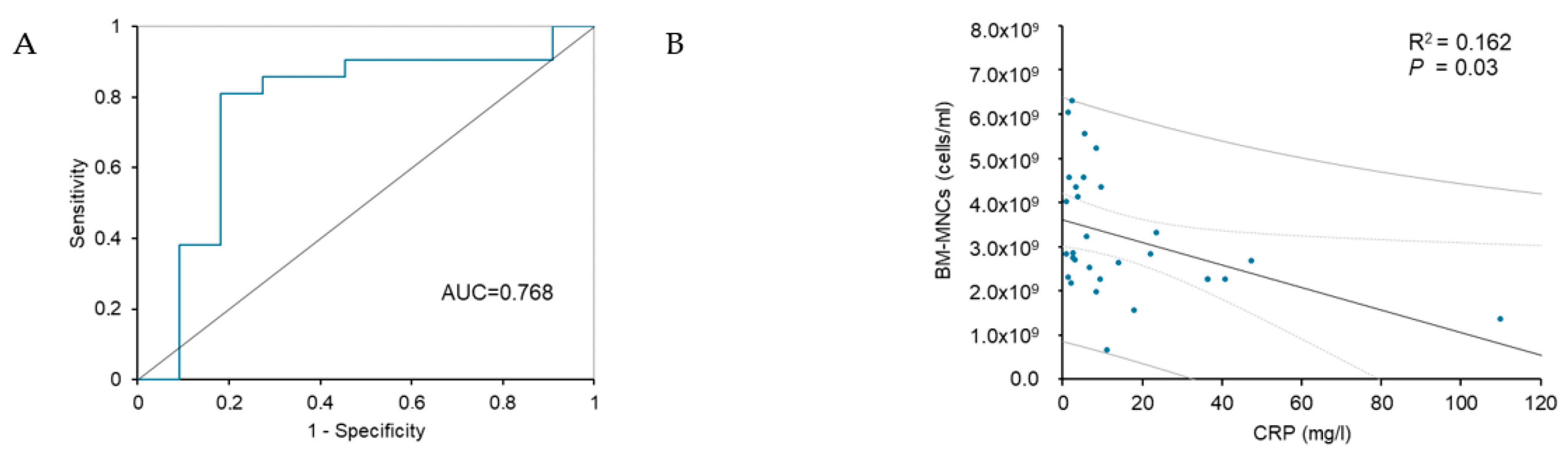
| CRP (mg/L) | 13.3 ± 21.3 | 5.2 ± 3.45 | 36.6 ± 37.01 | <0.001 * |
| Creatinine (µmol/L) | 95.9 ± 34.6 | 98.7 ± 41.02 | 102.5 ± 39.78 | 0.914 |
| Treatment history | ||||
| Statins (N, %) | 22 (67%) | 11 (85%) | 3 (50%) | 0.262 |
| Antiplatelet drugs | 24 (72%) | 12 (92%) | 3 (50%) | 0.071 |
| Aspirin | 16 (48%) | 8 (62%) | 2 (33%) | 0.1463 |
| Clopidogrel | 17 (52%) | 8 (62%) | 3 (50%) | 0.3361 |
| RAS-acting agents | 20 (61%) | 9 (69%) | 3 (50%) | 0.617 |
| Statins and RAS-acting agents | 17 (52%) | 9 (69%) | 2 (33%) | 0.319 |
| Naftidrofuryl | 28 (85%) | 12 (92%) | 6 (100%) | 1.000 |
| Post-MI | 31 (94%) | 12 (92%) | 5 (83%) | 1.000 |
| Parameters of limb ischaemia | ||||
| TcPO2 < 10 mmHg | 19 (58%) | 6 (46%) | 4 (67%) | 0.629 |
| ABI | 0.51 ± 0.38 | 0.57 ± 0.37 | 0.18 ±0.36 | 0.101 |
| Pain scale (0–10) | 5.75 ± 1.6 | 5.61 ± 1.71 | 6.33 ± 0.58 | 0.907 |
| Group Analysis | Responders (N = 22) | Non-Responders (N = 11) | p Value |
|---|---|---|---|
| BM-MNCs (109 cells/mL) | 3.53 ± 1.5 | 2.86 ± 1.13 | 0.213 |
| Viability of BM-MNCs (%) | 99.70 ± 0.3 | 99.27 ± 0.86 | 0.464 |
| MSCs (104 cells/mL) | 0.79 ± 0.61 | 1.46 ± 1.56 | 0.123 |
| Subgroup analysis | super-responders (limb salvage and complete ischemic wound healing, N = 13) | super-non-responders (major limb amputation, N = 6) | p value |
| BM-MNCs (109 cells/mL) | 3.68 ± 1.51 | 2.26 ± 0.74 | 0.049 * |
| Viability of BM-MNCs (%) | 99.71 ± 0.32 | 99.05 ± 0.94 | 0.263 |
| MSCs (104 cells/mL) | 0.83 ± 0.52 | 1.68 ± 2.14 | 0.639 |
| Candidate Variable | Responder | Super-Responder | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age ≥ 50 years | 1.097 (0.993–1.212) | 0.056 | 0.972 (0.844–1.095) | 0.689 |
| Rutherford class | 2.803 (0.221–35.552) | 0.411 | 3.026 (0.222–41.289) | 0.389 |
| Body mass index (kg/m2) | 0.977 (0.819–1.166) | 0.800 | 1.118 (0.847–1.476) | 0.412 |
| Smoker | 0.808 (0.169–3.858) | 0.653 | 0.500 (0.050–4.978) | 0.712 |
| CRP (mg/L) | 0.958 (0.905–1.014) | 0.044 * | 0.563 (0.242–1.312) | <0.0001 * |
| Creatinine (µmol/L) | 0.990 (0.965–1.017) | 0.919 | 0.984 (0.950–1.020) | 0.843 |
| TcPO2 (mmHg) | 1.134 (0.933–1.380) | 0.021 * | 1.104 (0.926–1.317) | 0.071 |
| ABI | 5.084 (0.347–126.09) | 0.199 | 46.635 (0.393–5533.96) | 0.035 |
| BM-MNCs (109 cells/mL) | 1.560 (0.896–3.138) | 0.189 | 1.0 (0.999–1.0) | 0.035 |
| All Patients (N = 33) | ATV Group (N = 22) | Non-ATV Group (N = 11) | p Value | |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 64.9 ± 10 | 64.3 ± 11 | 65.9 ± 9.2 | 0.983 |
| Sex (males) | 31 (94%) | 21 (95%) | 10 (91%) | 1.000 |
| Rutherford class (1–6) | 5 ± 0.30 | 5 ± 0.21 | 5 ± 0.45 | 0.829 |
| Body mass index (kg/m2) | 27 ± 4.1 | 27.5 ± 3.8 | 26.9 ± 4.9 | 0.080 |
| Risk factors of limb ischaemia | ||||
| Diabetes mellitus (N, %) | 11 (33%) | 8 (22%) | 3 (27%) | 0.709 |
| Arterial hypertension | 27 (82%) | 19 (86%) | 8 (73%) | 0.375 |
| Hyperlipidaemia | 23 (70%) | 17 (77%) | 6 (55%) | 0.240 |
| Smoker | 10 (30%) | 5 (23%) | 5 (45%) | 0.240 |
| Blood examination | ||||
| CRP (mg/L) | 13.3 ± 21.3 | 12.8 ± 24.6 | 14.2 ± 14 | 0.341 |
| Creatinine (µmol/L) | 95.9 ± 34.6 | 97 ± 37.6 | 94 ± 29.7 | 0.977 |
| Parameters of limb ischaemia | ||||
| TcPO2 < 10 mmHg | 19 (58%) | 11 (50%) | 8 (73%) | 0.278 |
| ABI | 0.51 ± 0.38 | 0.51 ± 0.36 | 0.52 ± 0.47 | 0.951 |
| Pain scale (0–10) | 5.75 ± 1.6 | 5.74 ± 1.52 | 5.78 ± 1.86 | 0.951 |
| ATV Group (N = 22) | Non-ATV Group (N = 11) | |||||||
|---|---|---|---|---|---|---|---|---|
| before BMCs | Three Months | Six Months | p Value | before BMCs | Three Months | Six Months | p Value | |
| Rutherford category | 4.95 ± 0.21 | 4.95 ± 0.60 | 4.55 ± 1.1 | 0.092 | 5.0 ± 0.45 | 5.0 ± 0.78 | 5.0 ± 0.93 | 0.655 |
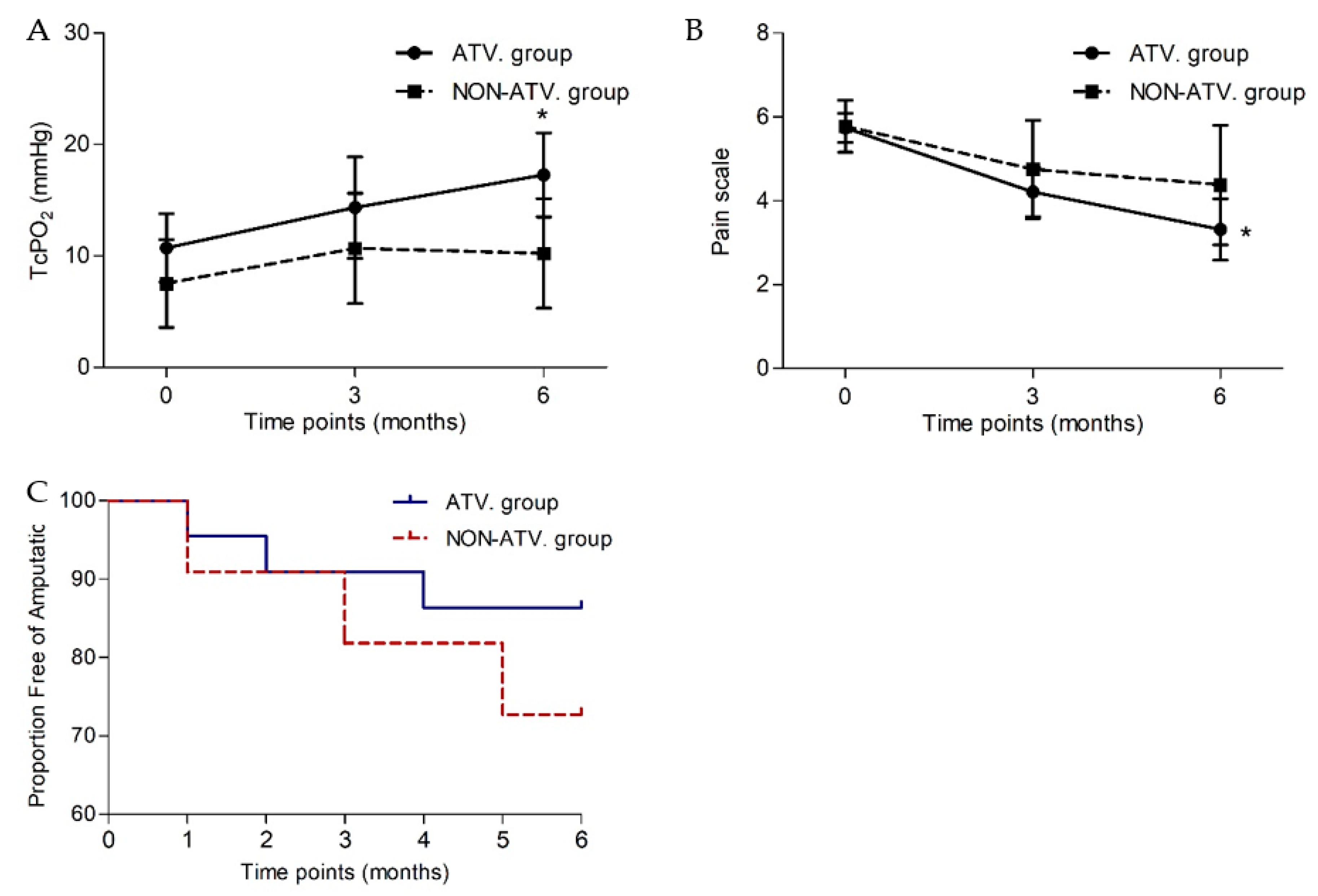
| TcPO2 (mmHg) | 10.72 ± 13.01 | 14.4 ± 19.9 | 17.3 ± 16.5 | 0.015 * | 7.5 ± 12.46 | 10.7 ± 14.9 | 10.2 ± 14.7 | 0.611 |
| ABI | 0.51 ± 0.36 | 0.44 ± 0.41 | 0.42 ± 0.34 | 0.532 | 0.52 ± 0.47 | 0.47 ± 0.47 | 0.38 ± 0.51 | 0.655 |
| Pain scale (0–10) | 5.74 ± 1.52 | 4.45 ± 2.7 | 3.6 ± 3.35 | 0.004 * | 5.78 ± 1.86 | 5.2 ± 3.42 | 4.8 ± 4.07 | 0.202 |
| ATV Group (N = 22) | non-ATV Group (N = 11) | p Value | |
|---|---|---|---|
| BM-MNCs (109 cells/mL) | 3.64 ± 1.53 | 2.58 ± 0.73 | 0.038 |
| Viability of BM-MNCs (%) | 99.5 ± 0.57 | 99.6 ± 0.67 | 0.537 |
| MSCs (104 cells/mL) | 0.79 ± 0.52 | 1.47 ± 1.62 | 0.281 |
| All Patients (N = 33) | RAS Group (N = 20) | Non-RAS Group (N = 13) | p Value | |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 64.9 ± 10 | 68.65 ± 8.82 | 59.0 ± 9.80 | 0.006 * |
| Sex (males) | 31 (94%) | 19 (95%) | 12 (92%) | 1.000 |
| Rutherford class (1–6) | 5 ± 0.30 | 4.95 ± 0.22 | 5.0 ± 0.41 | 0.652 |
| Body mass index (kg/m2) | 27 ± 4.1 | 26.56 ± 2.85 | 28.47 ± 5.57 | 0.385 |
| Risk factors of limb ischaemia | ||||
| Diabetes mellitus (N, %) | 11 (33%) | 8 (40%) | 3 (23%) | 0.277 |
| Arterial hypertension | 27 (82%) | 19 (95%) | 8 (62%) | 0.025 * |
| Hyperlipidaemia | 23 (70%) | 14 (70%) | 9 (69%) | 1.000 |
| Smoker | 10 (30%) | 4 (21%) | 6 (46%) | 0.244 |
| Blood examination | ||||
| CRP (mg/L) | 13.3 ± 21.3 | 16.09 ± 26.1 | 9.26 ± 10.77 | 0.367 |
| Creatinine (µmol/L) | 95.9 ± 34.6 | 100.1 ± 37.77 | 89.69 ± 29.8 | 0.258 |
| Parameters of limb ischaemia | ||||
| TcPO2 < 10 mmHg | 19 (58%) | 10 (50%) | 9 (69%) | 0.310 |
| ABI | 0.51 ± 0.38 | 0.59 ± 0.37 | 0.38 ± 0.37 | 0.165 |
| Pain scale (0–10) | 5.75 ± 1.6 | 5.56 ± 1.42 | 6.10 ± 1.91 | 0.426 |
| Parameters of Limb Ischaemia | RAS Group | Non-RAS Group | ||||
|---|---|---|---|---|---|---|
| Baseline (N = 20) | Six Months (N = 18) | p Value | Baseline (N = 13) | Six Months (N = 10) | p Value | |
| Rutherford class (1–6) | 4.95 ± 0.22 | 4.61 ± 0.41 | 0.164 | 5.0 ± 0.41 | 4.8 ± 1.03 | 0.276 |
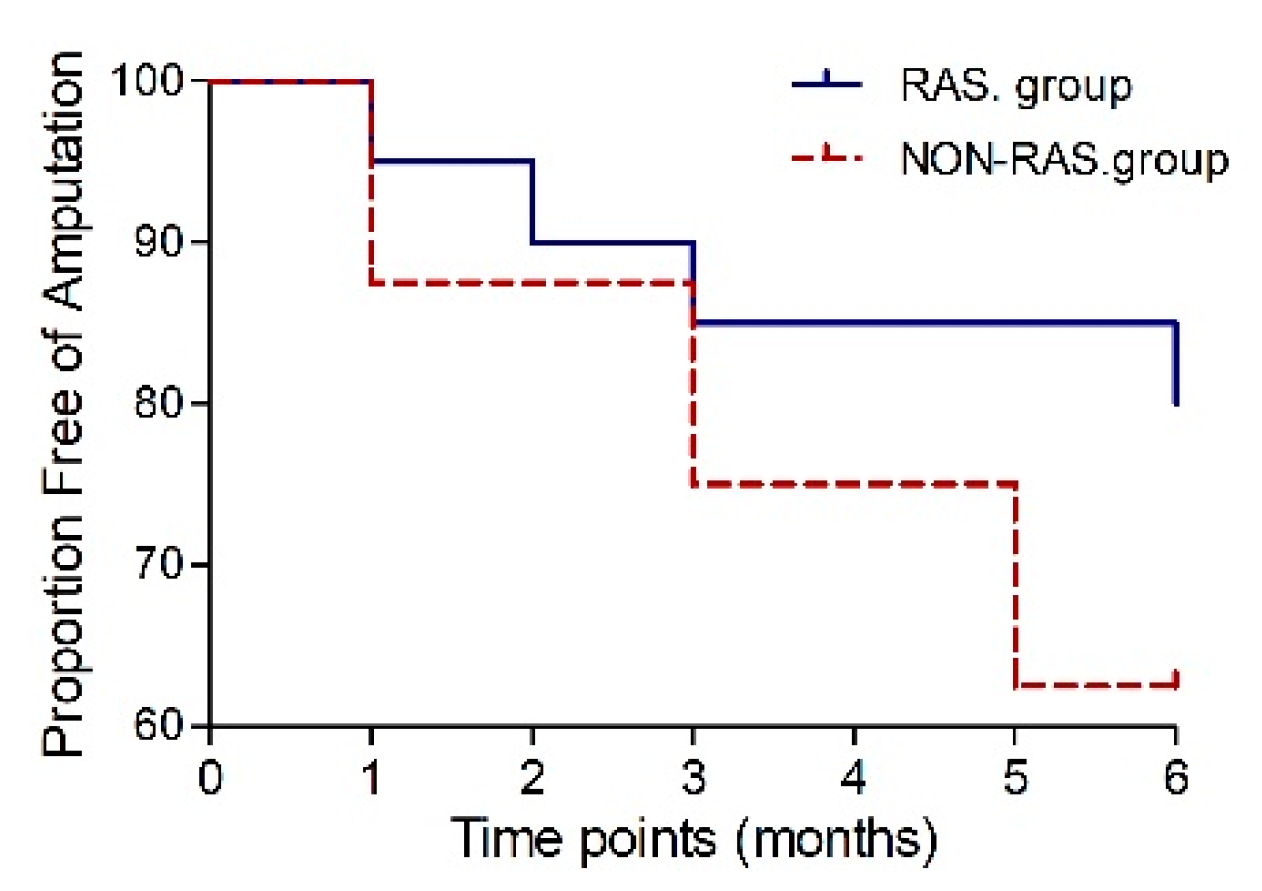
| TcPO2 (mmHg) | 11.4 ± 13.5 | 15.5 ± 14.5 | 0.077 | 6.7 ± 11.4 | 14.3 ± 18.8 | 0.201 |
| ABI | 0.59 ± 0.37 | 0.53 ± 0.41 | 0.408 | 0.38 ± 0.37 | 0.38 ± 0.26 | 0.500 |
| Pain scale (0–10) | 5.56 ± 1.42 | 3.33 ± 3.25 | 0.005 * | 6.10 ± 1.91 | 4.22 ± 3.83 | 0.139 |
| RAS Group (N = 18) | non-RAS Group (N = 12) | p Value | |
|---|---|---|---|
| BM-MNCs (109 cells/mL) | 3.44 ± 1.55 | 3.05 ± 1.61 | 0.462 |
| Viability of BM-MNCs (%) | 99.6 ± 0.49 | 99.5 ± 0.75 | 0.859 |
| MSCs (104 cells/mL) | 0.86 ± 0.60 | 1.23 ± 1.50 | 0.730 |
| All Patients (N = 33) | ATV and RAS Group (N = 17) | Non-ATV and Non-RAS Group (N = 8) | p Value | |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 64.9 ± 10 | 67.41 ± 9 | 62.25 ± 7.94 | 0.026 * |
| Sex (males) | 31 (94%) | 16 (94%) | 7 (88%) | 1.000 |
| Rutherford class (1–6) | 5 ± 0.30 | 4.94 ± 0.24 | 5 ± 0.54 | 0.817 |
| Body mass index (kg/m2) | 27 ± 4.1 | 26.37 ± 2.73 | 26.87 ± 5.50 | 0.763 |
| Risk factors of limb ischaemia | ||||
| Diabetes mellitus (N, %) | 11 (33%) | 7 (41%) | 2 (25%) | 0.661 |
| Arterial hypertension | 27 (82%) | 16 (94%) | 5 (63%) | 0.081 |
| Hyperlipidaemia | 23 (70%) | 13 (76%) | 5 (63%) | 0.640 |
| Smoker | 10 (30%) | 4 (24%) | 5 (63%) | 0.087 |
| Blood examination | ||||
| CRP (mg/L) | 13.3 ± 21.3 | 16.06 ± 27.51 | 13.43 ± 12.05 | 0.653 |
| Creatinine (µmol/L) | 95.9 ± 34.6 | 100.7 ± 41.31 | 93.06 ± 35.29 | 0.425 |
| Parameters of limb ischaemia | ||||
| TcPO2 < 10 mmHg | 9.6 ± 12.7 | 8 (47%) | 6 (75%) | 0.234 |
| ABI | 0.51 ± 0.38 | 0.54 ± 0.33 | 0.34 ± 0.32 | 0.540 |
| Pain scale (0–10) | 5.75 ± 1.6 | 5.80 ± 1.42 | 6.50 ± 1.87 | 0.063 |
| Parameters of Limb Ischaemia | ATV and RAS Group | Non-ATV and Non-RAS Group | ||||
|---|---|---|---|---|---|---|
| Baseline (N = 17) | Six Months (N = 15) | p Value | Baseline (N = 8) | Six Months (N = 7) | p Value | |
| Rutherford class (1–6) | 4.94 ± 0.24 | 4.56 ± 1.15 | 0.164 | 5.0 ± 0.54 | 5.0 ± 1.10 | 0.655 |
| TcPO2 (mmHg) | 11.6 ± 13.5 | 16.5 ± 15.0 | 0.033 * | 6.3 ± 11.8 | 10.9 ± 16.4 | 0.344 |
| ABI | 0.54 ± 0.33 | 0.47 ± 0.36 | 0.409 | 0.34 ± 0.32 | 0.18 ± 0.32 | 0.650 |
| Pain scale (0–10) | 5.80 ± 1.42 | 3.50 ± 3.43 | 0.009 * | 6.50 ± 1.87 | 5.17 ± 4.45 | 0.279 |
| ATV and RAS Group (N = 17) | Non-ATV and Non-RAS Group (N = 8) | p Value | |
|---|---|---|---|
| BM-MNCs (109 cells/mL) | 3.56 ± 1.60 | 2.60 ± 0.81 | 0.064 |
| Viability of BM-MNCs (%) | 99.6 ± 0.51 | 99.6 ± 0.75 | 0.642 |
| MSCs (104 cells/mL) | 0.78 ± 0.56 | 1.53 ± 1.89 | 0.549 |
| Responder | Age | CRP | RAS-Acting Agents Treatment | ||
|---|---|---|---|---|---|
| Responder | Spearman’s r | - | 0.430 | −0.442 | 0.351 |
| p value | - | 0.012 | 0.011 | 0.045 | |
| Statin treatment | Spearman’s r | - | - | - | 0.482 |
| p value | - | - | - | 0.005 | |
| RAS-acting agents treatment | Spearman’s r | 0.351 | 0.566 | - | - |
| p value | 0.045 | 0.0006 | - | - | |
| Statin and RAS-acting agents treatment | Spearman’s r | 0.618 | 0.454 | - | 1 |
| p value | 0.001 | 0.023 | - | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyselovic, J.; Adamičková, A.; Gažová, A.; Valášková, S.; Chomaničová, N.; Červenák, Z.; Madaric, J. Atorvastatin Treatment Significantly Increased the Concentration of Bone Marrow-Derived Mononuclear Cells and Transcutaneous Oxygen Pressure and Lowered the Pain Scale after Bone Marrow Cells Treatment in Patients with “No-Option” Critical Limb Ischaemia. Biomedicines 2024, 12, 922. https://doi.org/10.3390/biomedicines12040922
Kyselovic J, Adamičková A, Gažová A, Valášková S, Chomaničová N, Červenák Z, Madaric J. Atorvastatin Treatment Significantly Increased the Concentration of Bone Marrow-Derived Mononuclear Cells and Transcutaneous Oxygen Pressure and Lowered the Pain Scale after Bone Marrow Cells Treatment in Patients with “No-Option” Critical Limb Ischaemia. Biomedicines. 2024; 12(4):922. https://doi.org/10.3390/biomedicines12040922
Chicago/Turabian StyleKyselovic, Jan, Adriana Adamičková, Andrea Gažová, Simona Valášková, Nikola Chomaničová, Zdenko Červenák, and Juraj Madaric. 2024. "Atorvastatin Treatment Significantly Increased the Concentration of Bone Marrow-Derived Mononuclear Cells and Transcutaneous Oxygen Pressure and Lowered the Pain Scale after Bone Marrow Cells Treatment in Patients with “No-Option” Critical Limb Ischaemia" Biomedicines 12, no. 4: 922. https://doi.org/10.3390/biomedicines12040922
APA StyleKyselovic, J., Adamičková, A., Gažová, A., Valášková, S., Chomaničová, N., Červenák, Z., & Madaric, J. (2024). Atorvastatin Treatment Significantly Increased the Concentration of Bone Marrow-Derived Mononuclear Cells and Transcutaneous Oxygen Pressure and Lowered the Pain Scale after Bone Marrow Cells Treatment in Patients with “No-Option” Critical Limb Ischaemia. Biomedicines, 12(4), 922. https://doi.org/10.3390/biomedicines12040922







