Ethnic Aspects of Valproic Acid P-Oxidation
Abstract
1. Introduction
2. Effect of Variable Alleles of Genes Encoding Cytochrome P450 Isoenzymes on the P-Oxidation Rate of Valproic Acid
3. Cytochrome P450-Catalyzed Oxidation
4. Risk Factors for the Impaired P-Oxidation of Valproic Acid
5. Valproic Acid P-Oxidation and Ethnicity
5.1. The CYP2A6 Gene
5.2. The CYP2B6 Gene
5.3. The CYP2C9 Gene
5.4. The CYP2C19 Gene
5.5. The CYP2D6 Gene
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shnayder, N.A.; Grechkina, V.V.; Khasanova, A.K.; Bochanova, E.N.; Dontceva, E.A.; Petrova, M.M.; Asadullin, A.R.; Shipulin, G.A.; Altynbekov, K.S.; Al-Zamil, M.; et al. Therapeutic and toxic effects of valproic acid metabolites. Metabolites 2023, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Grechkina, V.V.; Trefilova, V.V.; Efremov, I.S.; Dontceva, E.A.; Narodova, E.A.; Petrova, M.M.; Soloveva, I.A.; Tepnadze, L.E.; Reznichenko, P.A.; et al. Valproate-induced metabolic syndrome. Biomedicines 2023, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- Nasyrova, R.F.; Ivanov, M.V.; Neznanov, N.G. Introduction to Psychopharmacogenetics; Publishing Center of V.M. Bekhterev: St. Petersburg, Russia, 2015; p. 272. [Google Scholar]
- Safdar, A.; Ismail, F. A comprehensive review on pharmacological applications and drug-induced toxicity of valproic acid. Saudi Pharm. J. 2023, 31, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Shnaider, N.A.; Dmitrenko, D.V. Chronic valproic acid intoxication in epileptology: Diagnosis and treatment. Neurol. Neuropsychiatry Psychosom. 2016, 8, 94–99. (In Russian) [Google Scholar] [CrossRef]
- Iannaccone, T.; Sellitto, C.; Manzo, V.; Colucci, F.; Giudice, V.; Stefanelli, B.; Iuliano, A.; Corrivetti, G.; Filippelli, A. Pharmacogenetics of carbamazepine and valproate: Focus on polymorphisms of drug metabolizing enzymes and transporters. Pharmaceuticals 2021, 14, 204. [Google Scholar] [CrossRef]
- Caudle, K.E.; Dunnenberger, H.M.; Freimuth, R.R.; Peterson, J.F.; Burlison, J.D.; Whirl-Carrillo, M.; Scott, S.A.; Rehm, H.L.; Williams, M.S.; Klein, T.E.; et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet. Med. Off. J. Am. Coll. Med. Genet. 2017, 19, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, J.M.J.L.; Nijenhuis, M.; Soree, B.; Guchelaar, H.J.; Swen, J.J.; van Schaik, R.H.N.; Weide, J.V.; Rongen, G.A.P.J.M.; Buunk, A.M.; de Boer-Veger, N.J.; et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. Eur. J. Hum. Genet. EJHG 2022, 30, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Bochanova, E.A.; Gusev, S.D. The frequency and structure of adverse drug reactions in the pharmacotherapy of epilepsy. Pers. Psychiatry Neurol. 2024, 4, 18–25. [Google Scholar] [CrossRef]
- Malygin, A.S.; Popov, N.S.; Demidova, M.A.; Kudryashova, M.N. Chromatography-tandem MASS spectrometry (HPLC-MS/MS) for the detection of valproic acid and its metabolites in blood plasma. Epilepsy Paroxysmal Cond. 2018, 10, 35–42. [Google Scholar] [CrossRef]
- Damegunta, S.R. Time Matters!: When is the right time to estimate serum valproic acid levels? Indian J. Psychol. Med. 2014, 36, 349–350. [Google Scholar] [CrossRef]
- Miroshnichenko, I.I.; Baymeeva, N.V.; Platova, A.I. Determination of serum/plasma concentrations of psychotropic drugs in therapeutic drug monitoring. Pharmacokinet. Pharmacodinamics 2021, 1, 3–13. [Google Scholar] [CrossRef]
- Reed, R.C.; Dutta, S. Does it really matter when a blood sample for valproic acid concentration is taken following once-daily administration of divalproex-ER? Ther. Drug Monit. 2006, 28, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Pagana, K.D.; Pagana, T.J.; Pagana, T.N. Mosby’s Diagnostic & Laboratory Test Reference; Elsevier: St. Louis, MO, USA, 2019; p. 14. [Google Scholar]
- Valproic Acid: The Test. Available online: http://labtestsonline.org/understanding/analytes/valproic-acid/tab/test (accessed on 12 December 2023).
- Brunton, L.L.; Chabner, B.A.; Knollmann, B.C. Pharmacotherapy of the Epilepsies, Valproic Acid. In Goodman & Gilman’s the Pharmacological Basis of Therapeutics; The McGraw-Hill Companies: New York, NY, USA, 2011; Chapter 12; p. 21. [Google Scholar]
- Wu, X.; Dong, W.; Li, H.; Yang, X.; Jin, Y.; Zhang, Z.; Jiang, Y. CYP2C9*3/*3 gene expression affects the total and free concentrations of valproic acid in pediatric patients with epilepsy. Pharmgenom. Pers. Med. 2021, 14, 417–430. [Google Scholar] [CrossRef]
- Sabin, O.; Pop, R.; Trifa, A.; Buzoianu, A.D. The influence of CYP2C9, CYP2C19 and ABCB1 polymorphisms on the plasma concentrations of valproic acid in epileptic patients. Int. J. Bioflux Soc. 2016, 8, 29–33. [Google Scholar]
- Song, C.; Li, X.; Mao, P.; Song, W.; Liu, L.; Zhang, Y. Impact of CYP2C19 and CYP2C9 gene polymorphisms on sodium valproate plasma concentration in patients with epilepsy. Eur. J. Hosp. Pharm. 2022, 29, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, P.N.; Orekhova, N.V.; Antoniuk, M.V.; Filatova, N.V.; Shnayder, N.A.; Dmitrenko, D.V.; Zobova, S.N.; Poverennova, I.E.; Yakunina, A.V.; Kalinin, V.A.; et al. Efficacy and safety of valproic acid preparations with delayed release of active substance in adults in real clinical practice from the position of pharmacokinetic and pharmacogenetic approach. Neurol. Neuropsychiatry Psychosom. 2017, 9 (Suppl. 1), 11–20. [Google Scholar] [CrossRef]
- Kantemirova, B.I.; Starodubtsev, A.K.; Sychev, D.A.; Belopasov, B.B.; Tsotsonava, J.M.; Griganov, V.I. Ways of improvement of pharmacotherapy of epilepsy in children: Focus on specific features of drug biotransformation. Epilepsy Paroxysmal Cond. 2012, 4, 14–18. [Google Scholar]
- Moskaleva, P.V.; Shnayder, N.A.; Nasyrova, R.F. Timing of pharmacogenetic testing: Before or after the development of adverse drug reactions? Pharmacogenet. Pharmacogenom. 2018, 2, 56–57. [Google Scholar]
- Wang, C.; Wang, P.; Yang, L.P.; Pan, J.; Yang, X.; Ma, H.Y. Association of CYP2C9, CYP2A6, ACSM2A, and CPT1A gene polymorphisms with adverse effects of valproic acid in Chinese patients with epilepsy. Epilepsy Res. 2017, 132, 64–69. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Song, M.; Yan, P.; Ju, X.; Liu, J.; Wang, C.; Shi, J. Effect of CYP2C19 polymorphisms on serum valproic level acid in Chinese Han patients with schizophrenia. Sci. Rep. 2021, 11, 23150. [Google Scholar] [CrossRef]
- Narjis, M.; Nada, M.; Mujeeb, U.S. Pharmacokinetic mechanisms underlying clinical cases of valproic acid autoinduction: A review. J. Affect. Disord. Rep. 2022, 10, 100426. [Google Scholar] [CrossRef]
- Fang, H.; Wang, X.; Hou, K.; Zhang, Y.; Shao, S.; Zhang, G.; Feng, Y.; Huang, L. The association of adjusted plasma valproic acid concentration with CYP2C9 gene polymorphism in patients with epilepsy: A systematic review and meta-analysis. Ann. Transl. Med. 2021, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Nagini, S. Cytochrome P450 structure, function and clinical significance: A review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Abdyrakhmanova, A.K.; Nasyrova, R.F. Oxidation of antipsychotics. Encyclopedia 2022, 2, 974–989. [Google Scholar] [CrossRef]
- Shnayder, N.A.; Abdyrakhmanova, A.K.; Nasyrova, R.F. Phase I of antipsychotics metabolism and its pharmacogenetic testing. Pers. Psychiatry Neurol. 2022, 2, 4–21. [Google Scholar] [CrossRef]
- Guengerich, F.P. Mechanisms of cytochrome P450-catalyzed oxidations. ACS Catal. 2018, 8, 10964–10976. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 enzymes and drug metabolism in humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, M.; So, R.; Yoshimura, Y.; Yamashita, R.; Yada, Y.; Kodama, M.; Nakajima, S.; Kishi, Y.; Takeda, T.; Yamada, N.; et al. Effect of smoking habits and concomitant valproic acid use on relapse in patients with treatment-resistant schizophrenia receiving clozapine: A 1-year retrospective cohort study. Acta Psychiatr. Scand. 2023, 148, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Mondragón-Jiménez, J.F.; Ojeda-Lara, B.; Alvarez-Aguilar, A.; Brito-Pérez, C.A.; Rosales-Hernández, F.J.; González-Pérez, M. Interaction of valproic acid vs. neurotransmitters and the relationship to nicotine by the quantum method. World J. Pharm. Res. 2017, 6, 96–103. [Google Scholar] [CrossRef][Green Version]
- Drokov, A.P.; Lipatova, L.V.; Shnayder, N.A.; Nasyrova, R.F. Pharmacogenetic Markers for Metabolic Impairments in Treatment with Valproic Acid. Neurosci. Behav. Phys. 2020, 50, 13–19. [Google Scholar] [CrossRef]
- Jia, Y.; Tang, L.; Yao, Y.; Zhuo, L.; Qu, D.; Chen, X.; Ji, Y.; Tao, J.; Zhu, Y. Low-intensity exercise combined with sodium valproate attenuates kainic acid-induced seizures and associated co-morbidities by inhibiting NF-κB signaling in mice. Front. Neurol. 2022, 13, 993405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Liu, L.S.; Chu, X.M.; Xie, H.; Cao, L.J.; Guo, C.; A, J.Y.; Cao, B.; Li, M.J.; Wang, G.J.; et al. Combined effects of a high-fat diet and chronic valproic acid treatment on hepatic steatosis and hepatotoxicity in rats. Acta Pharmacol. Sin. 2014, 35, 363–372. [Google Scholar] [CrossRef]
- Zhu, M.M.; Li, H.L.; Shi, L.H.; Chen, X.P.; Luo, J.; Zhang, Z.L. The pharmacogenomics of valproic acid. J. Hum. Genet. 2017, 62, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Ellisy, R.A.; Bakri, A.H.; Mangoura, S.A.; Abdelraheem, M.H.; Farghaly, H.S. Genetic polymorphisms associated with valproic acid therapy: Review article. SVU-Int. J. Med. Sci. 2022, 5, 355–361. [Google Scholar] [CrossRef]
- Verrotti, A.; Mencaroni, E.; Cofini, M.; Castagnino, M.; Leo, A.; Russo, E.; Belcastro, V. Valproic acid metabolism and its consequences on sexual functions. Curr. Drug Metab. 2016, 17, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, D.; Ramaswamy, R.; Kurlander, J.E.; DeRiggi, A.; Riba, M. Valproic acid in women and girls of childbearing age. Curr. Psychiatry Rep. 2017, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Glauser, T.A.; Cnaan, A.; Shinnar, S.; Hirtz, D.G.; Dlugos, D.; Masur, D.; Clark, P.O.; Adamson, P.C. Childhood Absence Epilepsy Study Team. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: Initial monotherapy outcomes at 12 months. Epilepsia 2013, 54, 141–155. [Google Scholar] [CrossRef]
- Stephen, J. Drug treatment of epilepsy in elderly people: Focus on valproic Acid. Drugs Aging 2003, 20, 141–152. [Google Scholar] [CrossRef]
- Perucca, E.; Aldenkamp, A.; Tallis, R.; Krämer, G. Role of valproate across the ages. Treatment of epilepsy in the elderly. Acta Neurol. Scand. Suppl. 2006, 184, 28–37. [Google Scholar] [CrossRef]
- Meseguer, E.S.; Elizalde, M.U.; Borobia, A.M.; Ramírez, E. Valproic Acid-Induced Liver Injury: A Case-Control Study from a Prospective Pharmacovigilance Program in a Tertiary Hospital. J. Clin. Med. 2021, 10, 1153. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012; Updated 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548284 (accessed on 10 March 2024).
- Ruiz-Giménez, J.; Sánchez-Alvarez, J.C.; Cañadillas-Hidalgo, F.; Serrano-Castro, P.J. Andalusian Epilepsy Society. Antiepileptic treatment in patients with epilepsy and other comorbidities. Seizure 2010, 19, 375–382. [Google Scholar] [CrossRef] [PubMed]
- de Leon, J. The effects of antiepileptic inducers in neuropsychopharmacology, a neglected issue. Part II: Pharmacological issues and further understanding. Rev. Psiquiatr. Salud Ment. 2015, 8, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Bilir, E.; Zengil, H.; Babaoglu, M.O.; Bozkurt, A.; Yasar, U. Inhibitory effect of valproic acid on cytochrome P450 2C9 activity in epilepsy patients. Basic Clin. Pharmacol. Toxicol. 2007, 100, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, P.; Capone, P.; Sisto, R. Contribution of Genetic Polymorphisms in Human Health. Int. J. Environ. Res. Public Health 2023, 20, 912. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, N.; Suyama, M. In silico identification of pseudo-exon activation events in personal genome and transcriptome data. RNA Biol. 2021, 18, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wu, L.X.; Tan, L.; Shang, F.F.; Zhou, H.H. Significance of Single-Nucleotide Variants in Long Intergenic Non-protein Coding RNAs. Front. Cell Dev. Biol. 2020, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Botton, M.R.; Whirl-Carrillo, M.; Del Tredici, A.L.; Sangkuhl, K.; Cavallari, L.H.; Agúndez, J.A.G.; Duconge, J.; Lee, M.T.M.; Woodahl, E.L.; Claudio-Campos, K.; et al. PharmVar GeneFocus: CYP2C19. Clin. Pharmacol. Ther. 2021, 109, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Kumondai, M.; Hishinuma, E.; Gutiérrez Rico, E.M.; Ito, A.; Nakanishi, Y.; Saigusa, D.; Hirasawa, N.; Hiratsuka, M. Heterologous expression of high-activity cytochrome P450 in mammalian cells. Sci. Rep. 2020, 10, 14193. [Google Scholar] [CrossRef]
- Giantin, M.; Rahnasto-Rilla, M.; Tolosi, R.; Lucatello, L.; Pauletto, M.; Guerra, G.; Pezzato, F.; Lopparelli, R.M.; Merlanti, R.; Carnier, P.; et al. Functional impact of cytochrome P450 3A (CYP3A) missense variants in cattle. Sci. Rep. 2019, 9, 19672. [Google Scholar] [CrossRef]
- Deng, N.; Zhou, H.; Fan, H.; Yuan, Y. Single nucleotide polymorphisms and cancer susceptibility. Oncotarget 2017, 8, 110635–110649. [Google Scholar] [CrossRef]
- Rigau, M.; Juan, D.; Valencia, A.; Rico, D. Intronic CNVs and gene expression variation in human populations. PLoS Genet. 2019, 15, e1007902. [Google Scholar] [CrossRef] [PubMed]
- Taleb, A.; Lin, W.; Xu, X.; Zhang, G.; Zhou, Q.G.; Naveed, M.; Meng, F.; Fukunaga, K.; Han, F. Emerging mechanisms of valproic acid-induced neurotoxic events in autism and its implications for pharmacological treatment. Biomed. Pharmacother. 2021, 137, 111322. [Google Scholar] [CrossRef]
- Lin, Y.L.; Bialer, M.; Cabrera, R.M.; Finnell, R.H.; Wlodarczyk, B.J. Teratogenicity of valproic acid and its constitutional isomer, amide derivative valnoctamide in mice. Birth Defects Res. 2019, 111, 1013–1023. [Google Scholar] [CrossRef]
- Nasyrova, R.F.; Neznanov, N.G. Clinical Psychopharmacogenetics; DEAN: St. Petersburg, Russia, 2019; p. 405. [Google Scholar]
- Nasyrova, R.F.; Sivakova, N.A.; Lipatova, L.V.; Ivashchenko, D.V.; Sosina, K.A.; Drokov, A.P.; Shnayder, N.A. Biological markers of efficacy and safety of antiepileptic drugs: Pharmacogenetics and pharmacokinetics. Sib. Med. Rev. 2017, 103, 17–25. (In Russian) [Google Scholar] [CrossRef]
- Monti, B.; Polazzi, E.; Contestabile, A. Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr. Mol. Pharmacol. 2009, 2, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.S.; Rocha, M.A.; Mello, M.L.S. Epigenetic studies in insects and the valproic acid perspective. Braz. J. Biol. 2022, 84, e256045. [Google Scholar] [CrossRef] [PubMed]
- Bűdi, T.; Tóth, K.; Nagy, A.; Szever, Z.; Kiss, Á.; Temesvári, M.; Háfra, E.; Garami, M.; Tapodi, A.; Monostory, K. Clinical significance of CYP2C9-status guided valproic acid therapy in children. Epilepsia 2015, 56, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.H.; Liu, Z.Q. Ethnic differences in drug metabolism. Clin. Chem. Lab. Med. 2000, 38, 899–903. [Google Scholar] [CrossRef]
- SNPedia. Available online: http://www.SNPedia.com (accessed on 8 December 2023).
- Marcus, J.; Novembre, J.; Tharsen, J.; Mueller, A. Geography of Genetic Variants Browser. Department of Human Genetics, University of Chicago. Available online: https://popgen.uchicago.edu/ggv/?data=%221000genomes%22&chr=1&pos=222087833 (accessed on 15 February 2024).
- National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 15 January 2023).
- Online Mendelian Inheritance in Man (OMIM®) 1966–2023, Johns Hopkins University. Available online: https://omim.org (accessed on 10 February 2024).
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 23 January 2024).
- Francis Lam, Y.W. Chapter 1—Principles of Pharmacogenomics: Pharmacokinetic, Pharmacodynamic, and Clinical Implications. In Pharmacogenomics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–53. [Google Scholar] [CrossRef]
- Safaa, M.; Alsanosi, M.; Skiffington, C.; Padmanabhan, S. Chapter 17—Pharmacokinetic Pharmacogenomics. In Handbook of Pharmacogenomics and Stratified Medicine; Elsevier: Amsterdam, The Netherlands, 2014; pp. 341–364. [Google Scholar] [CrossRef]
- Kiang, T.K.; Ho, P.C.; Anari, M.R.; Tong, V.; Abbott, F.S.; Chang, T.K. Contribution of CYP2C9, CYP2A6, and CYP2B6 to valproic acid metabolism in hepatic microsomes from individuals with the CYP2C9*1/*1 genotype. Toxicol. Sci. 2006, 94, 261–271. [Google Scholar] [CrossRef]
- Tornio, A.; Backman, J.T. Cytochrome P450 in Pharmacogenetics: An Update. Adv. Pharmacol. 2018, 83, 3–32. [Google Scholar] [CrossRef]
- Schaffenburg, W.C.; Lockshin, B.N.; DeKlotz, C.M.C. Chapter 3—Polymorphisms: Why Individual Drug Responses Vary. In Comprehensive Dermatologic Drug Therapy, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 21–33.e2. [Google Scholar] [CrossRef]
- Vuppalanchi, R.M. Chapter 22—Metabolism of Drugs and Xenobiotics. In Practical Hepatic Pathology: A Diagnostic Approach, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 319–326. [Google Scholar] [CrossRef]
- Langmia, I.M.; Just, K.S.; Yamoune, S.; Brockmöller, J.; Masimirembwa, C.; Stingl, J.C. CYP2B6 Functional variability in drug metabolism and exposure across populations-implication for drug safety, dosing, and individualized therapy. Front. Genet. 2021, 12, 692234. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A. Chapter 4—Genetic Factors Associated With Opioid Therapy and Opioid Addiction. In Fighting the Opioid Epidemic; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–88. [Google Scholar] [CrossRef]
- Sparreboom, A.; Evans, W.E.; Baker, S.D. 6—Chemotherapy in the Pediatric Patient. In Oncology of Infancy and Childhood; Elsevier: Amsterdam, The Netherlands, 2009; pp. 173–207. [Google Scholar] [CrossRef]
- Mittal, B.; Tulsyan, S.; Kumar, S.; Mittal, R.D.; Agarwal, G. Cytochrome P450 in Cancer Susceptibility and Treatment. Adv. Clin. Chem. 2015, 71, 77–139. [Google Scholar] [CrossRef] [PubMed]
- Sangkuhl, K.; Claudio-Campos, K.; Cavallari, L.H.; Agundez, J.A.G.; Whirl-Carrillo, M.; Duconge, J.; Del Tredici, A.L.; Wadelius, M.; Rodrigues Botton, M.; Woodahl, E.L.; et al. PharmVar GeneFocus: CYP2C9. Clin. Pharmacol. Ther. 2021, 110, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Van Booven, D.; Marsh, S.; McLeod, H.; Carrillo, M.W.; Sangkuhl, K.; Klein, T.E.; Altman, R.B. Cytochrome P450 2C9-CYP2C9. Pharmacogenet. Genom. 2010, 20, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Nevosadová, L.; Eliasson, E.; Lauschke, V.M. Global distribution of functionally important CYP2C9 alleles and their inferred metabolic consequences. Hum. Genom. 2023, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Amini-Shirazi, N.; Ghahremani, M.H.; Ahmadkhaniha, R.; Mandegary, A.; Dadgar, A.; Abdollahi, M.; Shadnia, S.; Pakdaman, H.; Kebriaeezadeh, A. Influence of CYP2C9 polymorphism on metabolism of valproate and its hepatotoxin metabolite in Iranian patients. Toxicol. Mech. Methods 2010, 20, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.C.; Abbott, F.S.; Zanger, U.M.; Chang, T.K. Influence of CYP2C9 genotypes on the formation of a hepatotoxic metabolite of valproic acid in human liver microsomes. Pharmacogenom. J. 2003, 3, 335–342. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Ahn, M.H.; Yee, J.; Lee, N.; Han, J.M.; Gwak, H.S. Influence of CYP2C9 and CYP2A6 on plasma concentrations of valproic acid: A meta-analysis. Eur. J. Clin. Pharmacol. 2020, 76, 1053–1058. [Google Scholar] [CrossRef]
- Umamaheswaran, G.; Deepak, G.S. Chapter 46—Pharmacogenomics in India. In Handbook of Pharmacogenomics and Stratified Medicine; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1037–1059. [Google Scholar] [CrossRef]
- Cacabelos, R.; Cacabelos, P.; Torrellas, C. Chapter 27—Personalized Medicine of Alzheimer’s Disease. In Handbook of Pharmacogenomics and Stratified Medicine; Elsevier: Amsterdam, The Netherlands, 2014; pp. 563–615. [Google Scholar] [CrossRef]
- Shao, Z.; Kyriakopoulou, L.G.; Ito, S. Chapter 14—Pharmacogenomics. In Handbook of Analytical Separations; Elsevier: Amsterdam, The Netherlands, 2020; pp. 321–353. [Google Scholar] [CrossRef]
- Nebert, D.W.; Zhang, G. Chapter 16—Pharmacogenomics. In Emery and Rimoin’s Principles and Practice of Medical Genetics and Genomics, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 445–486. [Google Scholar] [CrossRef]
- Dehbozorgi, M.; Kamalidehghan, B.; Hosseini, I.; Dehghanfard, Z.; Sangtarash, M.H.; Firoozi, M.; Ahmadipour, F.; Meng, G.Y.; Houshmand, M. Prevalence of the CYP2C19*2 (681 G>A), *3 (636 G>A) and *17 (-806 C>T) alleles among an Iranian population of different ethnicities. Mol. Med. Rep. 2018, 17, 4195–4202. [Google Scholar] [CrossRef]
- Payan, M.; Tajik, N.; Rouini, M.R.; Ghahremani, M.H. Genotype and allele frequency of CYP2C19*17 in a healthy Iranian population. Med. J. Islam. Repub. Iran 2015, 29, 269. [Google Scholar] [PubMed]
- Wolf, K.K.; Paine, M.F.; Watkins, P.B. Chapter 10.05—Metabolic barrier of the gastrointestinal tract. In Comprehensive Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 53–75. [Google Scholar] [CrossRef]
- Kawakami, M.; Takenoshita-Nakaya, S.; Takeba, Y.; Nishimura, Y.; Oda, M.; Watanabe, M.; Ohta, Y.; Kobayashi, S.; Ohtsubo, T.; Kobayashi, S.; et al. Evaluation of CYP2D6 protein expression and activity in the small intestine to determine its metabolic capability in the Japanese population. Biol. Pharm. Bull. 2017, 40, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Demkow, U. Chapter 11—Next generation sequencing in pharmacogenomics. In Clinical Applications for Next-Generation Sequencing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 217–240. [Google Scholar] [CrossRef]
- McGraw, J. Chapter 16—CYP450 and ethnicity. In Handbook of Pharmacogenomics and Stratified Medicine; Elsevier: Amsterdam, The Netherlands, 2014; pp. 323–340. [Google Scholar] [CrossRef]
- Hebert, M.F. Chapter 3—Impact of pregnancy on maternal pharmacokinetics of medications. In Clinical Pharmacology during Pregnancy; Elsevier: Amsterdam, The Netherlands, 2013; pp. 17–39. [Google Scholar] [CrossRef]
- Zobova, S.N.; Dmitrenko, D.V.; Shnayder, N.A.; Yakovleva, K.D.; Pervunina, A.V.; Pravdin, D.E.; Prusova, T.I. Role of carriage of single nucleotide variants of CYP2D6 and ABCB1 genes in the effectiveness of therapy with valproic acid preparations in patients with epilepsy. Pharmacogenet. Pharmacogenom. 2019, 2, 12. [Google Scholar] [CrossRef]
- Varela, N.M.; Cerpa, L.C.; Martínez, M.M.; Quiñones, L.A. Pharmacogenomics: Genetic Polymorphisms. In The ADME Encyclopedia; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Just, K.S.; Dormann, H.; Freitag, M.; Schurig, M.; Böhme, M.; Steffens, M.; Scholl, C.; Seufferlein, T.; Graeff, I.; Schwab, M.; et al. CYP2D6 in the Brain: Potential Impact on Adverse Drug Reactions in the Central Nervous System-Results from the ADRED Study. Front. Pharmacol. 2021, 12, 624104. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.; Chowdhury, A.R.; Feng, T.; Assenmacher, C.A.; Radaelli, E.; Guengerich, F.P.; Avadhani, N.G. Mitochondrially targeted cytochrome P450 2D6 is involved in monomethylamine-induced neuronal damage in mouse models. J. Biol. Chem. 2019, 294, 10336–10348. [Google Scholar] [CrossRef] [PubMed]
- Seripa, D.; Lozupone, M.; Stella, E.; Paroni, G.; Bisceglia, P.; La Montagna, M.; D’onofrio, G.; Gravina, C.; Urbano, M.; Priore, M.G.; et al. Psychotropic drugs and CYP2D6 in late-life psychiatric and neurological disorders. What do we know? Expert Opin. Drug Saf. 2017, 6, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Molden, E.; Jukić, M.M. CYP2D6 Reduced Function Variants and Genotype/Phenotype Translations of CYP2D6 Intermediate Metabolizers: Implications for Personalized Drug Dosing in Psychiatry. Front. Pharmacol. 2021, 12, 650750. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Bochanova, E.N.; Dmitrenko, D.V.; Nasyrova, R.F. Pharmacogenetics of carbamazepine. Epilepsy Paroxysmal Cond. 2019, 11, 364–378. [Google Scholar] [CrossRef]
- Shnayder, N.A.; Dmitrenko, D.V.; Pilugina, M.S. The pharmacogenetics antiepileptic drugs. Bull. Sib. Med. 2008, 7, 111–119. [Google Scholar] [CrossRef]
- Yakunina, A.V.; Povernova, I.E. The role of therapeutic drug monitoring in the use of antiepileptic drugs. Epilepsy Paroxysmal Cond. 2016, 8, 66–73. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Zhang, T.; Zhao, L. Risk Factors for valproic acid-induced hyperammonaemia in Chinese paediatric patients with epilepsy. Basic Clin. Pharmacol. Toxicol. 2018, 123, 628–634. [Google Scholar] [CrossRef]
- Nimesh, S.; Tomar, R.; Kumar, M.; Tyagi, N.; Shukla, P.K. A pharmacovigilance study of monitoring & focusing of adverse drug reactions induced by antiepileptic drugs used in epileptic patients. Pharm. Pharmacol. Int. J. 2019, 7, 100–104. [Google Scholar] [CrossRef]
- Lara, D.V.; Melo, D.O.; Silva, R.A.M.; Santos, P.C.J.L. Pharmacogenetic testing in psychiatry and neurology: An overview of reviews. Pharmacogenomics 2021, 22, 505–513. [Google Scholar] [CrossRef] [PubMed]
- van Schaik, R.H.N.; Müller, D.J.; Serretti, A.; Ingelman-Sundberg, M. Pharmacogenetics in psychiatry: An update on clinical usability. Front. Pharmacol. 2020, 11, 575540. [Google Scholar] [CrossRef]
- Hippman, C.; Nislow, C. Pharmacogenomic testing: Clinical evidence and implementation challenges. J. Pers. Med. 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Yee, S.W.; Giacomini, K.M. Pharmacogenetics of antidiabetic drugs. Adv. Pharmacol. 2018, 83, 361–389. [Google Scholar] [CrossRef]
- The Centre for Evidence-Based Medicine. Available online: https://www.cebm.net/ (accessed on 23 February 2024).
- Heneghan, C.; Aronson, J.K. Sodium valproate: Who knew what and when? Cumulative meta-analysis gives extra insights. BMJ Evid. Based Med. 2019, 24, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Grechkina, V.V.; Arkhipov, V.V.; Nasyrova, R.F. Pharmacogenetics-informed pharmacometabolomics as an innovative approach to assessing the safety and risk of pharmacotherapy with valproic acid. Saf. Risk Pharmacother. 2023, 11, 450–462. [Google Scholar] [CrossRef]
- Ji, Y.; Hebbring, S.; Zhu, H.; Jenkins, G.D.; Biernacka, J.; Snyder, K.; Drews, M.; Fiehn, O.; Zeng, Z.; Schaid, D.; et al. Glycine and a glycine dehydrogenase (GLDC) SNP as citalopram/escitalopram response biomarkers in depression: Pharmacometabolomics-informed pharmacogenomics. Clin. Pharmacol. Ther. 2011, 89, 97–104. [Google Scholar] [CrossRef]
- Ghodke-Puranik, Y.; Thorn, C.F.; Lamba, J.K.; Leeder, J.S.; Song, W.; Birnbaum, A.K.; Altman, R.B.; Klein, T.E. Valproic acid pathway: Pharmacokinetics and pharmacodynamics. Pharmacogenet. Genom. 2013, 23, 236–241. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Martinez-Lozano, N.; Rios, R.; Marcilla de Teruel, M.C.; Garaulet, M.; Cerón, J.J. Saliva as a non-invasive tool for assessment of metabolic and inflammatory biomarkers in children. Clin. Nutr. 2020, 39, 2471–2478. [Google Scholar] [CrossRef]
- de Sá Alves, M.; de Sá Rodrigues, N.; Bandeira, C.M.; Chagas, J.F.S.; Pascoal, M.B.N.; Nepomuceno, G.L.J.T.; da Silva Martinho, H.; Alves, M.G.O.; Mendes, M.A.; Dias, M.; et al. Identification of Possible Salivary Metabolic Biomarkers and Altered Metabolic Pathways in South American Patients Diagnosed with Oral Squamous Cell Carcinoma. Metabolites 2021, 11, 650. [Google Scholar] [CrossRef]
- Brunmair, J.; Gotsmy, M.; Niederstaetter, L.; Neuditschko, B.; Bileck, A.; Slany, A.; Feuerstein, M.L.; Langbauer, C.; Janker, L.; Zanghellini, J.; et al. Finger sweat analysis enables short interval metabolic biomonitoring in humans. Nat. Commun. 2021, 12, 5993. [Google Scholar] [CrossRef]
- Pham, Y.L.; Beauchamp, J. Breath Biomarkers in Diagnostic Applications. Molecules 2021, 26, 5514. [Google Scholar] [CrossRef]
- Trius-Soler, M.; Praticò, G.; Gürdeniz, G.; Garcia-Aloy, M.; Canali, R.; Natella, F.; Brouwer-Brolsma, E.M.; Andrés-Lacueva, C.; Dragsted, L.O. Biomarkers of moderate alcohol intake and alcoholic beverages: A systematic literature review. Genes Nutr. 2023, 18, 7. [Google Scholar] [CrossRef]
- Chachaj, A.; Matkowski, R.; Gröbner, G.; Szuba, A.; Dudka, I. Metabolomics of interstitial fluid, plasma and urine in patients with arterial hypertension: New insights into the underlying mechanisms. Diagnostics 2020, 10, 936. [Google Scholar] [CrossRef]
- Ashurov, Z.S. The evolution of personalized psychiatry. Pers. Psychiatry Neurol. 2023, 3, 1–2. [Google Scholar]


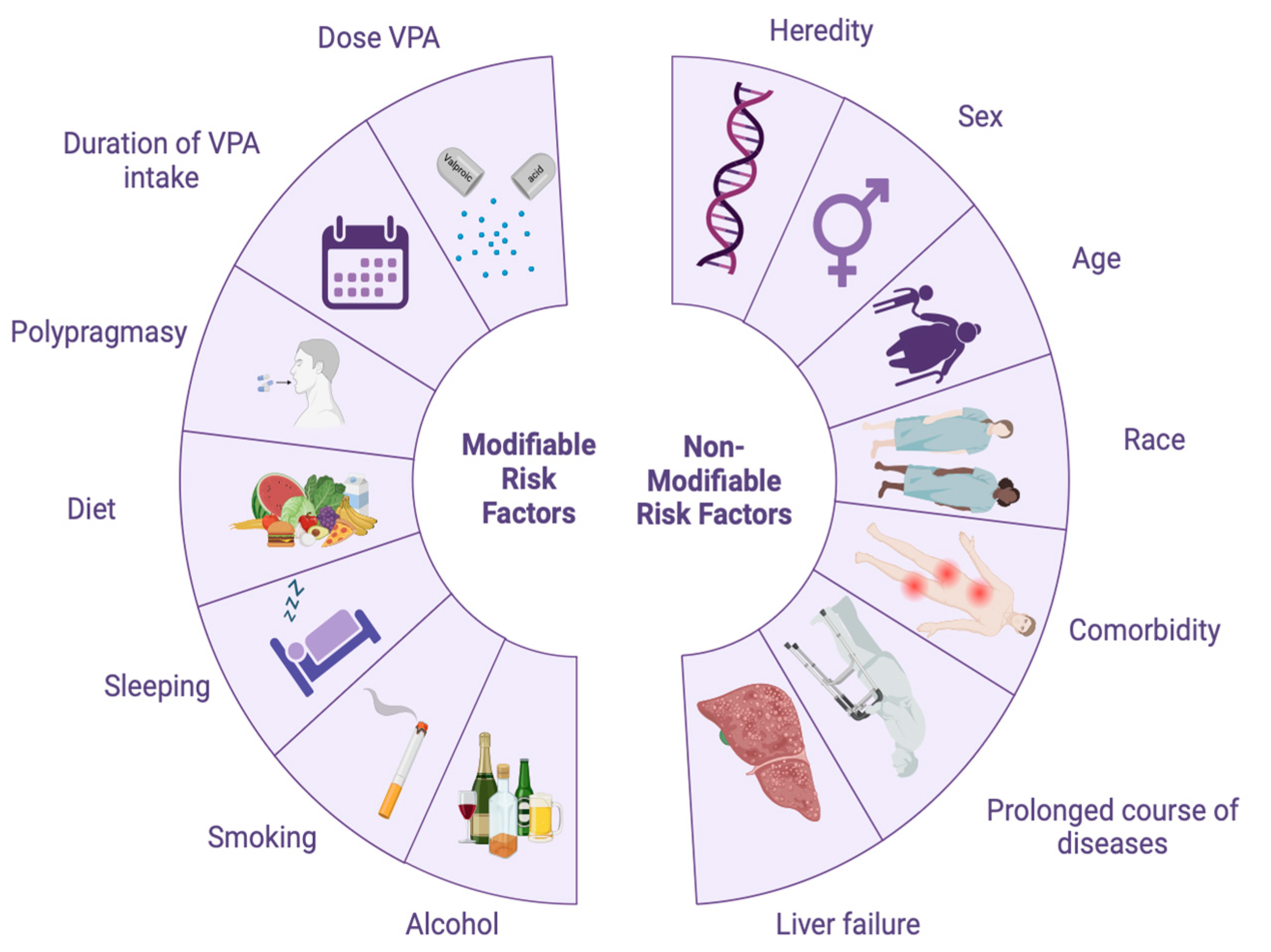


| Modifiable Risk Factors | Non-Modifiable Risk Factors |
|---|---|
| High doses of valproic acid | Heredity (monogenic hereditary liver and metabolic diseases) |
| Long-term use of valproic acid | Genetic predisposition to the impaired P-oxidation of valproic acid (IM and PM phenotypes) |
| Polypharmacy (simultaneous prescription of five or more drugs, especially those with a similar metabolic pathway) | Gender (female patients) |
| Poor nutrition | Age (elderly patients) |
| Stress | Ethnic and racial background of patients |
| Sleep disturbance | |
| Smoking | Comorbid illnesses and mental disorders |
| Alcohol abuse Low physical activity (sedentary lifestyle) | Duration of the disease Liver failure |
| Polytherapy (simultaneous administration of VPA and other psychotropic drugs with a similar metabolic pathway) |
| Isoenzyme and Tissue Expression Claster (Location) ** | Gene (OMIM), Location *** | Single Nucleotide Variant (RS ID) **** | Allele Frequency * | ||||
|---|---|---|---|---|---|---|---|
| North America | South America | Asia | Africa | Europe | |||
| CYP2A6 (cytochrome P450 family 2 subfamily A member 6). Liver—plasma proteins (mainly). Intracellular | CYP2A6 (122720), 19q13.2 | rs1801272 | 0.02 | 0.01 | 0.01 | - | 0.03 |
| rs111033610 | - | - | 0.01 | - | - | ||
| rs28399447 | - | - | 0.01 | - | - | ||
| rs4986891 | 0.01 | - | - | 0.045 | - | ||
| rs28399433 | 0.09 | 0.09 | 0.19 | 0.08 | 0.06 | ||
| rs28399447 | - | - | 0.01 | - | - | ||
| rs28399454 | 0.007 | 0.01 | 0.004 | 0.12 | - | ||
| rs1809810 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | ||
| rs72549435 | 0.007 | 0.005 | 0.004 | 0.01 | - | ||
| rs59552350 | 0.015 | - | - | 0.004 | - | ||
| rs4986891 | 0.015 | - | - | 0.004 | - | ||
| rs28399445 | - | 0.01 | - | 0.01 | - | ||
| CYP2C9 (cytochrome P450 family 2 subfamily C member 9). Liver—plasma proteins (mainly). Intracellular | CYP2C9 (601130), 10q23.33 | rs1799853 | 0.09 | 0.12 | 0.02 | - | 0.14 |
| rs1057910 | 0.03 | 0.03 | 0.1 | - | 0.08 | ||
| rs28371686 | 0.02 | 0.01 | - | 0.01 | - | ||
| rs7900194 | 0.02 | 0.04 | 0.005 | 0.05 | 0.004 | ||
| rs28371685 | 0.007 | 0.01 | 0.004 | 0.02 | 0.01 | ||
| rs72558187 | - | - | 0.04 | - | - | ||
|
CYP2B6 (cytochrome P450 family 2 subfamily B member 6). Liver—plasma proteins (mainly). Intracellular | CYP2B6 (123930), 19q13.2 | rs12721655 | 0.01 | 0.005 | 0.005 | - | - |
| rs28399499 | 0.005 | 0.02 | - | 0.07 | - | ||
| rs36079186 | - | - | 0.005 | 0.01 | - | ||
|
CYP3A4 (cytochrome P450 family 3 subfamily A member 4). Liver—metabolism (mainly). Membrane; intracellular | CYP3A4 (124010), 7q22.1 | rs4986910 | 0.01 | 0.01 | - | - | 0.01 |
| rs28371759 | - | - | 0.02 | - | - | ||
|
CYP2C19 (cytochrome P450 family 2 subfamily C member 19). Liver—metabolism (mainly). Intracellular | CYP2C19 (124020), 10q23.33 | rs4244285 | 0.12 | 0.11 | 0.3 | 0.18 | 0.1 |
| rs28399504 | 0.007 | 0.0004 | 0.0004 | - | 0.0004 | ||
| rs41291556 | 0.01 | - | - | - | - | ||
|
CYP2D6 (cytochrome P450 family 2 subfamily D member 6). Liver—plasma proteins (mainly). Membrane | CYP2D6, (124030) 22q13.2 | rs3892097 | 0.11 | 0.12 | 0.12 | 0.05 | 0.16 |
| rs5030865 | - | - | 0.01 | - | - | ||
| rs1065852 | 0.14 | 0.16 | 0.31 | 0.1 | 0.19 | ||
| rs28371706 | 0.01 | 0.01 | - | 0.2 | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shnayder, N.A.; Grechkina, V.V.; Trefilova, V.V.; Kissin, M.Y.; Narodova, E.A.; Petrova, M.M.; Al-Zamil, M.; Garganeeva, N.P.; Nasyrova, R.F. Ethnic Aspects of Valproic Acid P-Oxidation. Biomedicines 2024, 12, 1036. https://doi.org/10.3390/biomedicines12051036
Shnayder NA, Grechkina VV, Trefilova VV, Kissin MY, Narodova EA, Petrova MM, Al-Zamil M, Garganeeva NP, Nasyrova RF. Ethnic Aspects of Valproic Acid P-Oxidation. Biomedicines. 2024; 12(5):1036. https://doi.org/10.3390/biomedicines12051036
Chicago/Turabian StyleShnayder, Natalia A., Violetta V. Grechkina, Vera V. Trefilova, Mikhail Ya. Kissin, Ekaterina A. Narodova, Marina M. Petrova, Mustafa Al-Zamil, Natalia P. Garganeeva, and Regina F. Nasyrova. 2024. "Ethnic Aspects of Valproic Acid P-Oxidation" Biomedicines 12, no. 5: 1036. https://doi.org/10.3390/biomedicines12051036
APA StyleShnayder, N. A., Grechkina, V. V., Trefilova, V. V., Kissin, M. Y., Narodova, E. A., Petrova, M. M., Al-Zamil, M., Garganeeva, N. P., & Nasyrova, R. F. (2024). Ethnic Aspects of Valproic Acid P-Oxidation. Biomedicines, 12(5), 1036. https://doi.org/10.3390/biomedicines12051036








