Enrichment of Bioactive Lipids in Urinary Extracellular Vesicles and Evidence of Apoptosis in Kidneys of Hypertensive Diabetic Cathepsin B Knockout Mice after Streptozotocin Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Diet
2.3. Metabolic Cage Studies
2.4. STZ Injections
2.5. Blood Glucose Measurements
2.6. Blood Pressure
2.7. Urinary Extracellular Vesicle Isolation
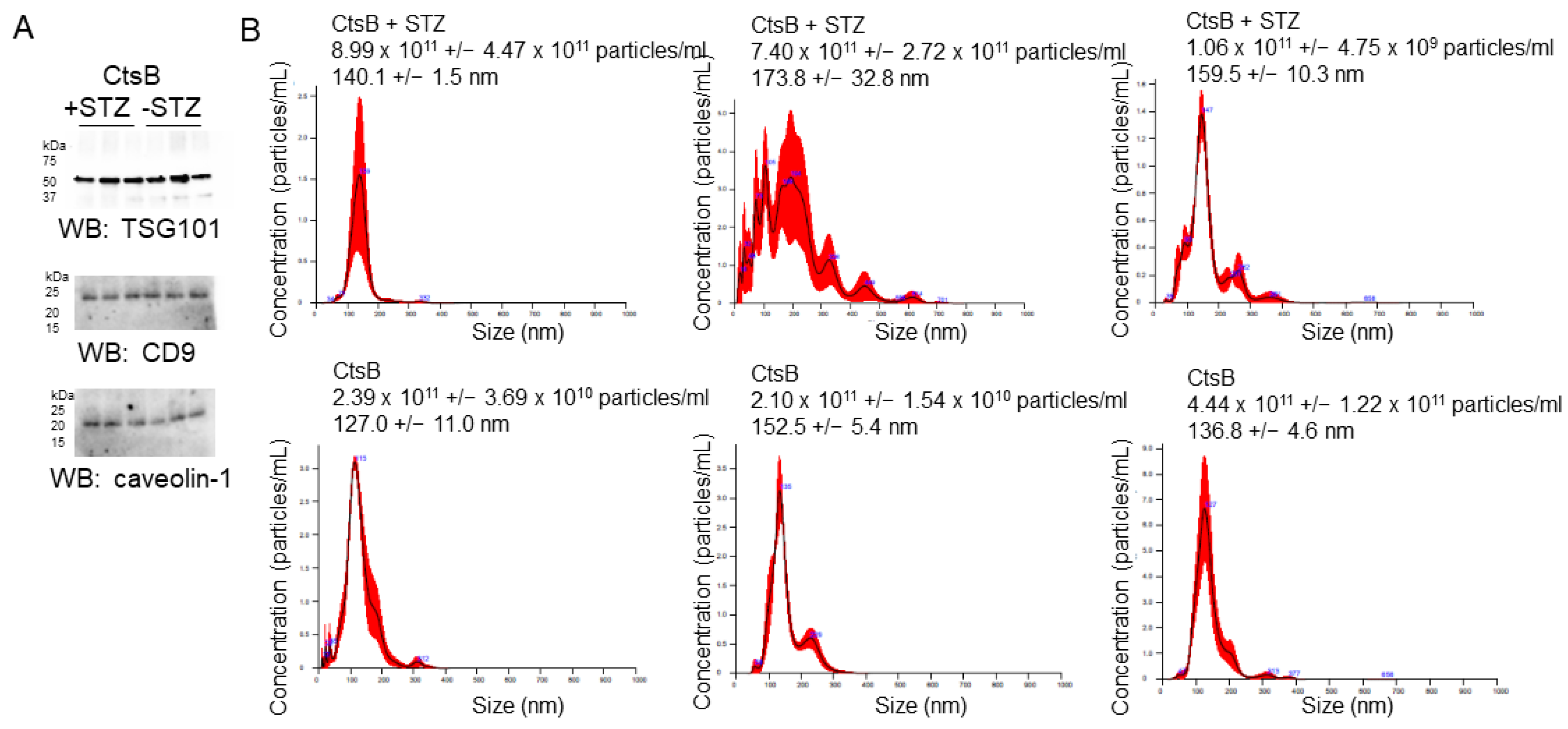
2.8. Nanoparticle Tracking Analysis
2.9. BCA Assay and Western Blotting
2.10. Lipid Extraction
2.11. LC-MS/MS
2.12. Lipid Quantitation
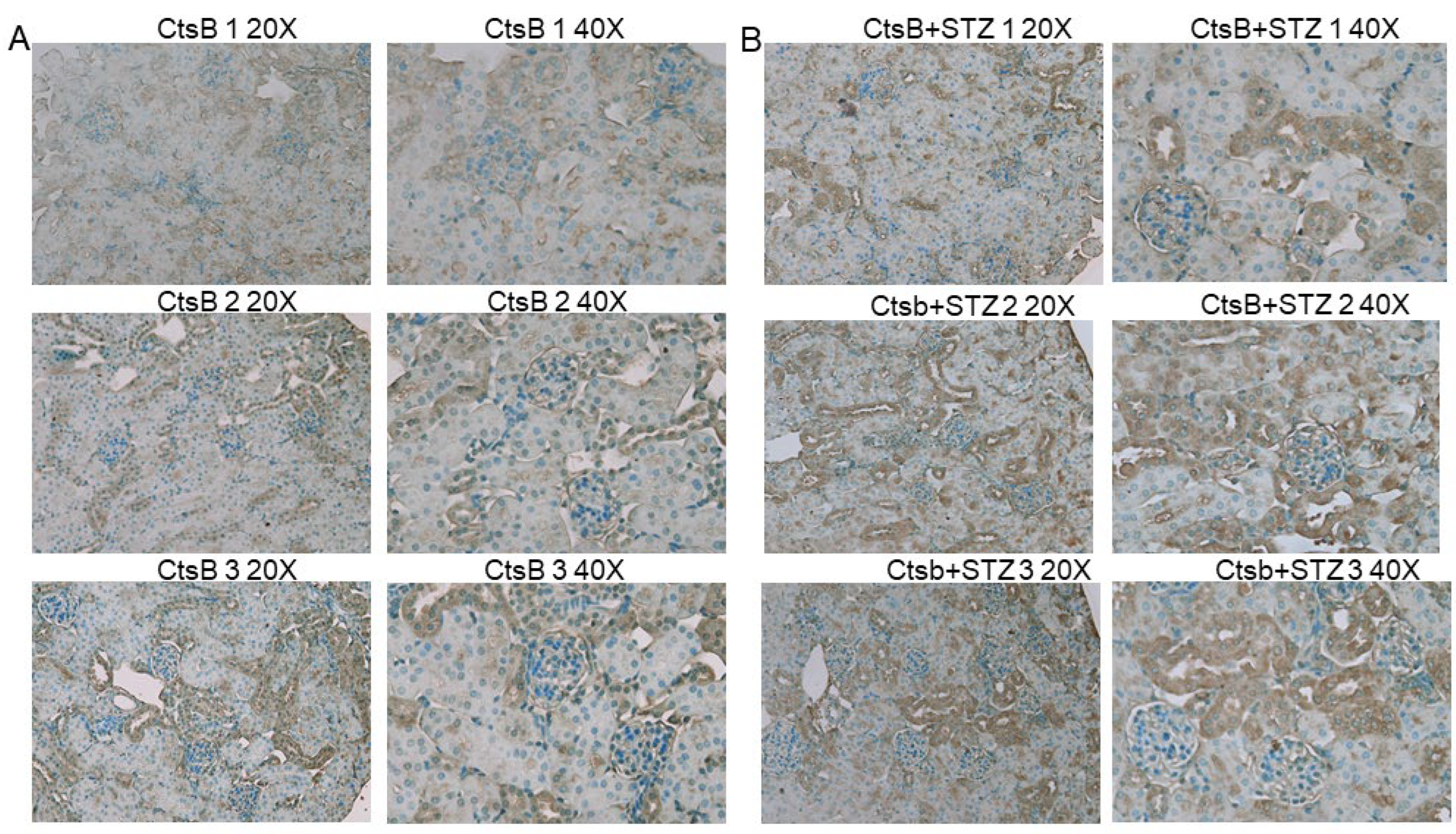
2.13. Immunohistochemistry
2.14. Data Analysis and Statistics
3. Results
3.1. STZ Increases Blood Glucose and Blood Pressure in CtsB Knockout Mice
3.2. Characterization of uEVs from CtsB Knockout Mice Treated with or without STZ
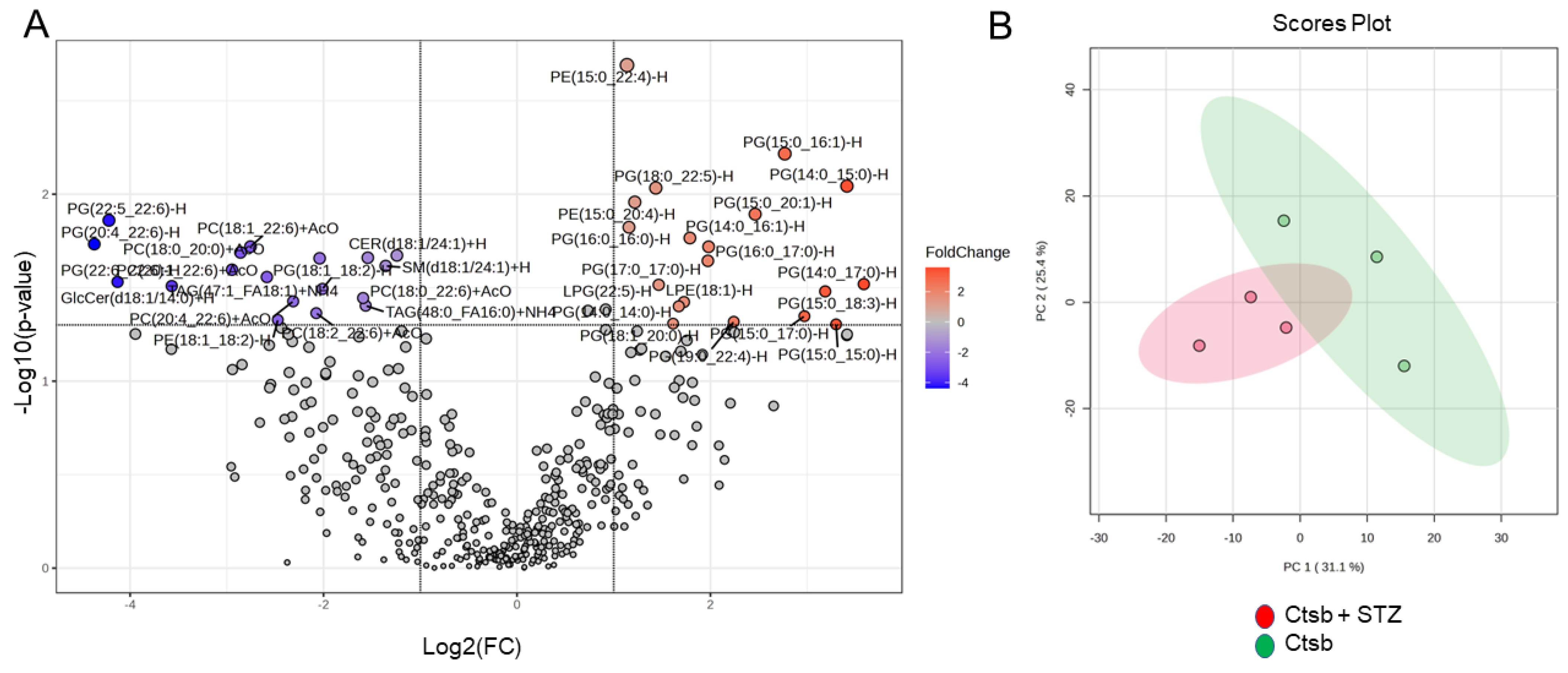
3.3. Lipidomic Analysis of uEVs from CtsB Knockout Mice Treated with or without STZ
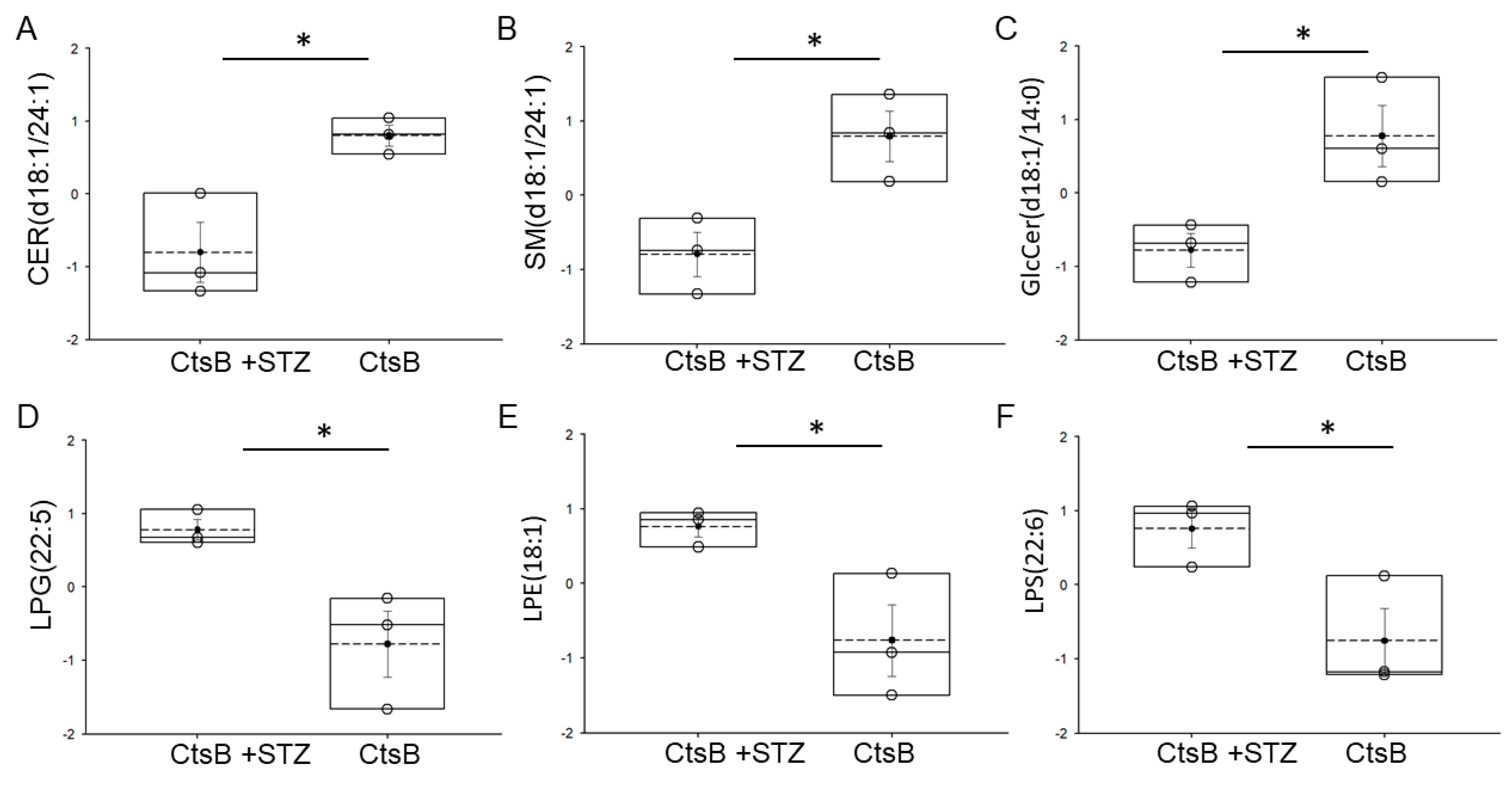
3.4. Detection of Bioactive Lipids in uEVs from CtsB Knockout Mice Treated with or without STZ
3.5. STZ Induces 4-HNE Upregulation in the Kidneys of CtsB Knockout Mice
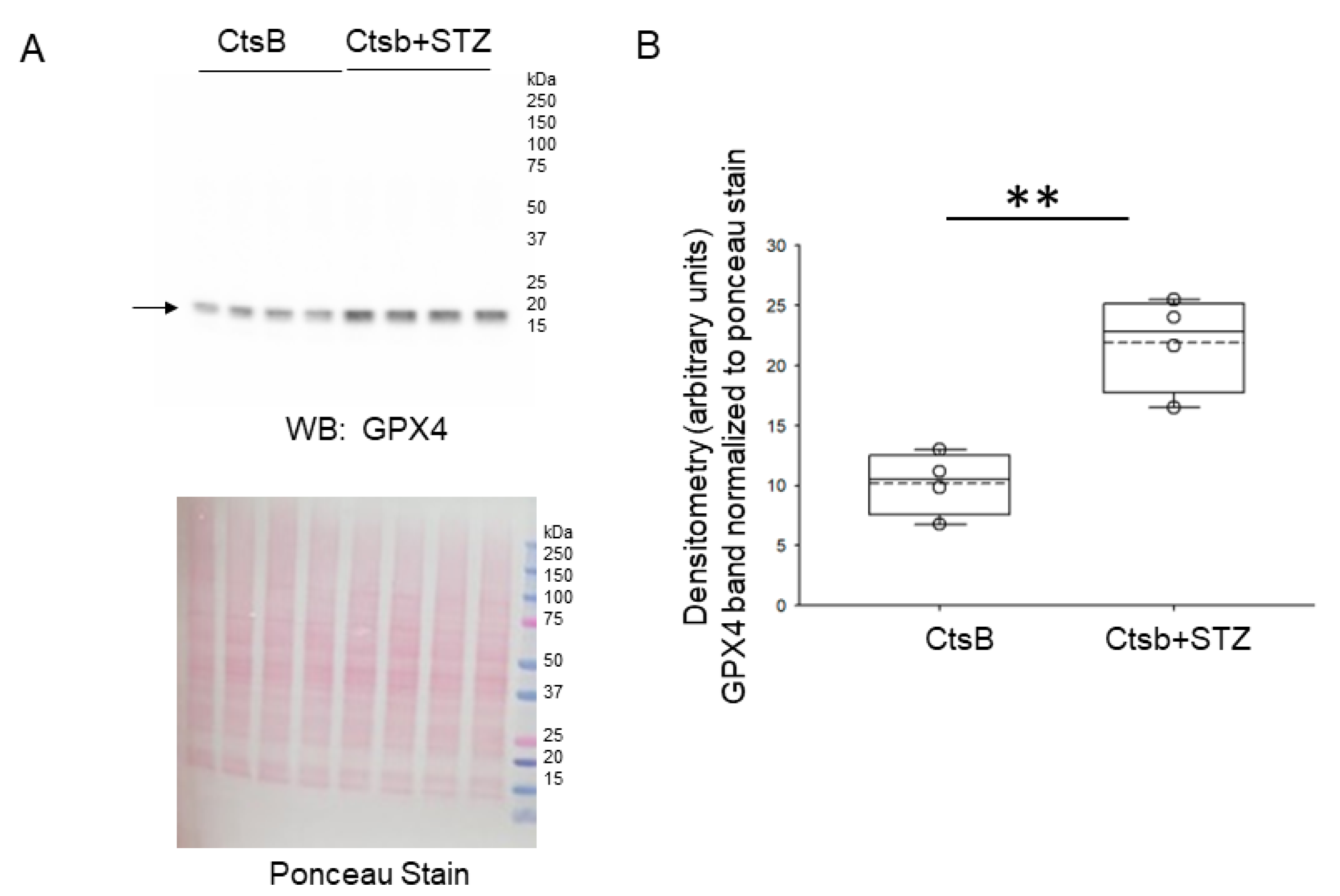
3.6. STZ Treatment of CtsB Knockout Mice Results in Increases in Renal GPX4 and XCT Protein Expression
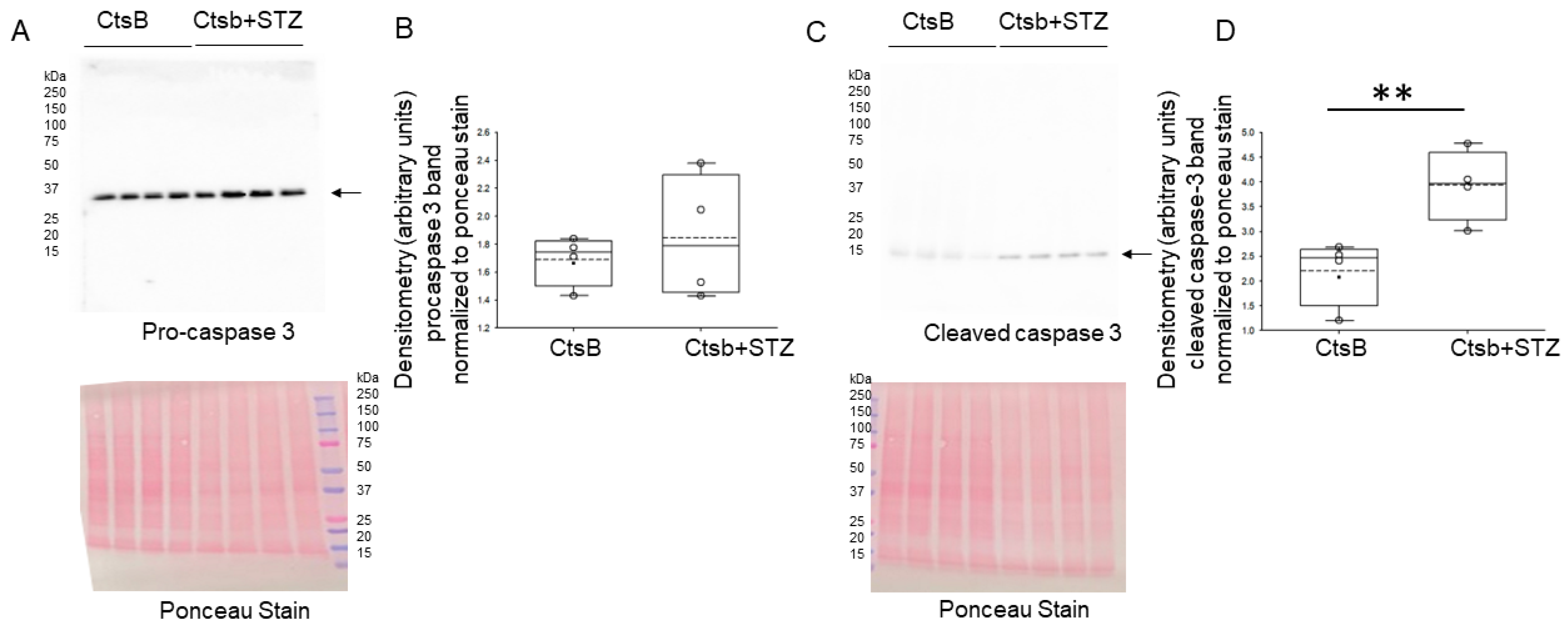
3.7. STZ Treatment Activates Caspase but Not ACSL4 in the Kidneys of CtsB Knockout Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alli, A.A. Extracellular Vesicles: Investigating the Pathophysiology of Diabetes-Associated Hypertension and Diabetic Nephropathy. Biology 2023, 12, 1138. [Google Scholar] [CrossRef]
- Beal, J.R.; Ma, Q.; Bagchi, I.C.; Bagchi, M.K. Role of Endometrial Extracellular Vesicles in Mediating Cell-to-Cell Communication in the Uterus: A Review. Cells 2023, 12, 2584. [Google Scholar] [CrossRef] [PubMed]
- Godakumara, K.; Dissanayake, K.; Hasan, M.M.; Kodithuwakku, S.P.; Fazeli, A. Role of extracellular vesicles in intercellular communication during reproduction. Reprod. Domest. Anim. 2022, 57 (Suppl. S5), 14–21. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Lu, F.; Chen, X.; Yang, D.; Cao, Y.; Zhang, W.; Chen, J.; Zheng, L.; Wang, G.; et al. Extracellular vesicles derived from oesophageal cancer containing P4HB promote muscle wasting via regulating PHGDH/Bcl-2/caspase-3 pathway. J. Extracell. Vesicles 2021, 10, e12060. [Google Scholar] [CrossRef]
- Lian, J.; Zhu, X.; Du, J.; Huang, B.; Zhao, F.; Ma, C.; Guo, R.; Zhang, Y.; Ji, L.; Yahaya, B.H.; et al. Extracellular vesicle-transmitted miR-671-5p alleviates lung inflammation and injury by regulating the AAK1/NF-kappaB axis. Mol. Ther. 2023, 31, 1365–1382. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, E.; Fujita, K.; Nonomura, N. Urinary Extracellular Vesicles: Ultracentrifugation Method. Methods Mol. Biol. 2021, 2292, 173–181. [Google Scholar] [CrossRef]
- Cappe, B.; Vadi, M.; Sack, E.; Wacheul, L.; Verstraeten, B.; Dufour, S.; Franck, J.; Xie, W.; Impens, F.; Hendrix, A.; et al. Systematic compositional analysis of exosomal extracellular vesicles produced by cells undergoing apoptosis, necroptosis and ferroptosis. J. Extracell. Vesicles 2023, 12, e12365. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Li, S.; Ning, L.G.; Lou, X.H.; Xu, G.Q. Necroptosis in inflammatory bowel disease and other intestinal diseases. World J. Clin. Cases 2018, 6, 745–752. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv. Protein Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar] [CrossRef]
- Liao, Z.; Tong, B.; Ou, Z.; Wei, J.; Lei, M.; Yang, C. The role of extracellular vesicles in iron homeostasis and ferroptosis: Focus on musculoskeletal diseases. Traffic 2023, 24, 384–396. [Google Scholar] [CrossRef]
- Wu, Q.; Ying, X.; Yu, W.; Li, H.; Wei, W.; Lin, X.; Zhang, X. Identification of ferroptosis-related genes in syncytiotrophoblast-derived extracellular vesicles of preeclampsia. Medicine 2022, 101, e31583. [Google Scholar] [CrossRef]
- Yelamanchili, S.V.; Lamberty, B.G.; Rennard, D.A.; Morsey, B.M.; Hochfelder, C.G.; Meays, B.M.; Levy, E.; Fox, H.S. Correction: MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease. PLoS Pathog. 2018, 14, e1007068. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, Z.; Loughran, P.A.; Fan, E.K.; Scott, M.J.; Li, Y.; Billiar, T.R.; Wilson, M.A.; Shi, X.; Fan, J. Frontline Science: Macrophage-derived exosomes promote neutrophil necroptosis following hemorrhagic shock. J. Leukoc. Biol. 2018, 103, 175–183. [Google Scholar] [CrossRef]
- Vennin, C.; Cattaneo, C.M.; Bosch, L.; Vegna, S.; Ma, X.; Damstra, H.G.J.; Martinovic, M.; Tsouri, E.; Ilic, M.; Azarang, L.; et al. Taxanes trigger cancer cell killing in vivo by inducing non-canonical T cell cytotoxicity. Cancer Cell 2023, 41, 1170–1185. [Google Scholar] [CrossRef]
- Zhou, G.; Gu, Y.; Zhou, F.; Zhang, H.; Zhang, M.; Zhang, G.; Wu, L.; Hua, K.; Ding, J. Adipocytes-Derived Extracellular Vesicle-miR-26b Promotes Apoptosis of Cumulus Cells and Induces Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 789939. [Google Scholar] [CrossRef]
- Jeon, J.S.; Kim, E.; Bae, Y.U.; Yang, W.M.; Lee, H.; Kim, H.; Noh, H.; Han, D.C.; Ryu, S.; Kwon, S.H. microRNA in Extracellular Vesicles Released by Damaged Podocytes Promote Apoptosis of Renal Tubular Epithelial Cells. Cells 2020, 9, 1409. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Larionov, A.; Dahlke, E.; Kunke, M.; Zanon Rodriguez, L.; Schiessl, I.M.; Magnin, J.L.; Kern, U.; Alli, A.A.; Mollet, G.; Schilling, O.; et al. Cathepsin B increases ENaC activity leading to hypertension early in nephrotic syndrome. J. Cell Mol. Med. 2019, 23, 6543–6553. [Google Scholar] [CrossRef]
- Numata, M.; Chu, H.W.; Dakhama, A.; Voelker, D.R. Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus-induced inflammation and infection. Proc. Natl. Acad. Sci. USA 2010, 107, 320–325. [Google Scholar] [CrossRef]
- Kandasamy, P.; Zarini, S.; Chan, E.D.; Leslie, C.C.; Murphy, R.C.; Voelker, D.R. Pulmonary surfactant phosphatidylglycerol inhibits Mycoplasma pneumoniae-stimulated eicosanoid production from human and mouse macrophages. J. Biol. Chem. 2011, 286, 7841–7853. [Google Scholar] [CrossRef]
- Kayser, B.D.; Lhomme, M.; Prifti, E.; Da Cunha, C.; Marquet, F.; Chain, F.; Naas, I.; Pelloux, V.; Dao, M.C.; Kontush, A.; et al. Phosphatidylglycerols are induced by gut dysbiosis and inflammation, and favorably modulate adipose tissue remodeling in obesity. FASEB J. 2019, 33, 4741–4754. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, R.; Chen, Y.; Guo, W.; Wang, J.; Cai, Z. In situ localization of lipids on mouse kidney tissues with acute cadmium toxicity using atmospheric pressure-MALDI mass spectrometry imaging. Talanta 2022, 245, 123466. [Google Scholar] [CrossRef]
- Kurano, M.; Jubishi, D.; Okamoto, K.; Hashimoto, H.; Sakai, E.; Morita, Y.; Saigusa, D.; Kano, K.; Aoki, J.; Harada, S.; et al. Dynamic modulations of urinary sphingolipid and glycerophospholipid levels in COVID-19 and correlations with COVID-19-associated kidney injuries. J. Biomed. Sci. 2022, 29, 94. [Google Scholar] [CrossRef]
- Dang, V.D.; Jella, K.K.; Ragheb, R.R.T.; Denslow, N.D.; Alli, A.A. Lipidomic and proteomic analysis of exosomes from mouse cortical collecting duct cells. FASEB J. 2017, 31, 5399–5408. [Google Scholar] [CrossRef]
- Chacko, K.M.; Nouri, M.Z.; Schramm, W.C.; Malik, Z.; Liu, L.P.; Denslow, N.D.; Alli, A.A. Tempol Alters Urinary Extracellular Vesicle Lipid Content and Release While Reducing Blood Pressure during the Development of Salt-Sensitive Hypertension. Biomolecules 2021, 11, 1804. [Google Scholar] [CrossRef]
- Deng, G.; Li, Y.; Ma, S.; Gao, Z.; Zeng, T.; Chen, L.; Ye, H.; Yang, M.; Shi, H.; Yao, X.; et al. Caveolin-1 dictates ferroptosis in the execution of acute immune-mediated hepatic damage by attenuating nitrogen stress. Free Radic. Biol. Med. 2020, 148, 151–161. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, S.; Lu, Y.; Wang, Q.; Wang, S.; Xun, Y. CAV1 alleviated CaOx stones formation via suppressing autophagy-dependent ferroptosis. PeerJ 2022, 10, e14033. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Z.; Pan, X.; Zhang, J.; Wang, X.; Wang, M.; Li, H.; Yan, M.; Chen, W. Caveolin-1 promotes cancer progression via inhibiting ferroptosis in head and neck squamous cell carcinoma. J. Oral. Pathol. Med. 2022, 51, 52–62. [Google Scholar] [CrossRef]
- Lopez, J.P.; Nouri, M.Z.; Ebrahim, A.; Chacko, K.M.; Schramm, W.C.; Gholam, M.F.; Ozrazgat-Baslanti, T.; Denslow, N.D.; Alli, A.A. Lipid Profiles of Urinary Extracellular Vesicles Released during the Inactive and Active Phases of Aged Male Mice with Spontaneous Hypertension. Int. J. Mol. Sci. 2022, 23, 15397. [Google Scholar] [CrossRef]
- Lugo, C.I.; Liu, L.P.; Bala, N.; Morales, A.G.; Gholam, M.F.; Abchee, J.C.; Elmoujahid, N.; Elshikha, A.S.; Avdiaj, R.; Searcy, L.A.; et al. Human Alpha-1 Antitrypsin Attenuates ENaC and MARCKS and Lowers Blood Pressure in Hypertensive Diabetic db/db Mice. Biomolecules 2022, 13, 66. [Google Scholar] [CrossRef]
- Qin, H.; Bollag, W.B. The caveolin-1 scaffolding domain peptide decreases phosphatidylglycerol levels and inhibits calcium-induced differentiation in mouse keratinocytes. PLoS ONE 2013, 8, e80946. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, C.; Deng, L.; Xie, E.; Yadav, N.; Tie, Y.; Cheng, Z.; Deng, J. Novel function of fluvastatin in attenuating oxidized low-density lipoprotein-induced endothelial cell ferroptosis in a glutathione peroxidase4- and cystine-glutamate antiporter-dependent manner. Exp. Ther. Med. 2021, 22, 1275. [Google Scholar] [CrossRef] [PubMed]
- Alli, A.A.; Desai, D.; Elshika, A.; Conrad, M.; Proneth, B.; Clapp, W.; Atkinson, C.; Segal, M.; Searcy, L.A.; Denslow, N.D.; et al. Kidney tubular epithelial cell ferroptosis links glomerular injury to tubulointerstitial pathology in lupus nephritis. Clin. Immunol. 2023, 248, 109213. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Z.; Zhang, J.; Guan, M.; Zheng, Y.; Wang, Y.; Wu, H.; Su, W.; Li, P. Naringenin regulates cigarette smoke extract-induced extracellular vesicles from alveolar macrophage to attenuate the mouse lung epithelial ferroptosis through activating EV miR-23a-3p/ACSL4 axis. Phytomedicine 2024, 124, 155256. [Google Scholar] [CrossRef]



| Antibody | Company | Catalog Number | Application |
|---|---|---|---|
| caveolin-1 | Cell Signaling Tech; Danvers, MA, USA | 3267 | Western blotting |
| CD9 | Abcam; Waltham, MA, USA | ab223052 | Western blotting |
| TSG101 | Abcam; Waltham, MA, USA | ab30871 | Western blotting |
| GPX4 | Abcam; Waltham, MA, USA | ab125066 | Western blotting |
| XCT | Abcam; Waltham, MA, USA | ab175186 | Western blotting |
| ACLS4 | Santa Cruz Biotech; Dallas, TX, USA | Sc-271800 | Immunohistochemistry |
| CD93 | Santa Cruz Biotech; Dallas, TX, USA | Sc-365172 | Immunohistochemistry |
| Caspase-3 | Proteintech; Rosemont, IL, USA | 66470 | Western blotting |
| Cleaved caspase-3 | Cell Signaling Tech; Danvers, MA, USA | 9661 | Western blotting |
| 4-Hydroxynonenal | R&D Systems; Minneapolis, MN, USA | MAB3249 | Immunohistochemistry |
| Parameter | CtsB STZ | CtsB | p-Value |
|---|---|---|---|
| Body weight | 25.065 | 27.148 | p = 0.549 |
| Blood glucose | 396.250 | 149.750 | p = 0.001 |
| Blood pressure | 139.250 | 105.000 | p ≤ 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schramm, W.C.; Bala, N.; Arekar, T.; Malik, Z.; Chacko, K.M.; Lewis, R.L.; Denslow, N.D.; Scindia, Y.; Alli, A.A. Enrichment of Bioactive Lipids in Urinary Extracellular Vesicles and Evidence of Apoptosis in Kidneys of Hypertensive Diabetic Cathepsin B Knockout Mice after Streptozotocin Treatment. Biomedicines 2024, 12, 1038. https://doi.org/10.3390/biomedicines12051038
Schramm WC, Bala N, Arekar T, Malik Z, Chacko KM, Lewis RL, Denslow ND, Scindia Y, Alli AA. Enrichment of Bioactive Lipids in Urinary Extracellular Vesicles and Evidence of Apoptosis in Kidneys of Hypertensive Diabetic Cathepsin B Knockout Mice after Streptozotocin Treatment. Biomedicines. 2024; 12(5):1038. https://doi.org/10.3390/biomedicines12051038
Chicago/Turabian StyleSchramm, Whitney C., Niharika Bala, Tanmay Arekar, Zeeshan Malik, Kevin M. Chacko, Russell L. Lewis, Nancy D. Denslow, Yogesh Scindia, and Abdel A. Alli. 2024. "Enrichment of Bioactive Lipids in Urinary Extracellular Vesicles and Evidence of Apoptosis in Kidneys of Hypertensive Diabetic Cathepsin B Knockout Mice after Streptozotocin Treatment" Biomedicines 12, no. 5: 1038. https://doi.org/10.3390/biomedicines12051038
APA StyleSchramm, W. C., Bala, N., Arekar, T., Malik, Z., Chacko, K. M., Lewis, R. L., Denslow, N. D., Scindia, Y., & Alli, A. A. (2024). Enrichment of Bioactive Lipids in Urinary Extracellular Vesicles and Evidence of Apoptosis in Kidneys of Hypertensive Diabetic Cathepsin B Knockout Mice after Streptozotocin Treatment. Biomedicines, 12(5), 1038. https://doi.org/10.3390/biomedicines12051038







