A Novel RP-UHPLC-MS/MS Approach for the Determination of Tryptophan Metabolites Derivatized with 2-Bromo-4′-Nitroacetophenone
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Standards and Reagents Preparation
2.3. Calibration Solutions
2.4. Sample Treatment and Derivatization Procedure
2.5. UHPLC-MS/MS Analysis
2.6. Method Validation
2.7. Data Analysis
3. Results and Discussion
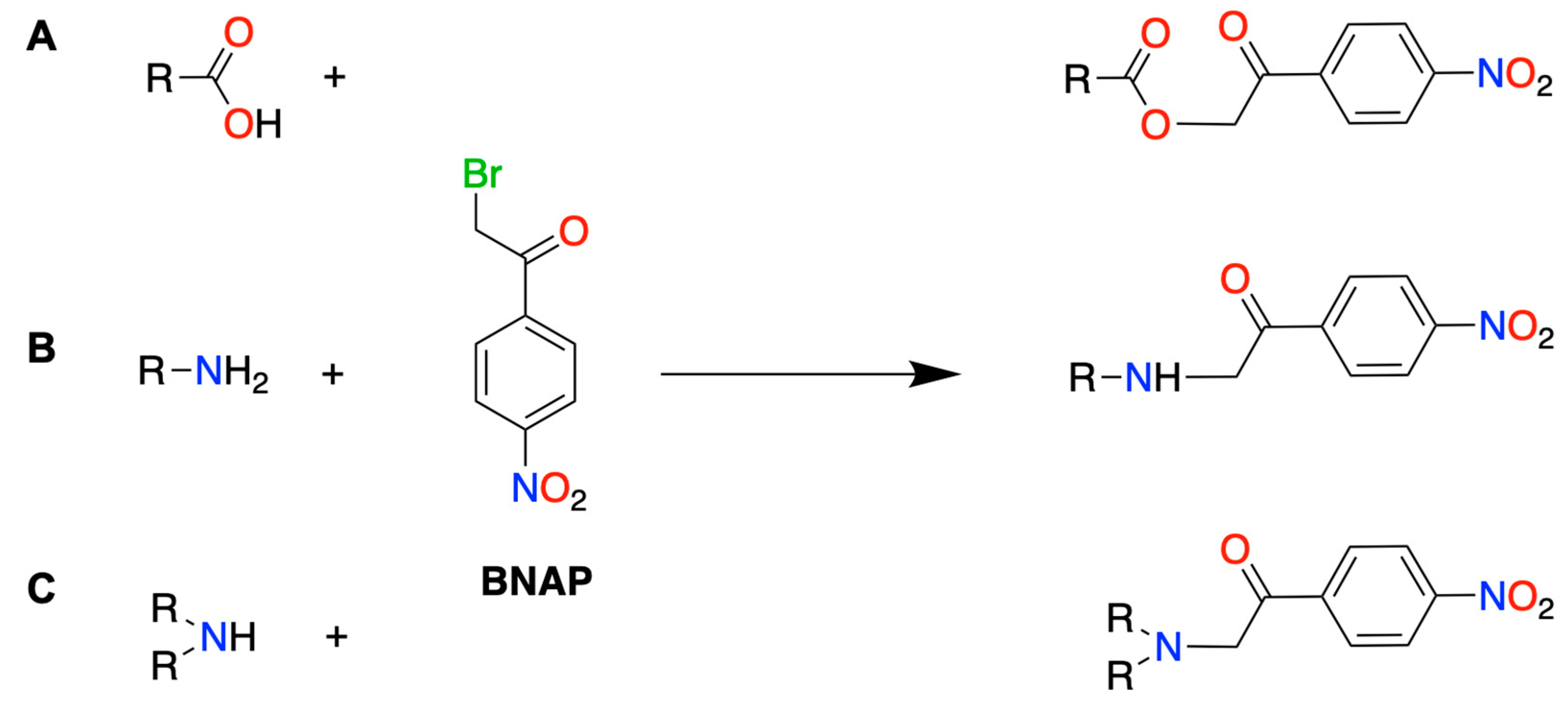
3.1. Optimization of Derivatization
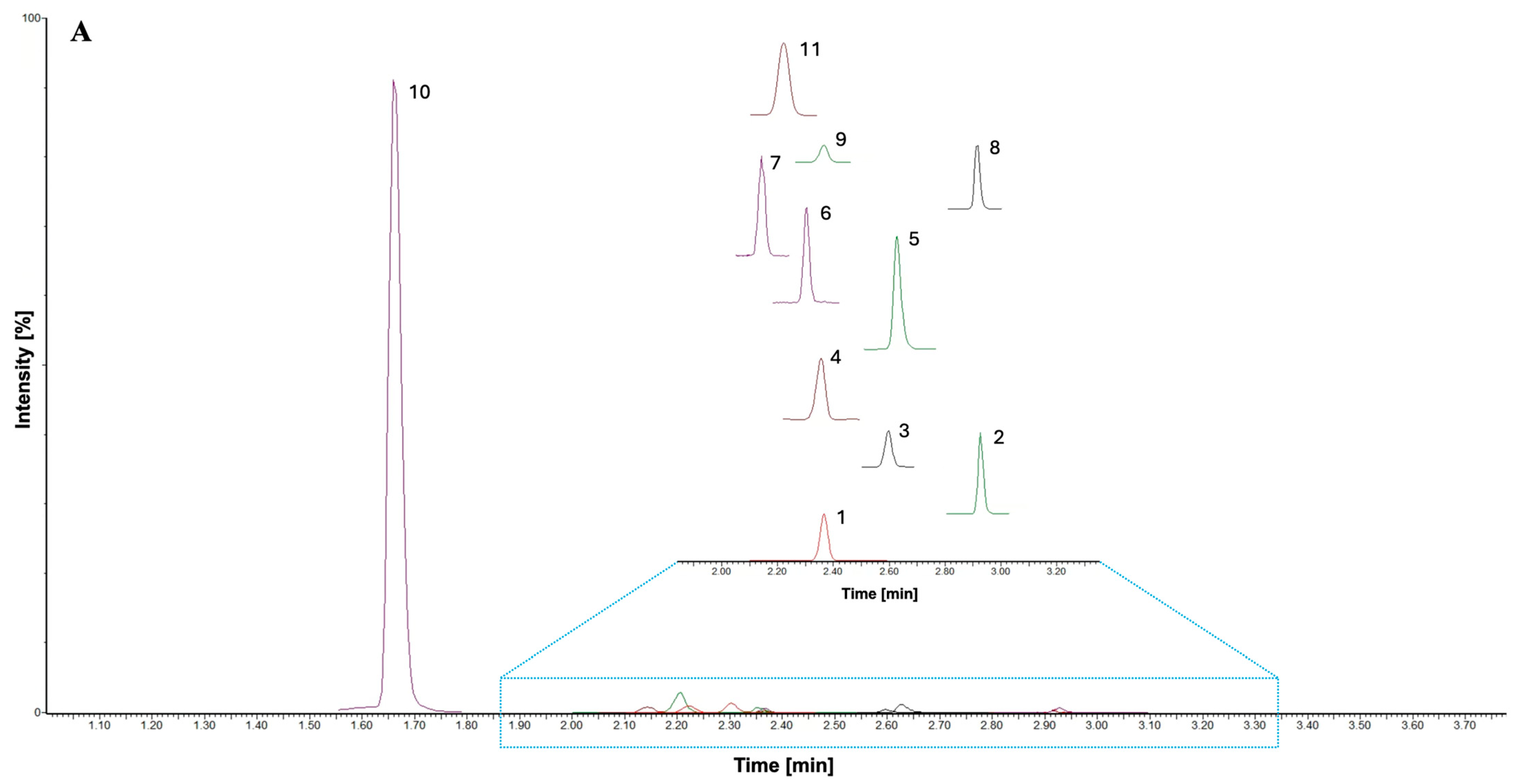
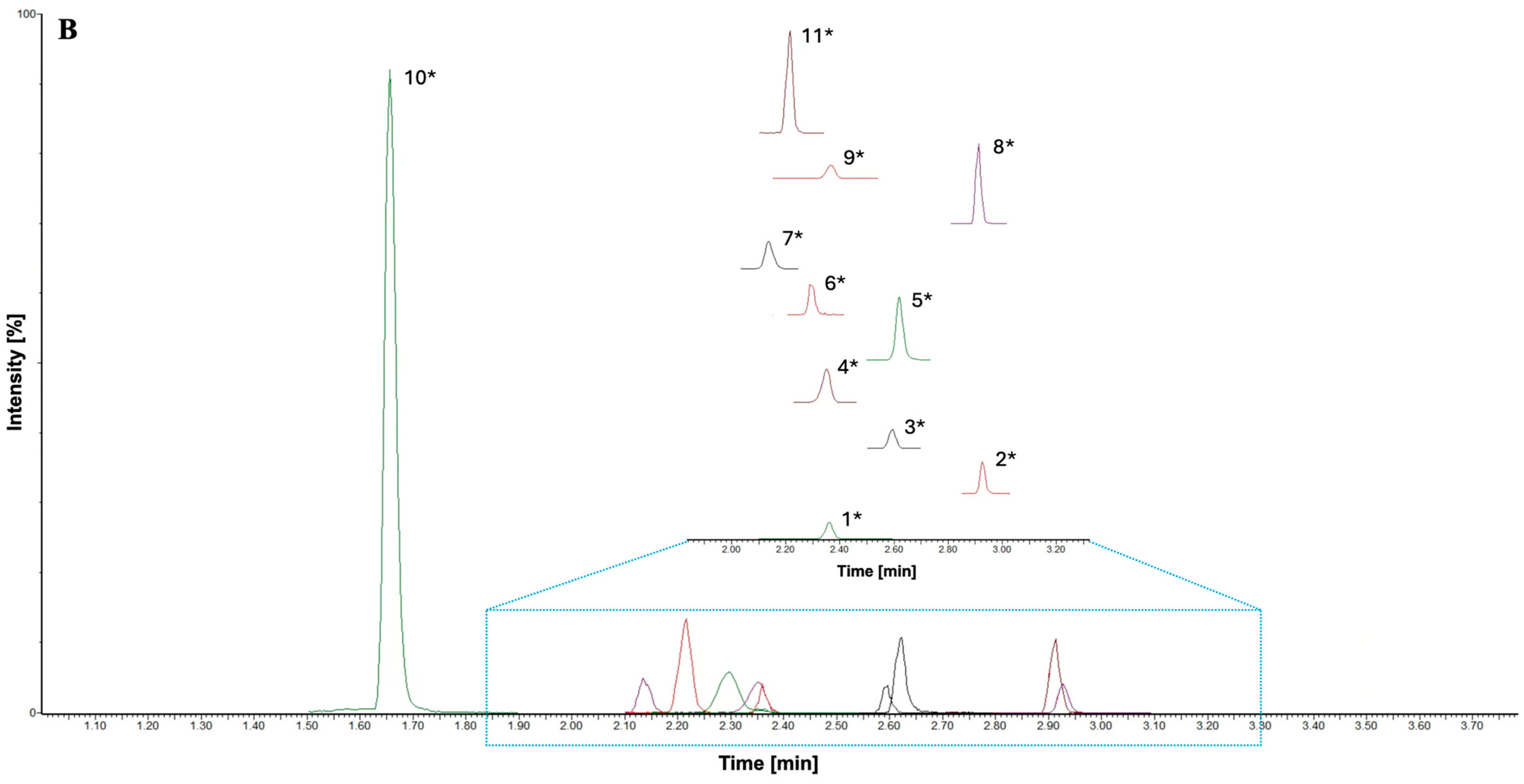
3.2. RP-UHPLC-MS/MS Method Development
3.3. UHPLC-MS/MS Method Validation
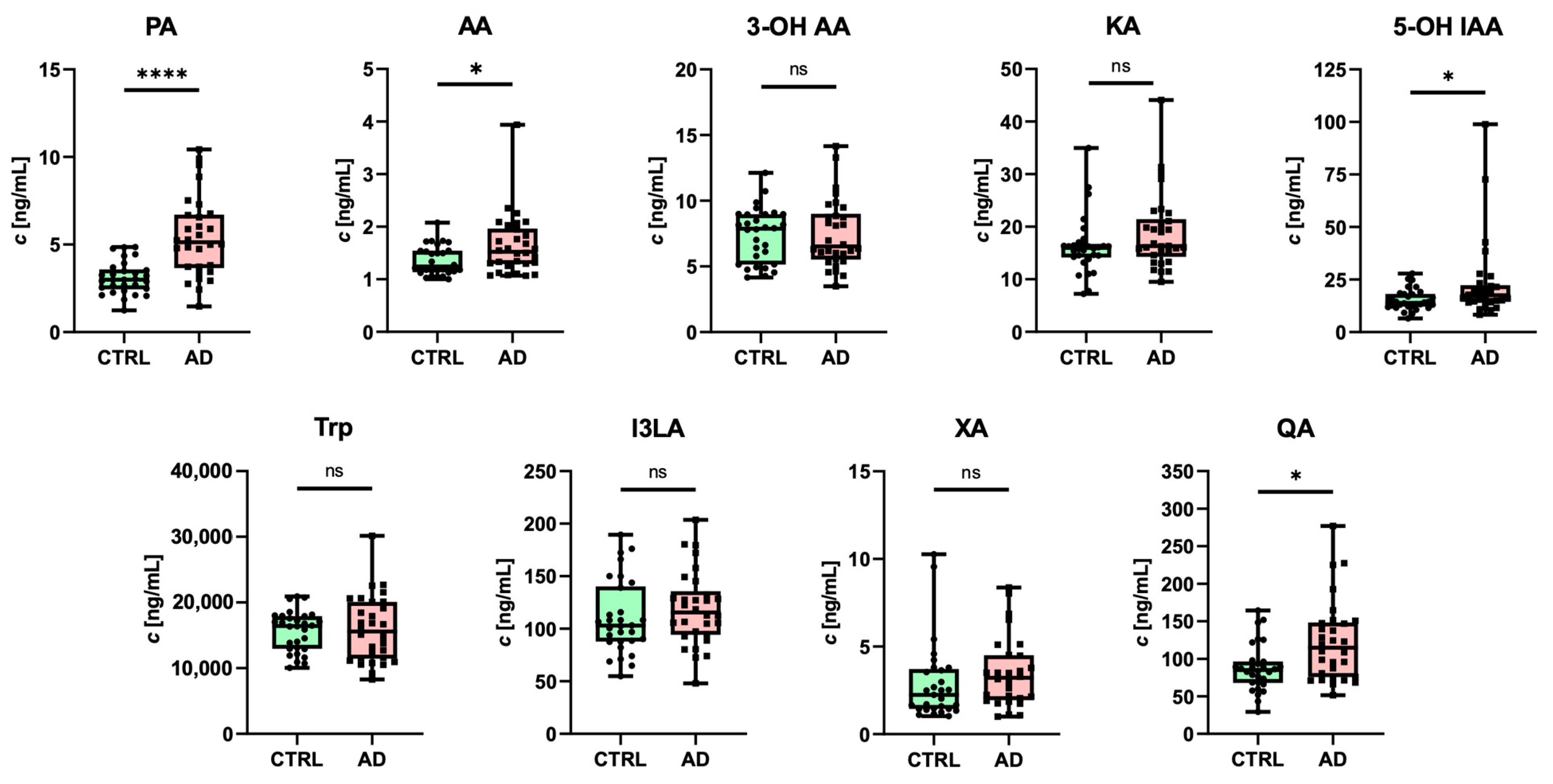
3.4. Quantification of Trp Metabolites in Human Plasma Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Chen, H.; Shi, G.; Yang, M.; Zheng, F.; Zheng, Z.; Zhang, S.; Zhong, S. Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry Quantitative Profiling of Tryptophan Metabolites in Human Plasma and Its Application to Clinical Study. J. Chromatogr. B 2019, 1128, 121745. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Panahi, H.K.S.; Kavyani, B.; Heng, B.; Tan, V.; Braidy, N.; Guillemin, G.J. The Role of Kynurenine Pathway and NAD+ Metabolism in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Aging Dis. 2022, 13, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, A.; Mavel, S.; Nadal-Desbarats, L.; Galineau, L.; Attucci, S.; Dufour, D.; Sokol, H.; Emond, P. Validation of a Global Quantitative Analysis Methodology of Tryptophan Metabolites in Mice Using LC-MS. Talanta 2019, 195, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.; Rashidi, N.; Nurgali, K.; Apostolopoulos, V. The Role of Tryptophan Metabolites in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 9968. [Google Scholar] [CrossRef] [PubMed]
- Grifka-Walk, H.M.; Jenkins, B.R.; Kominsky, D.J. Amino Acid Trp: The Far Out Impacts of Host and Commensal Tryptophan Metabolism. Front. Immunol. 2021, 12, 653208. [Google Scholar] [CrossRef] [PubMed]
- Valente-Silva, P.; Ruas, J.L. Tryptophan-Kynurenine Metabolites in Exercise and Mental Health. In Hormones, Metabolisme and the Benefits of Exercise, 1st ed.; Spiegelman, B., Ed.; Springer: Berlin, Germany, 2017; pp. 83–92. [Google Scholar] [CrossRef]
- Wong, C.B.; Tanaka, A.; Kuhara, T.; Xiao, J.Z. Potential Effects of Indole-3-Lactic Acid, a Metabolite of Human Bifidobacteria, on NGF-Induced Neurite Outgrowth in PC12 Cells. Microorganisms 2020, 8, 398. [Google Scholar] [CrossRef]
- Karakawa, S.; Nishimoto, R.; Harada, M.; Arashida, N.; Nakayama, A. Simultaneous Analysis of Tryptophan and Its Metabolites in Human Plasma Using Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry. Chromatography 2019, 40, 127–133. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G.; Prabhakar, N.K.; Mannan, A. Kynurenine Metabolism and Alzheimer’s Disease: The Potential Targets and Approaches. Neurochem. Res. 2022, 47, 1459–1476. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent Evidence for an Expanded Role of the Kynurenine Pathway of Tryptophan Metabolism in Neurological Diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef]
- Galla, Z.; Rajda, C.; Rácz, G.; Grecsó, N.; Baráth, Á.; Vécsei, L.; Bereczki, C.; Monostori, P. Simultaneous Determination of 30 Neurologically and Metabolically Important Molecules: A Sensitive and Selective Way to Measure Tyrosine and Tryptophan Pathway Metabolites and Other Biomarkers in Human Serum and Cerebrospinal Fluid. J. Chromatogr. A 2021, 1635, 461775. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan Metabolism as a Common Therapeutic Target in Cancer, Neurodegeneration and Beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Nadar, R.; Kim, S.; Liu, K.; Govindarajulu, M.; Cook, P.; Watts Alexander, C.S.; Dhanasekaran, M.; Moore, T. The Influence of Kynurenine Metabolites on Neurodegenerative Pathologies. Int. J. Mol. Sci. 2024, 25, 853. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Takao, M.; Hatsuta, H.; Nishina, Y.; Komiya, T.; Sengoku, R.; Nakano, Y.; Uchino, A.; Sumikura, H.; Saito, Y.; et al. Homovanillic Acid and 5-Hydroxyindole Acetic Acid as Biomarkers for Dementia with Lewy Bodies and Coincident Alzheimer’s Disease: An Autopsy-Confirmed Study. PLoS ONE 2017, 12, e0171524. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.B.; Byrne, L.M.; Lowe, A.J.; Tortelli, R.; Heins, M.; Flik, G.; Johnson, E.B.; De Vita, E.; Scahill, R.I.; Giorgini, F.; et al. Kynurenine Pathway Metabolites in Cerebrospinal Fluid and Blood as Potential Biomarkers in Huntington’s Disease. J. Neurochem. 2021, 158, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, S.; He, Y.; Xu, M.; Qiao, X.; Zhu, Y.; Wu, W. Kynurenine Pathway Metabolites as Biomarkers in Alzheimer’s Disease. Dis. Markers 2022, 2022, 9484217. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Ramprasath, T.; Wang, H.; Zou, M.-H. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol. Life Sci. 2017, 74, 2899–2916. [Google Scholar] [CrossRef] [PubMed]
- Eser, B.; Özkan, Y.; Sepici Dinçel, A. Determination of Tryptophan and Kynurenine by LC-MS/MS by Using Amlodipine as an Internal Standard. J. Am. Soc. Mass. Spectrom. 2020, 31, 379–385. [Google Scholar] [CrossRef]
- Sugishita, T.; Tokunaga, M.; Kami, K.; Terai, K.; Yamamoto, H.; Shinohara, H.; Kinugasa, Y. Determination of the Minimum Sample Amount for Capillary Electrophoresis-Fourier Transform Mass Spectrometry (CE-FTMS)-Based Metabolomics of Colorectal Cancer Biopsies. Biomedicines 2023, 11, 1706. [Google Scholar] [CrossRef]
- Pautova, A.; Khesina, Z.; Getsina, M.; Sobolev, P.; Revelsky, A.; Beloborodova, N. Determination of Tryptophan Metabolites in Serum and Cerebrospinal Fluid Samples Using Microextraction by Packed Sorbent, Silylation and GC–MS Detection. Molecules 2020, 25, 3258. [Google Scholar] [CrossRef]
- Sadok, I.; Gamian, A.; Staniszewska, M.M. Chromatographic Analysis of Tryptophan Metabolites. J. Sep. Sci. 2017, 40, 3020–3045. [Google Scholar] [CrossRef]
- Tong, Q.; Song, J.; Yang, G.; Fan, L.; Xiong, W.; Fang, J. Simultaneous Determination of Tryptophan, Kynurenine, Kynurenic Acid, Xanthurenic Acid and 5-Hydroxytryptamine in Human Plasma by LC-MS/MS and Its Application to Acute Myocardial Infarction Monitoring. Biomed. Chromatogr. 2018, 32, e4156. [Google Scholar] [CrossRef] [PubMed]
- Hényková, E.; Vránová, H.P.; Amakorová, P.; Pospíšil, T.; Žukauskaite, A.; Vlčková, M.; Urbánek, L.; Novák, O.; Mareš, J.; Kaňovský, P.; et al. Stable Isotope Dilution Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry Quantitative Profiling of Tryptophan-Related Neuroactive Substances in Human Serum and Cerebrospinal Fluid. J. Chromatogr. A 2016, 1437, 145–157. [Google Scholar] [CrossRef]
- Badawy, A.A.B.; Morgan, C.J. Rapid Isocratic Liquid Chromatographic Separation and Quantification of Tryptophan and Six Kynurenine Metabolites in Biological Samples with Ultraviolet and Fluorimetric Detection. Int. J. Tryptophan Res. 2010, 3, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.; García-Marchena, N.; O’Shea, E.; Requena-Ocaña, N.; Flores-López, M.; Araos, P.; Serrano, A.; Suárez, J.; Rubio, G.; Rodríguez de Fonseca, F.; et al. Plasma Tryptophan and Kynurenine Pathway Metabolites in Abstinent Patients with Alcohol Use Disorder and High Prevalence of Psychiatric Comorbidity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109958. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.L.; Mutengwa, V.S.; Abdalla, A.; Yeoman, M.S.; Patel, B.A. Determination of Tryptophan Metabolism from Biological Tissues and Fluids Using High Performance Liquid Chromatography with Simultaneous Dual Electrochemical Detection. Analyst 2019, 144, 6011–6018. [Google Scholar] [CrossRef] [PubMed]
- Pedraz-Petrozzi, B.; Marszalek-Grabska, M.; Kozub, A.; Szalaj, K.; Trzpil, A.; Stachniuk, A.; Lamadé, E.K.; Gilles, M.; Deuschle, M.; Turski, W.A.; et al. LC–MS/MS-Based Quantification of Tryptophan, Kynurenine, and Kynurenic Acid in Human Placental, Fetal Membranes, and Umbilical Cord Samples. Sci. Rep. 2023, 13, 12554. [Google Scholar] [CrossRef] [PubMed]
- Schwieler, L.; Trepci, A.; Krzyzanowski, S.; Hermansson, S.; Granqvist, M.; Piehl, F.; Venckunas, T.; Brazaitis, M.; Kamandulis, S.; Lindqvist, D.; et al. A Novel, Robust Method for Quantification of Multiple Kynurenine Pathway Metabolites in the Cerebrospinal Fluid. Bioanalysis 2020, 12, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Baxter-Parker, G.; Chu, A.; Petocz, P.; Samman, S.; Gieseg, S.P. Simultaneous analysis of neopterin, kynurenine and tryptophan by amine-HPLC shows minor oxidative stress from short-term exhaustion exercise. Pteridines 2019, 30, 21–32. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, P.; Zhong, D. Derivatization Methods for LC-MS Analysis of Endogenous Compounds. Bioanalysis 2015, 7, 2557–2581. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Li, S.; Zhu, Q.F.; Wang, Q.; Hussain, D.; Feng, Y.Q. Derivatization for Liquid Chromatography-Electrospray Ionization-Mass Spectrometry Analysis of Small-Molecular Weight Compounds. TrAC Trends Anal. Chem. 2019, 119, 115608. [Google Scholar] [CrossRef]
- Willacey, C.C.W.; Naaktgeboren, M.; Lucumi Moreno, E.; Wegrzyn, A.B.; van der Es, D.; Karu, N.; Fleming, R.M.T.; Harms, A.C.; Hankemeier, T. LC–MS/MS Analysis of the Central Energy and Carbon Metabolites in Biological Samples Following Derivatization by Dimethylaminophenacyl Bromide. J. Chromatogr. A 2019, 1608, 460413. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Moldoveanu, S.C.; Galaon, T. Derivatization Procedures and Their Analytical Performances for HPLC Determination in Bioanalysis. Biomed. Chromatogr. 2021, 35, e5008. [Google Scholar] [CrossRef] [PubMed]
- Jankech, T.; Gerhardtova, I.; Majerova, P.; Piestansky, J.; Jampilek, J.; Kovac, A. Derivatization of Carboxylic Groups Prior to Their LC Analysis—A Review. Anal. Chim. Acta. 2024, 1300, 342435. [Google Scholar] [CrossRef]
- Tömösi, F.; Kecskeméti, G.; Cseh, E.K.; Szabó, E.; Rajda, C.; Kormány, R.; Szabó, Z.; Vécsei, L.; Janáky, T. A Validated UHPLC-MS Method for Tryptophan Metabolites: Application in the Diagnosis of Multiple Sclerosis. J. Pharm. Biomed. Anal. 2020, 185, 113246. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.Y.; Wang, C.Y.; Lee, C.H.; Kao, H.L.; Wu, W.K.; Kuo, C.H. Development of an Efficient and Sensitive Chemical Derivatization-Based LC-MS/MS Method for Quantifying Gut Microbiota-Derived Metabolites in Human Plasma and Its Application in Studying Cardiovascular Disease. J. Proteome Res. 2021, 20, 3508–3518. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Xiao, H.M.; Zhan, J.T.; Yuan, B.F.; Feng, Y.Q. Simultaneous Determination of Indole Metabolites of Tryptophan in Rat Feces by Chemical Labeling Assisted Liquid Chromatography-Tandem Mass Spectrometry. Chin. Chem. Lett. 2022, 33, 4746–4749. [Google Scholar] [CrossRef]
- European Medicines Agency—ICH Guideline M10 on Bioanalytical Method Validation. EMA Guidance Document. 2019. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf (accessed on 5 February 2024).
- Guo, K.; Li, L. High-Performance Isotope Labeling for Profiling Carboxylic Acid-Containing Metabolites in Biofluids by Mass Spectrometry. Anal. Chem. 2010, 82, 8789–8793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Zhang, Z.Q.; Dong, W.C.; Jiang, Y. A New Derivatization Method to Enhance Sensitivity for the Determination of Low Levels of Valproic Acid in Human Plasma. J. Chromatogr. Sci. 2014, 52, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, S.T.; Harahap, Y. Harmita Bioanalytical Method Validation of Valproic Acid in Human Plasma In-Vitro after Derivatization With 2,4-Dibomoacetophenon by High Performance Liquid Chromatography- Photo Diode Array and Its Application to In-Vivo Study. IJPTP 2013, 4, 644–648. [Google Scholar]
- Shi, Y.; Xiong, J.; Sun, D.; Liu, W.; Wei, F.; Ma, S.; Lin, R. Simultaneous Quantification of the Major Bile Acids in Artificial Calculus Bovis by High-Performance Liquid Chromatography with Precolumn Derivatization and Its Application in Quality Control. J. Sep. Sci. 2015, 38, 2753–2762. [Google Scholar] [CrossRef]
- Song, P.; Mabrouk, O.S.; Hershey, N.D.; Kennedy, R.T. In Vivo Neurochemical Monitoring Using Benzoyl Chloride Derivatization and Liquid Chromatography−Mass Spectrometry. Anal. Chem. 2012, 84, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Little, T.A. Establishing Acceptance Criteria for Analytical Methods. BioPharm Int. 2016, 29, 44–48. [Google Scholar]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue Applicability Grade Index (BAGI) and Software: A New Tool for the Evaluation of Method Practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Chouraki, V.; Preis, S.R.; Yang, Q.; Beiser, A.; Li, S.; Larson, M.G.; Weinstein, G.; Wang, T.J.; Gerszten, R.E.; Vasan, R.S.; et al. Association of Amine Biomarkers with Incident Dementia and Alzheimer’s Disease in the Framingham Study. Alzheimer’s Dement. 2017, 13, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Gulaj, E.; Pawlak, K.; Bien, B.; Pawlak, D. Kynurenine and Its Metabolites in Alzheimer’s Disease Patients. Adv. Med. Sci. 2010, 55, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.R.; Lim, C.K.; Blennow, K.; Zetterberg, H.; Chatterjee, P.; Martins, R.N.; Brew, B.J.; Guillemin, G.J.; Lovejoy, D.B. Correlation between Plasma and CSF Concentrations of Kynurenine Pathway Metabolites in Alzheimer’s Disease and Relationship to Amyloid-β and Tau. Neurobiol. Aging 2019, 80, 11–20. [Google Scholar] [CrossRef]
- Eggertsen, P.P.; Hansen, J.; Andersen, M.L.; Nielsen, J.F.; Olsen, R.K.J.; Palmfeldt, J. Simultaneous Measurement of Kynurenine Metabolites and Explorative Metabolomics Using Liquid Chromatography-Mass Spectrometry: A Novel Accurate Method Applied to Serum and Plasma Samples from a Large Healthy Cohort. J. Pharm. Biomed. Anal. 2023, 227, 115304. [Google Scholar] [CrossRef]
- Meier, T.B.; Drevets, W.C.; Teague, T.K.; Wurfel, B.E.; Mueller, S.C.; Bodurka, J.; Dantzer, R.; Savitz, J. Kynurenic acid is reduced in females and oral contraceptive users: Implications for depression. Brain Behav. Immun. 2018, 67, 59–64. [Google Scholar] [CrossRef]
- Whiley, L.; Chappell, K.E.; D’Hondt, E.; Lewis, M.R.; Jiménez, B.; Snowden, S.G.; Soininen, H.; Kłoszewska, I.; Mecocci, P.; Tsolaki, M.; et al. Metabolic Phenotyping Reveals a Reduction in the Bioavailability of Serotonin and Kynurenine Pathway Metabolites in Both the Urine and Serum of Individuals Living with Alzheimer’s Disease. Alzheimers Res. Ther. 2021, 13, 20. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Ghojazadeh, M.; Abolhasani, S.; Alikhah, H.; Khaki-Khatibi, F. Correlation of Serum Levels of Vitronectin, Malondialdehyde and Hs-CRP With Disease Severity in Coronary Artery Disease. J. Cardiovasc. Thorac. Res. 2015, 7, 113–117. [Google Scholar] [CrossRef]
- Vondroušová, J.; Mikoška, M.; Syslová, K.; Böhmová, A.; Tejkalová, H.; Vacek, L.; Kodym, P.; Krsek, D.; Horáček, J. Monitoring of Kynurenine Pathway Metabolites, Neurotransmitters and Their Metabolites in Blood Plasma and Brain Tissue of Individuals with Latent Toxoplasmosis. J. Pharm. Biomed. Anal. 2019, 170, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Sun, S.; Chen, H.; Liu, M.; Zhang, L.; Wu, Z.; Li, L.; Wu, G. Analysis of Tryptophan and Its Metabolites by High-Performance Liquid Chromatography. In Amino Acid Analysis. Methods in Molecular Biology, 2nd ed.; Alterman, M., Ed.; Humana: New York, NY, USA, 2019; Volume 2030, pp. 131–142. [Google Scholar] [CrossRef]
- Shao, Y.; Ouyang, Y.; Li, T.; Liu, X.; Xu, X.; Li, S.; Xu, G.; Le, W. Alteration of Metabolic Profile and Potential Biomarkers in the Plasma of Alzheimer’s Disease. Aging Dis. 2020, 11, 1459–1470. [Google Scholar] [CrossRef] [PubMed]

| Analyte | QC LLOQ [ng/mL] | QC Low [ng/mL] | QC Medium [ng/mL] | QC High [ng/mL] |
|---|---|---|---|---|
| PA | 1 | 2 | 40 | 80 |
| AA | ||||
| 3-OH AA | ||||
| KA | ||||
| XA | ||||
| NA | 5 | 10 | 200 | 400 |
| 5-OH IAA | ||||
| QA | 10 | 20 | 400 | 800 |
| I3LA | ||||
| 3-OH KYN | 25 | 50 | 1000 | 2000 |
| Trp | 500 | 1000 | 20,000 | 40,000 |
| Analyte | Precursor Ion | Product Ion (Qualitative) * | Product Ion (Quantitative) | Cone [V] | Ce [eV] |
|---|---|---|---|---|---|
| PA | 287 | 106 | 78 | 35 | 20 |
| PA-d4 | 291 | 110 | 82 | 35 | 20 |
| NA | 287 | 90 | 241 | 30 | 30 |
| NA-d4 | 291 | - | 245 | 30 | 30 |
| AA | 301 | 92 | 120 | 25 | 15 |
| AA-ring-13C6 | 307 | - | 126 | 25 | 15 |
| 3-OH AA | 317 | 108 | 136 | 20 | 25 |
| 3-OH AA-d3 | 320 | - | 139 | 20 | 20 |
| KA | 353 | 172 | 144 | 30 | 20 |
| KA-d5 | 358 | 177 | 149 | 30 | 20 |
| XA | 369 | 188 | 160 | 30 | 25 |
| XA-d4 | 373 | 192 | 164 | 30 | 25 |
| QA | 494 | 164 | 313 | 35 | 15 |
| QA-d3 | 497 | - | 316 | 30 | 20 |
| Trp | 368 | 159 | 170 | 25 | 25 |
| Trp-indole-d5 | 373 | - | 164 | 25 | 25 |
| 3-OH KYN | 551 | 190 | 152 | 30 | 30 |
| 3-OH KYN-d3 | 554 | - | 155 | 30 | 20 |
| I3LA | 369 | 170 | 118 | 25 | 35 |
| I3LA-d5 | 374 | - | 123 | 25 | 30 |
| 5-OH IAA | 355 | 118 | 146 | 30 | 30 |
| 5-OH IAA-d6 | 361 | - | 152 | 30 | 25 |
| Analyte | tR [min] | Linear Range [ng/mL] | R2 | LOD [ng/mL] | LLOQ [ng/mL] | Carryover [%] |
|---|---|---|---|---|---|---|
| PA | 2.31 | 1–100 | 0.9978 | 0.40 | 1 | 8.8 |
| NA | 2.38 | 5–500 | 0.9958 | 2.43 | 5 | 0.8 |
| AA | 2.94 | 1–100 | 0.9980 | 0.25 | 1 | 6.0 |
| 3-OH AA | 2.61 | 1–100 | 0.9947 | 0.15 | 1 | 0.2 |
| KA | 2.23 | 1–100 | 0.9957 | 0.60 | 1 | 2.8 |
| XA | 2.15 | 1–100 | 0.9966 | 0.57 | 1 | 0.4 |
| QA | 2.92 | 10–1000 | 0.9961 | 4.50 | 10 | 1.9 |
| Trp | 1.67 | 500–50,000 | 0.9994 | 0.86 * | 500 | 2.4 |
| 3-OH KYN | 2.38 | 25–2500 | 0.9942 | 9.43 | 25 | 0.7 |
| I3LA | 2.64 | 10–1000 | 0.9955 | 7.81 | 10 | 0.4 |
| 5-OH IAA | 2.36 | 5–500 | 0.9982 | 1.33 | 5 | 0.3 |
| Intra-Day (n = 5) | Inter-Day (n = 15) | n = 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Analyte | QC Level | Nominal c [ng/mL] | Found [ng/mL] | Accuracy [%] | RSD [%] | Found [ng/mL] | Accuracy [%] | RSD [%] | Recovery [%] |
| PA | LLOQ | 1 | 1.00 | 100.4 | 6.6 | 1.00 | 100.2 | 5.5 | 90.4 |
| Low | 2 | 2.14 | 106.6 | 4.8 | 2.10 | 105.2 | 5.9 | 100.0 | |
| Medium | 40 | 40.48 | 101.2 | 2.9 | 40.04 | 100.1 | 3.2 | 97.2 | |
| High | 80 | 79.68 | 99.6 | 3.0 | 79.71 | 99.6 | 3.1 | 103.6 | |
| NA | LLOQ | 5 | 5.96 | 97.6 | 5.9 | 4.90 | 98.1 | 5.3 | 90.7 |
| Low | 10 | 10.87 | 97.9 | 8.2 | 9.89 | 98.9 | 7.2 | 103.7 | |
| Medium | 200 | 200.33 | 99.6 | 2.1 | 196.87 | 98.4 | 5.0 | 97.6 | |
| High | 400 | 393.93 | 98.2 | 4.3 | 385.98 | 96.5 | 3.1 | 98.7 | |
| AA | LLOQ | 1 | 1.01 | 101.0 | 6.5 | 1.01 | 101.1 | 5.3 | 92.7 |
| Low | 2 | 2.00 | 100.2 | 4.2 | 2.00 | 100.4 | 4.6 | 109.0 | |
| Medium | 40 | 38.88 | 97.2 | 3.4 | 39.82 | 99.6 | 3.8 | 98.6 | |
| High | 80 | 78.61 | 98.3 | 5.4 | 79.61 | 99.5 | 4.1 | 101.3 | |
| 3-OH AA | LLOQ | 1 | 0.98 | 97.9 | 4.9 | 0.98 | 97.7 | 6.7 | 83.3 |
| Low | 2 | 2.03 | 101.6 | 6.7 | 2.03 | 101.6 | 5.8 | 94.6 | |
| Medium | 40 | 39.72 | 99.3 | 4.8 | 40.67 | 101.7 | 3.2 | 97.8 | |
| High | 80 | 81.40 | 101.7 | 4.4 | 82.18 | 102.7 | 4.1 | 97.8 | |
| KA | LLOQ | 1 | 1.03 | 103.0 | 7.0 | 1.03 | 102.7 | 6.0 | 100.8 |
| Low | 2 | 2.08 | 104.2 | 6.8 | 2.03 | 101.5 | 5.6 | 89.5 | |
| Medium | 40 | 40.24 | 100.6 | 2.8 | 42.03 | 105.1 | 2.3 | 105.2 | |
| High | 80 | 79.13 | 98.9 | 6.5 | 83.66 | 104.6 | 5.7 | 102.7 | |
| XA | LLOQ | 1 | 1.01 | 100.5 | 4.7 | 1.02 | 101.7 | 6.4 | 88.1 |
| Low | 2 | 2.19 | 109.4 | 3.0 | 2.12 | 106.0 | 4.7 | 80.5 | |
| Medium | 40 | 40.02 | 100.0 | 4.7 | 39.28 | 98.2 | 4.8 | 85.4 | |
| High | 80 | 83.35 | 104.2 | 2.2 | 80.45 | 100.6 | 2.9 | 88.0 | |
| QA | LLOQ | 10 | 9.99 | 99.9 | 4.5 | 10.04 | 100.4 | 5.6 | 85.1 |
| Low | 20 | 19.98 | 99.9 | 3.5 | 20.36 | 101.8 | 5.3 | 84.0 | |
| Medium | 400 | 422.78 | 105.7 | 6.6 | 409.44 | 102.4 | 4.4 | 95.4 | |
| High | 800 | 807.93 | 101.0 | 4.3 | 805.38 | 100.7 | 5.2 | 97.3 | |
| Trp | LLOQ | 500 | 503.24 | 100.6 | 1.4 | 498.16 | 99.6 | 4.9 | 95.5 |
| Low | 1000 | 1008.51 | 100.9 | 4.7 | 1003.68 | 100.4 | 5.3 | 80.6 | |
| Medium | 20,000 | 19,826.20 | 99.1 | 6.4 | 19,853.62 | 99.3 | 5.7 | 90.2 | |
| High | 40,000 | 39,031.26 | 97.6 | 6.0 | 39,083.29 | 97.7 | 4.7 | 88.4 | |
| 3-OH KYN | LLOQ | 25 | 26.44 | 105.8 | 0.5 | 26.75 | 107.0 | 3.2 | 104.4 |
| Low | 50 | 54.67 | 109.3 | 6.5 | 54.04 | 108.1 | 5.6 | 98.2 | |
| Medium | 1000 | 1096.64 | 109.7 | 3.3 | 1095.45 | 109.6 | 4.5 | 96.9 | |
| High | 2000 | 2179.48 | 109.0 | 4.0 | 2201.95 | 110.1 | 4.4 | 98.7 | |
| I3LA | LLOQ | 10 | 10.17 | 101.7 | 7.3 | 10.11 | 101.1 | 6.4 | 87.3 |
| Low | 20 | 20.70 | 103.5 | 7.1 | 20.35 | 101.7 | 6.1 | 87.4 | |
| Medium | 400 | 381.10 | 95.3 | 5.8 | 378.80 | 94.7 | 7.4 | 83.4 | |
| High | 800 | 759.23 | 94.9 | 3.3 | 764.41 | 95.5 | 3.9 | 80.7 | |
| 5-OH IAA | LLOQ | 5 | 5.12 | 102.5 | 7.0 | 5.096 | 101.9 | 5.4 | 98.7 |
| Low | 10 | 10.00 | 99.9 | 2.1 | 10.00 | 100.0 | 3.7 | 96.9 | |
| Medium | 200 | 186.59 | 93.3 | 6.5 | 195.08 | 97.5 | 5.6 | 99.8 | |
| High | 400 | 395.50 | 98.9 | 2.5 | 399.55 | 99.9 | 3.4 | 98.2 | |
| Autosampler (n = 5) | Freeze–Thaw (n = 5) | |||||||
|---|---|---|---|---|---|---|---|---|
| Analyte | QC Level | Nominal c [ng/mL] | Found [ng/mL] | Accuracy [%] | RSD [%] | Found [ng/mL] | Accuracy [%] | RSD [%] |
| PA | LLOQ | 1 | 1.00 | 100.2 | 8.6 | 1.04 | 101.1 | 4.9 |
| Low | 2 | 2.05 | 102.4 | 9.8 | 2.17 | 108.6 | 1.0 | |
| Medium | 40 | 39.45 | 98.6 | 4.4 | 38.91 | 97.3 | 6.5 | |
| High | 80 | 79.07 | 98.8 | 0.6 | 79.55 | 99.4 | 1.4 | |
| NA | LLOQ | 5 | 5.11 | 102.3 | 10.2 | 5.19 | 103.9 | 14.3 |
| Low | 10 | 10.20 | 102.0 | 4.0 | 10.00 | 100.0 | 8.1 | |
| Medium | 200 | 207.19 | 103.6 | 3.8 | 188.04 | 94.0 | 8.1 | |
| High | 400 | 408.91 | 102.2 | 3.5 | 390.74 | 97.7 | 5.7 | |
| AA | LLOQ | 1 | 1.02 | 101.6 | 3.5 | 0.97 | 97.2 | 14.3 |
| Low | 2 | 2.03 | 101.2 | 3.3 | 1.92 | 95.9 | 8.6 | |
| Medium | 40 | 40.74 | 101.8 | 1.6 | 41.51 | 103.8 | 4.2 | |
| High | 80 | 80.62 | 100.8 | 3.3 | 85.85 | 107.3 | 7.5 | |
| 3-OH AA | LLOQ | 1 | 1.01 | 100.5 | 14.4 | 0.91 | 90.8 | 6.5 |
| Low | 2 | 1.99 | 99.4 | 6.8 | 2.05 | 102.3 | 9.7 | |
| Medium | 40 | 39.40 | 98.5 | 2.0 | 40.54 | 101.3 | 3.5 | |
| High | 80 | 80.58 | 100.7 | 2.8 | 81.43 | 101.8 | 4.0 | |
| KA | LLOQ | 1 | 1.01 | 101.2 | 7.3 | 0.95 | 95.2 | 9.8 |
| Low | 2 | 1.99 | 99.3 | 8.0 | 2.10 | 105.1 | 10.4 | |
| Medium | 40 | 42.21 | 105.5 | 1.5 | 41.70 | 104.2 | 7.8 | |
| High | 80 | 81.58 | 102.0 | 1.8 | 84.70 | 105.9 | 7.8 | |
| XA | LLOQ | 1 | 0.99 | 99.0 | 7.7 | 1.014 | 101.4 | 12.4 |
| Low | 2 | 2.033 | 101.6 | 8.1 | 1.897 | 94.9 | 6.3 | |
| Medium | 40 | 39.44 | 98.6 | 4.6 | 38.24 | 95.6 | 5.5 | |
| High | 80 | 78.24 | 97.8 | 2.4 | 77.71 | 97.1 | 2.5 | |
| QA | LLOQ | 10 | 10.17 | 101.7 | 5.9 | 10.16 | 101.6 | 9.4 |
| Low | 20 | 19.93 | 99.7 | 10.6 | 21.15 | 105.8 | 4.7 | |
| Medium | 400 | 405.13 | 101.3 | 2.4 | 395.47 | 98.9 | 3.8 | |
| High | 800 | 797.64 | 99.7 | 8.8 | 817.14 | 102.1 | 8.9 | |
| Trp | LLOQ | 500 | 506.88 | 101.4 | 7.5 | 492.62 | 98.5 | 14.2 |
| Low | 1000 | 1013.99 | 101.4 | 4.7 | 955.40 | 95.5 | 8.3 | |
| Medium | 20,000 | 20,438.98 | 102.2 | 2.5 | 19,603.80 | 98.0 | 7.4 | |
| High | 40,000 | 39,922.96 | 99.8 | 2.5 | 40,087.04 | 100.2 | 4.9 | |
| 3-OH KYN | LLOQ | 25 | 24.42 | 97.4 | 6.6 | 27.58 | 110.3 | 12.3 |
| Low | 50 | 50.70 | 101.4 | 3.4 | 53.02 | 106.0 | 6.0 | |
| Medium | 1000 | 1039.83 | 104.0 | 4.3 | 1033.51 | 103.4 | 3.7 | |
| High | 2000 | 2138.14 | 106.9 | 45.7 | 2239.98 | 112.0 | 5.5 | |
| I3LA | LLOQ | 10 | 10.38 | 103.7 | 7.0 | 9.57 | 95.7 | 12.0 |
| Low | 20 | 20.27 | 101.4 | 11.6 | 20.66 | 103.3 | 6.7 | |
| Medium | 400 | 405.01 | 101.3 | 1.3 | 429.28 | 107.3 | 4.6 | |
| High | 800 | 785.55 | 98.2 | 4.2 | 815.93 | 102.0 | 4.8 | |
| 5-OH IAA | LLOQ | 5 | 4.95 | 99.0 | 6.3 | 4.47 | 94.8 | 6.3 |
| Low | 10 | 9.02 | 90.4 | 6.6 | 10.98 | 109.8 | 11.8 | |
| Medium | 200 | 188.79 | 94.4 | 5.8 | 180.13 | 90.1 | 5.2 | |
| High | 400 | 388.75 | 97.2 | 14.0 | 373.21 | 93.3 | 8.9 | |
| Analyte | QC Level | TC RSD [%] | FR RSD [%] | % of B RSD [%] |
|---|---|---|---|---|
| PA | LLOQ | 4.9 | 4.4 | 8.7 |
| Low | 4.1 | 5.2 | 4.9 | |
| Medium | 3.6 | 5.6 | 4.9 | |
| High | 4.6 | 1.8 | 4.5 | |
| NA | LLOQ | 3.3 | 6.0 | 6.2 |
| Low | 7.0 | 5.5 | 1.0 | |
| Medium | 7.8 | 4.8 | 6.3 | |
| High | 1.7 | 4.4 | 4.7 | |
| AA | LLOQ | 1.8 | 3.6 | 6.5 |
| Low | 4.2 | 1.8 | 1.4 | |
| Medium | 3.3 | 2.7 | 3.0 | |
| High | 3.0 | 1.7 | 3.4 | |
| 3-OH AA | LLOQ | 7.9 | 1.5 | 6.9 |
| Low | 4.1 | 5.4 | 7.5 | |
| Medium | 5.8 | 8.2 | 7.1 | |
| High | 4.2 | 7.8 | 5.3 | |
| KA | LLOQ | 1.3 | 5.1 | 3.4 |
| Low | 4.8 | 1.3 | 6.3 | |
| Medium | 5.0 | 5.8 | 4.3 | |
| High | 4.4 | 3.7 | 7.4 | |
| XA | LLOQ | 4.6 | 4.5 | 8.1 |
| Low | 7.7 | 5.5 | 4.7 | |
| Medium | 8.4 | 7.4 | 2.4 | |
| High | 8.9 | 5.9 | 7.0 | |
| QA | LLOQ | 3.5 | 1.3 | 4.0 |
| Low | 3.3 | 6.0 | 1.6 | |
| Medium | 6.2 | 9.1 | 4.6 | |
| High | 2.7 | 2.3 | 5.7 | |
| Trp | LLOQ | 3.9 | 2.5 | 4.1 |
| Low | 6.3 | 1.8 | 4.9 | |
| Medium | 2.5 | 6.4 | 4.8 | |
| High | 4.3 | 6.5 | 4.2 | |
| 3-OH KYN | LLOQ | 6.4 | 8.0 | 5.9 |
| Low | 7.3 | 4.7 | 6.8 | |
| Medium | 2.3 | 7.9 | 5.6 | |
| High | 2.2 | 5.1 | 7.4 | |
| I3LA | LLOQ | 2.9 | 5.5 | 1.9 |
| Low | 4.1 | 4.5 | 7.3 | |
| Medium | 6.0 | 8.0 | 2.3 | |
| High | 5.7 | 2.4 | 2.6 | |
| 5-OH IAA | LLOQ | 5.5 | 2.8 | 1.1 |
| Low | 2.0 | 2.0 | 3.8 | |
| Medium | 6.3 | 5.3 | 3.0 | |
| High | 5.9 | 8.8 | 8.9 |
| Group | CTRL | AD |
|---|---|---|
| Number of individuals (n) | 30 | 30 |
| Age (mean ± SD) | 78.5 ± 4.3 | 82.6 ± 4.7 |
| Sex (male/female) | 10/20 | 11/19 |
| MMSE (mean ± SD) | - | 19.9 ± 7.6 * |
| MoCA (mean ± SD) | - | 16.7 ± 5.8 * |
| Analyte | Sex | Concentration (Mean ± SD) [ng/mL] | p-Value | |
|---|---|---|---|---|
| CTRL | AD | |||
| PA | F | 2.80 ± 0.74 | 5.11 ± 2.27 | 0.0002 |
| M | 3.70 ± 0.89 | 5.96 ± 2.04 | 0.0062 | |
| AA | F | 1.36 ± 0.30 | 1.66 ± 0.63 | 0.0790 |
| M | 1.53 ± 0.45 | 1.61 ± 0.44 | 0.6987 | |
| 3-OH AA | F | 7.38 ± 1.95 | 7.25 ± 2.87 | 0.8715 |
| M | 7.40 ± 2.20 | 7.85 ± 1.83 | 0.6301 | |
| KA | F | 14.42 ± 3.16 | 19.36 ± 7.81 | 0.0151 |
| M | 20.23 ± 6.76 | 17.87 ± 5.69 | 0.4198 | |
| 5-OH IAA | F | 13.80 ± 3.35 | 23.32 ± 19.23 | 0.0405 |
| M | 18.46 ± 6.11 | 22.86 ± 17.48 | 0.4818 | |
| Trp | F | 15,773.02 ± 2620.40 | 17,172.81 ± 4902.50 | 0.2825 |
| M | 15,378.28 ± 3649.57 | 13,703.87 ± 4202.85 | 0.3676 | |
| I3LA | F | 90.94 ± 16.76 | 114.75 ± 37.01 | 0.0154 |
| M | 149.24 ± 27.78 | 127.07 ± 29.65 | 0.1097 | |
| XA | F | 2.83 ± 1.97 | 2.34 ± 1.30 | 0.7063 |
| M | 3.07 ± 1.21 | 4.95 ± 2.09 | 0.0289 | |
| QA | F | 87.60 ± 32.72 | 127.79 ± 56.37 | 0.0112 |
| M | 87.98 ± 27.40 | 114.02 ± 45.39 | 0.1513 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankech, T.; Gerhardtova, I.; Majerova, P.; Piestansky, J.; Fialova, L.; Jampilek, J.; Kovac, A. A Novel RP-UHPLC-MS/MS Approach for the Determination of Tryptophan Metabolites Derivatized with 2-Bromo-4′-Nitroacetophenone. Biomedicines 2024, 12, 1003. https://doi.org/10.3390/biomedicines12051003
Jankech T, Gerhardtova I, Majerova P, Piestansky J, Fialova L, Jampilek J, Kovac A. A Novel RP-UHPLC-MS/MS Approach for the Determination of Tryptophan Metabolites Derivatized with 2-Bromo-4′-Nitroacetophenone. Biomedicines. 2024; 12(5):1003. https://doi.org/10.3390/biomedicines12051003
Chicago/Turabian StyleJankech, Timotej, Ivana Gerhardtova, Petra Majerova, Juraj Piestansky, Lubica Fialova, Josef Jampilek, and Andrej Kovac. 2024. "A Novel RP-UHPLC-MS/MS Approach for the Determination of Tryptophan Metabolites Derivatized with 2-Bromo-4′-Nitroacetophenone" Biomedicines 12, no. 5: 1003. https://doi.org/10.3390/biomedicines12051003
APA StyleJankech, T., Gerhardtova, I., Majerova, P., Piestansky, J., Fialova, L., Jampilek, J., & Kovac, A. (2024). A Novel RP-UHPLC-MS/MS Approach for the Determination of Tryptophan Metabolites Derivatized with 2-Bromo-4′-Nitroacetophenone. Biomedicines, 12(5), 1003. https://doi.org/10.3390/biomedicines12051003








